Porous tantalum rods improve the hip joint function of patients with avascular necrosis of the femoral head after femoral neck fracture surgery: study protocol of a randomized controlled trial
He-ling Zhang*, Ming-hao Deng, Jian-cheng Yang
Department of Orthopedic Trauma, Qinghai University Affiliated Hospital, Xining, Qinghai Province, China
INTRODUCTION
History and current related studies
Femoral neck fracture can render the blood supply to the femoral head insuf ficient, leading to avascular necrosis of the femoral head. This often increases treatment dif ficulty and can have a strong in fluence on the recovery of hip joint function (Li et al., 2015; Sharma et al., 2015; Hu et al., 2016; Kuo et al., 2016; Varghese et al., 2016; Zhang et al., 2016). Although core decompression for treatment of avascular necrosis of the femoral head has been largely used in clinical settings, the mid-term therapeutic effects are not satisfactory in some patients (Yang et al., 2010).
Biocompatibility is de fined as the ability of a biomaterial,prosthesis, or medical device to perform with an appropriate host response. The biocompatibility of a bone implant and host in fluences the therapeutic effects. Therefore, biomaterials that are less likely to induce negative host responses may increase the therapeutic effects of such treatments for orthopedic diseases. A porous tantalum rod is a bone trabeculalike metal implant that is used to support weight-bearing areas of necrotic bone, preventing further collapse of the necrotic area. This implant has exhibited favorable effects in the early treatment of avascular necrosis of the femoral head(Zhong, 2012). Although previous reports have focused on porous tantalum rod implantation for clinical treatment of avascular necrosis of the femoral head after fermoral neck fracture, few have assessed the long-term therapeutic effects or biocompatibility of porous tantalum rods.
Main objective
We aim to con firm 1) whether, compared with core decompression alone, core decompression with porous tantalum rod implantation improves the hip joint function of patients with avascular necrosis of the femoral head after femoral neck fracture surgery, 2) whether the porous tantalum rod shows favorable biocompatibility with the human body,and 3) whether this treatment method is feasible for treating avascular necrosis of the femoral head after femoral neck fracture surgery.
Novelty of this study
A literature search conducted on 30 June 2016 identi fied no similar studies published since June 2006 (according to Web of Science and ClinicalTrial.gov). Therefore, we believe our research to be innovative.
METHODS/DESIGN
Study design
A prospective, single-center, randomized controlled openlabel trial.
Study setting
Department of Orthopedic Trauma, Qinghai University Af filiated Hospital, China.
Study procedure
A total of 100 patients with avascular necrosis of the femoral head after femoral head fracture surgery who are scheduled to receive treatment in the Department of Orthopedic Trauma, Qinghai University Af filiated Hospital, China will be randomly assigned to undergo core decompression and porous tantalum rod implantation (experimental group, n =50) or core decompression only (control group, n = 50). All patients will be followed up for 1 year (Figure 1).
Inclusion criteria
Patients meeting all of the following conditions will be considered for admission:
· Patients with fracture nonunion as indicated by a clear fracture line 12 months after femoral neck fracture;avascular necrosis of the femoral head as shown by cystic degeneration, sclerosis, and uneven density on X-ray images and CT scans
· Patients with avascular necrosis of the femoral head after femoral neck fracture surgery who underwent internal fixation between 1 January 2016 and 31 May 2017
· Age 18-80 years
· Any sex and nationality
· Provision of signed informed consent to participate in the trial
Exclusion criteria
Patients will be excluded from this trial if they present with one of the following:
· Poor general health
· Chronic disease or surgical contraindications
· Alcohol abuse or long-term use of hormone drugs
· Unable or declines to cooperate with treatment and examination because of language and/or mental disorders
· Unable or declines to cooperate with rehabilitation treatment because of mental and/or psychological disorders
Withdrawal criteria
Patients will be withdrawn from the trial if any of the following occurs:
· Withdrawal of informed consent and refusal to continue treatment
· Unable to undergo further treatment because of severe adverse events
· Poor compliance during the trial
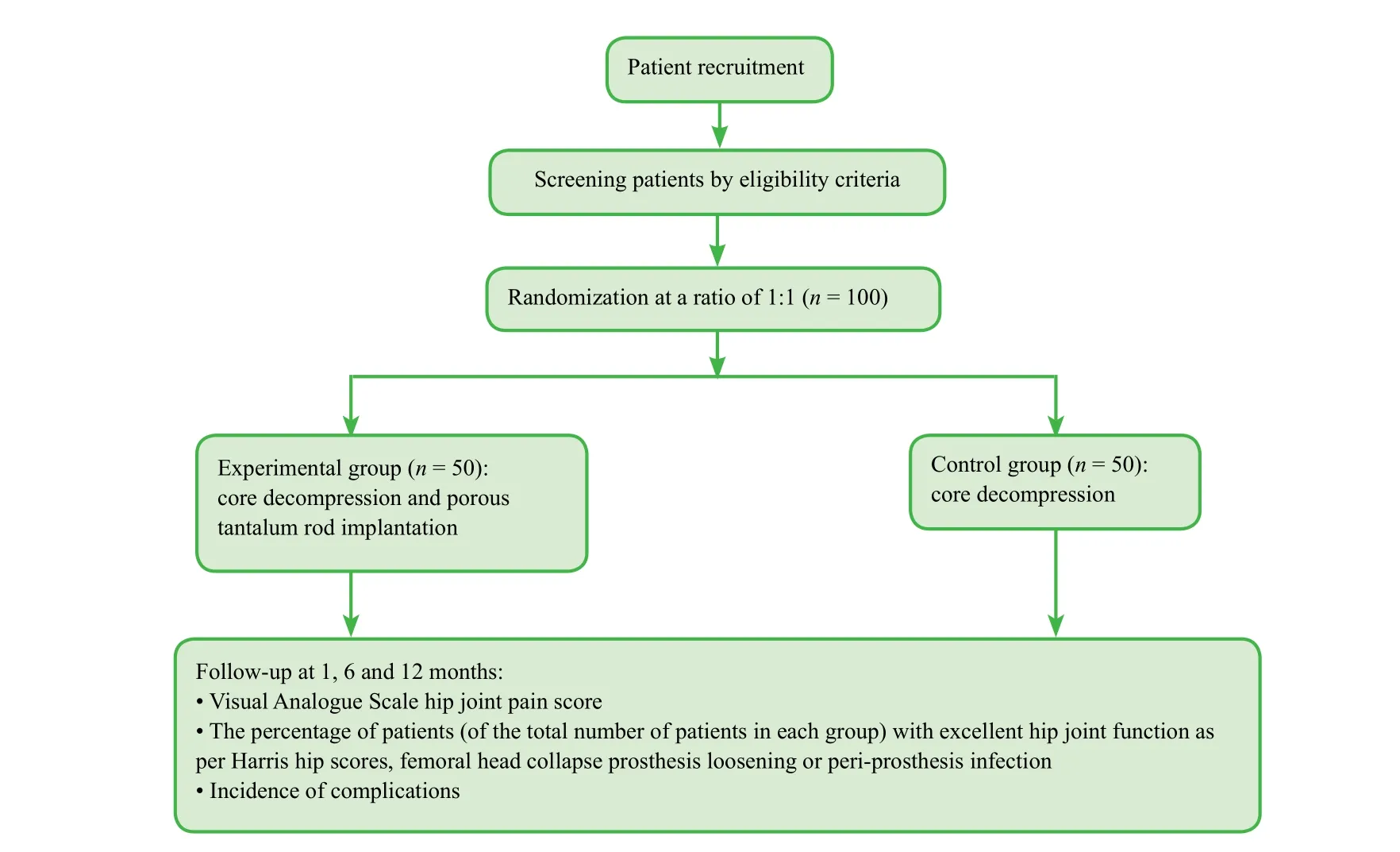
Figure 1: Flow chart of the trial.
Baseline evaluation
Prior to randomization, we will collect patient baseline data,including demographic data (age, sex, level of education,body mass index) and general disease history (current,previous, and genetic history).
Sample size
In accordance with a previous study (Gong et al., 2010),we hypothesized that the percentage of patients with excellent hip joint function as per Harris hip scores 12 months after surgery will be 92%. We used PASS 14.0 software(Jerry Hintze, Kayville, UT, USA) to calculate sample size, with a signi ficance level of α = 0.05 (two-sided), β≤ 0.1, and power = 90%. The final effective sample size was determined to be n = 93 per group. Taking a 20% rate of lost patients into consideration, we would require 112 patients per group. After screening against the inclusion and exclusion criteria, we plan to include 50 patients per group.
Recruitment
Potential patients from the wards in our hospital will be informed about the trial through a recruitment advertisement on the hospital bulletin board. Those interested in participating will be asked to contact the project manager.After providing written informed consent, all potential participants will be screened according to the above inclusion and exclusion criteria.
Randomization
One day prior to treatment, we will use a random number table generated by SPSS 19.0 software (IBM Corporation,Armonk, NY, USA) to number the 100 patients in successive order. The patients assigned even numbers will be included in the experimental group and those assigned odd numbers will comprise the control group.
Blinding
This is an open-label trial. Group information and therapeutic regimen are fully exposed to physicians, patients,and assessors.
Intervention
Experimental group
· A titanium alloy guiding pin (Shenzhen Baoxing Biological Preparation Co., Ltd., China; 3.2 mm in length) with good biocompatibility will be drilled at a site 2.0 cm inferior to the inferior greater trochanter in the necrotic area of the femoral head. With the assistance of a C-arm fluoroscopic X-ray system, a guiding pin will be positioned at the center of the femoral neck relative to the subchondral bone and necrotic area and inserted to a point 5 mm inferior to the surface of the femoral head cartilage. Necrotic bone in the necrotic area and dead bone in the area of cystic degeneration will be curetted and removed using a standard hollow biopsy tool (6 mm in diameter). The pore will then be ex-panded using an annular bit (10 mm in diameter) and core decompression will be performed.
· After core decompression, porous titanium rods (Trabecular Metal, Zimmer Inc., USA; cylinders, 10 mm in diameter, 70-130 mm in length, 25 mm length of screw thread at the end, 5 mm gradient, 14 mm thickness, with good mechanical performance, excellent biocompatibility,and favorable stabilization in the initial stage after implantation) will be placed with the lateral part of the rod implant parallel to the level of cortical bone and slightly embedded in the cortical bone.
Control group
· With the assistance of a C-arm fluoroscopic X-ray system, a Kirschner wire (2.5 mm in diameter) will be positioned at the center of the femoral neck relative to the subchondral bone and necrotic area and inserted to a point 5 mm inferior to the surface of the femoral head cartilage.The necrotic bone in the necrotic area and dead bone in the area of cystic degeneration will be curetted and removed using a standard hollow biopsy tool (6 mm in diameter). The pore will then be expanded using an annular bit (9-12 mm in diameter) and core decompression will be performed.
Postoperative management
· Routine drainage, preventive anti-infection treatment and functional rehabilitation exercise should be performed in each group. After surgery, intravenous administration of antibiotics should be required for no more than 24 hours.The drainage strips are expected to be removed within 48 hours after surgery.
· Lower limb functional exercises, including leg-raising,kicking, and ankle flexion and extension, under non-weight bearing conditions, are required and rehabilitation treatments using tendon-soothing maneuvers are necessary for promoting limb blood circulation and preventing muscular atrophy.
· The two groups of patients will be asked to lie in bed for 3 months after the surgery. They will be allowed to bear partial weight between 3 and 9 months after surgery and bear total weight 9 months after surgery. All patients will receive follow up assessments once every 3 months for more than 1 year. X-ray images and CT scans should be collected by an experienced radiologist during the follow up to judge the disease progression. Follow-ups will be immediately discontinued if femoral head collapse occurs.
Outcome measures
Primary outcome measure
· We will use the percentage of patients (of the total number of patients in each group) whose hip joint function is graded as excellent, as per Harris hip scores 12 months after surgery, to evaluate the recovery of hip joint function.The Harris hip score ranges from 0-100 points with higher scores indicating better hip joint function. Hip joint function is scored as follows: ≥ 90 is excellent, 80-89 very good,70-79 good, and < 70 poor.
Secondary outcome measures
· The percentage of patients (of the total number of patients in each group) with excellent hip joint function prior to, 1, and 6 months after surgery: the evaluation criteria are the same as above.
· Hip joint pain score on a Visual Analogue Scale (VAS)prior to and 1, 6, and 12 months after surgery: VAS hip joint pain score will be used to evaluate the severity of pain and ranges from 0-10, with higher scores indicating more severe pain. A score of 0 represents no pain, scores > 0 and≤ 3 represent mild pain, scores > 3 and ≤ 6 moderate pain,and scores > 6 and ≤ 10 severe pain (Knop et al., 2001).· The percentage of patients presenting with femoral head collapse, prosthesis loosening, or peri-prosthesis infection 6 and 12 months after surgery will be used to evaluate the biocompatibility of the biomaterial with the host site. If X-ray images and CT scans show evidence of femoral head collapse, prosthesis loosening, and/or periprosthesis infection, then the biocompatibility between the porous tantalum rod implant and the host will be considered to be poor. A lower percentage value indicates better biocompatibility between the porous tantalum rod implant and host.
· The incidences of complications 6 and 12 months after surgery are used to evaluate the safety of various implantation methods. The percentage of patients (of the total number of patients in each group) presenting with adverse events will be calculated.
The schedule of outcome measurement assessments is shown in Table 1.
Adverse events
Possible adverse events include any expected or unexpected symptoms. If severe adverse events occur, information including the date of occurrence and measures taken related to the treatment of the adverse events will be reported to the principal investigator and the institutional review board within 24 hours.
Data collection, management, analysis, and open access
Data collection: Case report forms with demographic data,disease diagnosis, accompanying diseases, drug allergy history, and adverse events will be collected, processed using Epidata software, collated, and then electrically recorded using a double-data entry strategy by data managers.

Table 1: Timing of outcome assessment
Data management: The locked electronic database will not be altered in any way, and will only be available to the project manager. Paper and electronic data regarding screening, informed consent, and clinical outcomes will be preserved at the Af filiated Hospital of Nantong University,China.
Data analysis: The electronic database will be made available to a professional statistician for statistical analysis. An outcome analysis report will be made by the statistician and submitted to the lead researchers. An independent data monitoring committee will supervise and manage the trial data with the goal of ensuring a scienti fic and stringent trial process, resulting in accurate and complete data.
Data open access: Anonymized trial data will be published at http://www. figshare.com.
Statistical analysis
Statistical analysis will be performed by a statistician using SPSS 19.0 software (IBM Corporation) and will follow the intention-to-treat principle. Normally distributed measurement data will be expressed as a mean, standard deviation,min, and max. Non-normally distributed measurement data will be expressed as a lower quartile (q1), median,and upper quartile (q3). The chi-square test will be used to compare the percentage of patients with excellent hip joint function as per Harris hip scores 12 months after surgery,the percentage of patients presenting with femoral head collapse, prosthesis loosening, peri-prosthesis infection, and incidence of complications 6 and 12 months after surgery.The Mann-Whitney U test will be used to compare the VAS score prior to and 1, 6, and 12 months after surgery. Statistical signi ficance will be accepted when α = 0.05.
Auditing
The progress of the trial will be reported to the ethics committee of Qinghai University Af filiated Hospital, China every 3 months and the trial status will be updated in the registration database.
Confidentiality
Valuable trial data will be transcribed, dated, and uploaded to a dedicated computer by two staff members. Data will be scheduled, checked, locked by an investigator, passwordprotected, and will not be altered. Data regarding this trial protocol recorded on paper will be preserved by Qinghai University Af filiated Hospital, China.
DISCUSSION
Significance of this study
With this study, we hope to provide quantitative evidence for the ef ficacy and biocompatibility of porous tantalum rod implantation in the treatment of avascular necrosis of the femoral head in patients who have undergone femoral neck fracture surgery.
Advantages and limitations of this study
Advantages: this study is a prospective, single-center,randomized, controlled, open-label trial with the aim of investigating the ef ficacy and biocompatibility of porous tantalum rod implantation in the treatment of avascular necrosis of the femoral head after femoral neck fracture surgery. Compared to a previous report (Zhong, 2012), this study protocol is more normative.
Limitations: the sample size (n = 50 per group) is small.Additionally, we will not collect X-ray radiological findings of the hip joint. Finally, the follow-up period is very short. In future work, patients should be followed up for more than 1 year to determine the long-term therapeutic effects of porous tantalum rod implantation on avascular necrosis of the femoral head after femoral neck fracture surgery.
Contributions to future research
This study is powered to assess whether porous tantalum rod implantation can promote the recovery of hip joint function in patients with avascular necrosis of the femoral head after femoral neck fracture surgery, and whether this treatment is associated with decreased collapse of the femoral head,prosthesis loosening, and peri-prosthesis infection. This treatment method is expected to increase the percentage of patients with excellent hip joint function 12 months after surgery, as per Harris hip scores, to be ≥ 92%.
Trial status
Recruiting is ongoing at the time of submission.
Conflicts of interest
None declared.
Author contributions
HLZ conceived and designed the study protocol. MHD and HLZ wrote the manuscript. JCY authorized the manuscript.All authors approved the final version of this manuscript for publication.
Plagiarism check
This paper was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
Gong NJ, Wang SJ, Liu WG, Yin QF (2010) The porous tantalum screw and quadratus femoris muscle-pedicle bone graft in treating Ficat stage II necrosis of the femoral head. Shandong Daxue Xuebao: Yixue Ban 48:99-101.
Hu LY, Jia QY, Yu Y, Cao Y, Zheng SQ (2016) Clinical effects of internal fixation with Herbert screws for the treatment of Pipkin femoral head fractures. Zhongguo Gu Shang 29:162-166.
Li Q, Xie XR, Wang QB, Lu J (2015) Treatment of fresh subtrochanteric fracture combined with old femoral neck fracture with hemiarthoplasty through anterolateral approach. Zhongguo Gu Shang 28:1056-4059.
Knop C, Oeser M, Bastian L, Lange U, Zdichavsky M, Blauth M(2011) Development and validation of the Visual Analogue Scale(VAS) Spine Score. Unfallchirurg 104:488-497.
Kuo FC, Kuo SJ, Ko JY (2016) Overgrowth of the moral neck after hip fractures in children. J Orthop Surg Res 11:50.
Sharma N, Bache E, Clare T (2015) Bilateral femoral neck fractures in a young patient suffering from hypophosphatasia due to a first time epileptic seizure. J Orthop Case Rep 5:66-68.
Varghese VD, Livingston A, Boopalan PR, Jepegnanam TS (2016)Valgus osteotomy for nonunion and neglected neck of femur fractures. World J Orthop 7:301-307.
Yang J, Kang DP, Shem B, Zhou ZK, Pei FX, Sun GJ (2010) Treatment of early stage osteonecrosis of the femoral head with multiple small-diameter drilling core decompression. Zhonghua Guke Zazhi 30:58-61.
Zhang YY, Zuo JL, Gao ZL (2016) Case-control study on methods of limb length control in hip arthroplasty. Zhongguo Gu Shang 29:102-106.
Zhong GH (2012) Core decompression with bone graft and porous tantalum rod insertion for avascular necrosis of the femoral head.Zhongguo Zuzhi Gongcheng Yanjiu 16:5501-5505.
 Clinical Trials in Orthopedic Disorder2016年3期
Clinical Trials in Orthopedic Disorder2016年3期
- Clinical Trials in Orthopedic Disorder的其它文章
- Chondromyxoid fibroma of the distal one-third of the fibula: a rare tumour at rare site
- Traction apophysitis of the medial malleolus
- Effects of a combined nerve block on intraoperative stress and postoperative immune function in elderly patients subjected to total hip replacement: study protocol for a randomized controlled trial
- Digital navigation enhances cervical pedicle screw placement accuracy and safety: study protocol of a randomized controlled trial
- Safety and effectiveness of proximal femoral nail antirotation for the treatment of intertrochanteric femoral fracture: study protocol for a prospective case series
- Commentary on "clinical efficacy of tranexamic acid administration via different routes during total hip arthroplasty:study protocol for a prospective randomized controlled trial”
