Two Different Cd(Ⅱ)Coordination Polymers Based on a Flexible Bis(imidazole)and Different Dicarboxylate Co-ligands:Syntheses,Crystal Structures and Properties
LU Jiu-FuZHAO Cai-BinJIN Ling-XiaSHI JuanLI Li-HuaGE Hong-Guang
(Shannxi Province Key Laboratory of Catalytic Foundation and Application,College of Chemical&Environment Science, Shaanxi University of Technology,Hanzhong,Shaanxi 723001,China)
Two Different Cd(Ⅱ)Coordination Polymers Based on a Flexible Bis(imidazole)and Different Dicarboxylate Co-ligands:Syntheses,Crystal Structures and Properties
LU Jiu-Fu*ZHAO Cai-BinJIN Ling-XiaSHI JuanLI Li-HuaGE Hong-Guang*
(Shannxi Province Key Laboratory of Catalytic Foundation and Application,College of Chemical&Environment Science, Shaanxi University of Technology,Hanzhong,Shaanxi 723001,China)
Two novel Cd(Ⅱ)coordination polymers,[Cd2(μ2-H2O)(1,5-Bip)(Tpa)2]n(1),[Cd(1,5-Bip)(Oba)]n(2),where 1,5-Bip=1,5-bis(imidazole)pentane,H2Tpa=terephthalic acid,H2Oba=4,4′-oxybis(benzoic acid),were synthesized under hydrothermal conditions and characterized by single crystal X-ray diffraction,powder XRD,FT-IR,TGA and elemental analysis technique.Single crystal X-ray analysis revealed that complex 1 features a 3D network with a 5-connected(46.64)topology and complex 2 shows a 2D layer with a(4,4)topology.Furthermore,the photoluminescence properties in the solid state at room temperature and thermal behaviors of these complexes were also investigated.CCDC:1043520,1;1444145,2.
hydrothermal synthesis;coordination polymer;topology;photoluminescence property
0 Introduction
Construction of new metal coordination polymers (MCPs)has attracted attention due to their diverse structural topologies and potential applications as functional materials such as gas storage,magnetism, catalysis,and luminescence[1-5].Up to now,the selection of suitable organic ligands is crucial for constructing extended coordination frameworks,which has been prepared on the basis of carboxylate-type O-donors and amine-or N-donors.Thereinto,di-and polycarboxylic acids are widely used as bridging ligands to constructcoordinationframeworkswithversatile structures[8],such as terephthalic acid(H2Tpa),4,4′-oxybis(benzoic acid)(H2Oba)[9-12].Meanwhile,the 1,5-bis(imidazole)pentane(1,5-Bip)bearing alkyl spacers is a good choice of N-donor ligands,in which the flexible nature of spacers allows the ligands to bend and rotate when it coordinates to metal centers[13-14], and it possesses trans and cis conformations which favor the possibility of generating fascinating structures.
Considering all these above-mentioned points and our previous work[15-17],we selected the flexible 1,5-Bip ligand and H2Tpa,H2Oba ligands as an interesting candidate to construct two novel MCPs,[Cd2(μ2-H2O) (1,5-Bip)(Tpa)2]n(1)and[Cd(1,5-Bip)(Oba)]n(2)(H2Tpa =terephthalic acid,H2Oba=4,4′-oxybis(benzoic acid) and1,5-Bip=1,5-bis(imidazole)pentane)(Scheme 1).
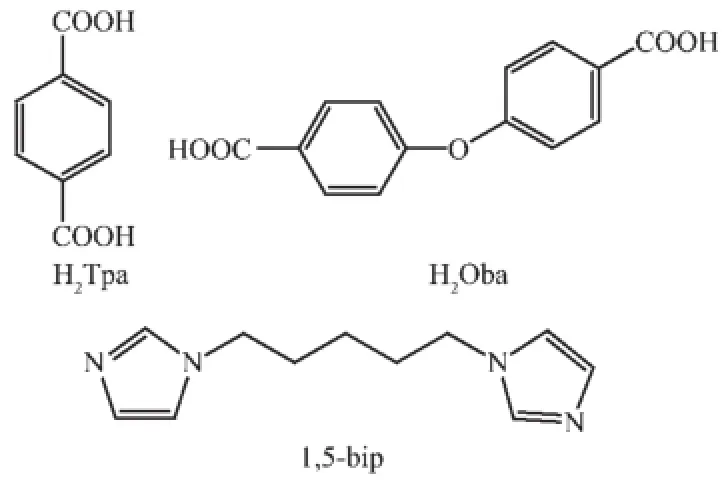
Scheme 1Structures of the organic ligands
The single crystal structures and photoluminescence properties of 1 and 2 have been investigated.
1 Experimental
1.1 Materials and physical measurements
Allreagentsusedinthesyntheseswere purchasedfromBeijingLabmaterialTechnology Development Co.,Ltd.Elemental analyses for carbon, hydrogen,and nitrogen atoms were performed on a Vario ELⅢelemental analyzer.The infrared spectra (4 000~400 cm-1)were recorded by using KBr pellet on an Avatar 360 E.S.P.IR spectrometer.Powder X-ray diffraction(PXRD)data were collected on a Rigaku UltimaⅣX-ray diffractometer with Cu Kα radiation (λ=0.154 056 nm).Thermogravimetric analysis(TGA) was performed on a TA-SDT Q600 thermal analyzer under N2atmosphere with a heating rate of 10℃· min-1in the range of 30~800℃.The luminescent spectra for polycrystalline samples were measured at room temperature on a Perkin Elmer LS 55 fluorescence spectrometer with a xenon arc lamp as the light source.
1.2 Synthesis of complex
1.2.1 Synthesis of[Cd2(μ2-H2O)(1,5-Bip)(Tpa)2]n(1)
A mixture of Cd(NO3)2·4H2O(0.2 mmol,58.2 mg),1,5-Bip(0.20 mmol,40.8 mg),NaOH(0.2 mmol, 8 mg),H2Tpa(0.2 mmol,31.5 mg)and 8 mL H2O were placedina25mLTeflon-linedstainlesssteel container,which was heated to 150℃for 3 days,and then cooled to room temperature over 24 hours. Colorless block crystals of 1 were collected.Yield: 43%based on Cadmium.Elemental analysis(%):Calcd. for C27H28Cd2N4O10:C 40.84,H 3.52,N 7.06;Found:C 40.53,H 3.72,N 7.81.IR(cm-1):3 440(s),3 125(w), 2 918(w),1 615(s),1 557(m),1 500(w),1 420(m),1 352 (m),1 270(w),1 153(w),1 091(w),997(w)and 721(m). 1.2.2Synthesis of[Cd(1,5-Bip)(Oba)]n(2)
The synthetic procedure of 2 was similar to that for 1,except that H2Tpa(0.2 mmol)was used instead of H2Oba.Colorless block crystals of 2 were collected. Yield:67%based on Cadmium.Elemental analysis(%): Calcd.for C25H24CdN4O5:C 52.37,H 4.19,N 9.78; Found:C 52.53,H 4.72,N 9.81.IR(cm-1):3 416(w), 3 119(m),2 944(m),2 885(w),1 611(s),1 564(s),1 512 (s),1 375(s),1 274(m),1 026(m)and 832(m).
1.3 Determination of crystal structures
Single crystal data of 1(0.26 mm×0.21 mm×0.17 mm)or 2(0.23 mm×0.18 mm×0.14 mm)were collected on a Bruker SMART APEXⅡCCD diffractometer equipped with graphite-monochromatized Mo Kα radiation(λ=0.071 073 nm)by using the φ-ω scan mode at room temperature.Absorption corrections based on multi-scan were obtained using the SADABS program[18]. All the structures were solved with direct methods and refined with a full-matrix least-squarest on F2using SHELX 97[19-20].Hydrogens were located by geometric calculations,and their positions and thermal parameters were fixed during the structure refinement.Crystallographic data and experimental details of structural analyses for complexes 1 and 2 are summarized in Table 1.Selected bond lengths and bond angles of 1 and 2 are given in Table 2.
CCDC:1043520,1;1444145,2.
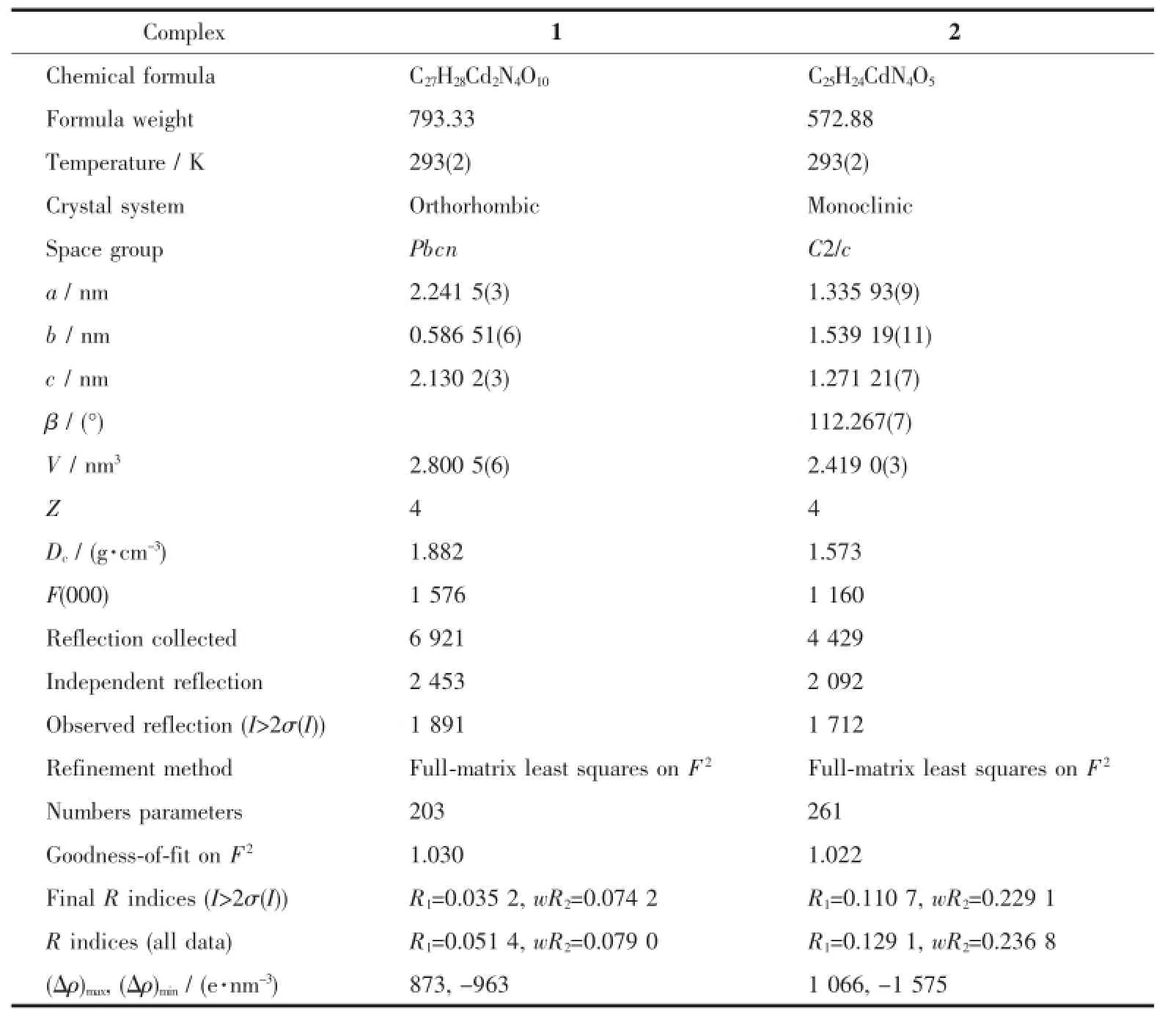
Table 1Crystallographic data and structure refinement for 1 and 2
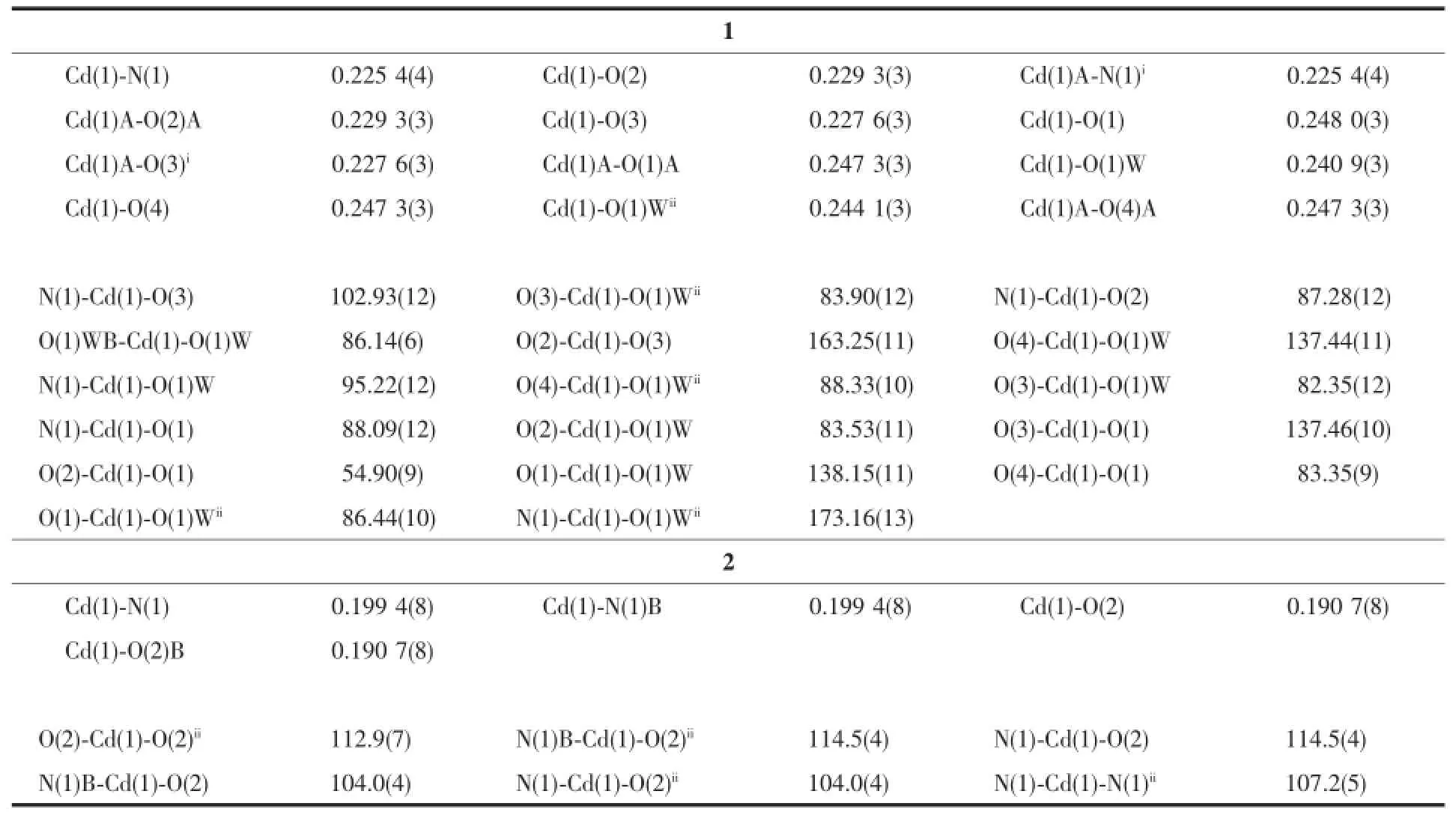
Table 2Selected bond lengths(nm)and angles(°)for 1 and 2
2 Results and discussion
2.1 Description of the structures
2.1.1 [Cd2(μ2-H2O)2(1,5-Bip)(Tpa)2]n(1)
Single X-ray diffraction study revealed that the asymmetric unit of 1 contains two Cd(Ⅱ)cations,two μ2-coordinated H2O molecule,two Tpa2-anions and one 1,5-Bip ligand(Fig.1a).As shown in Fig.1b,the two crystallographic symmetric Cd(Ⅱ)ions have the samedistorteddecahedralcoordinationgeometry, which are seven-coordinated by four carboxylate oxygen atoms from two Tpa2-ligands,one nitrogen atom of 1,5-Bip ligand and two μ2-coordinated H2O molecule. A μ2-coordinated H2O molecule connected two adjacent Cd(Ⅱ)ions to form a 1D zigzag chain(Fig.1c),and Tpa2-anions as a μ2-bridge connect the abovementioned 1D zigzag chain to form a 2D layer,as depicted in Fig.1d.Then,neighboring 2D layers constructed by Cd(Ⅱ)ions,μ2-coordinated H2O molecule and Tpa2-anion were further connected by 1,5-Bip ligands to give a novel 3D framework with 1D channels whose internal dimension is ca.1.177 02 nm×1.119 67 nm along b axis(Fig.1e).
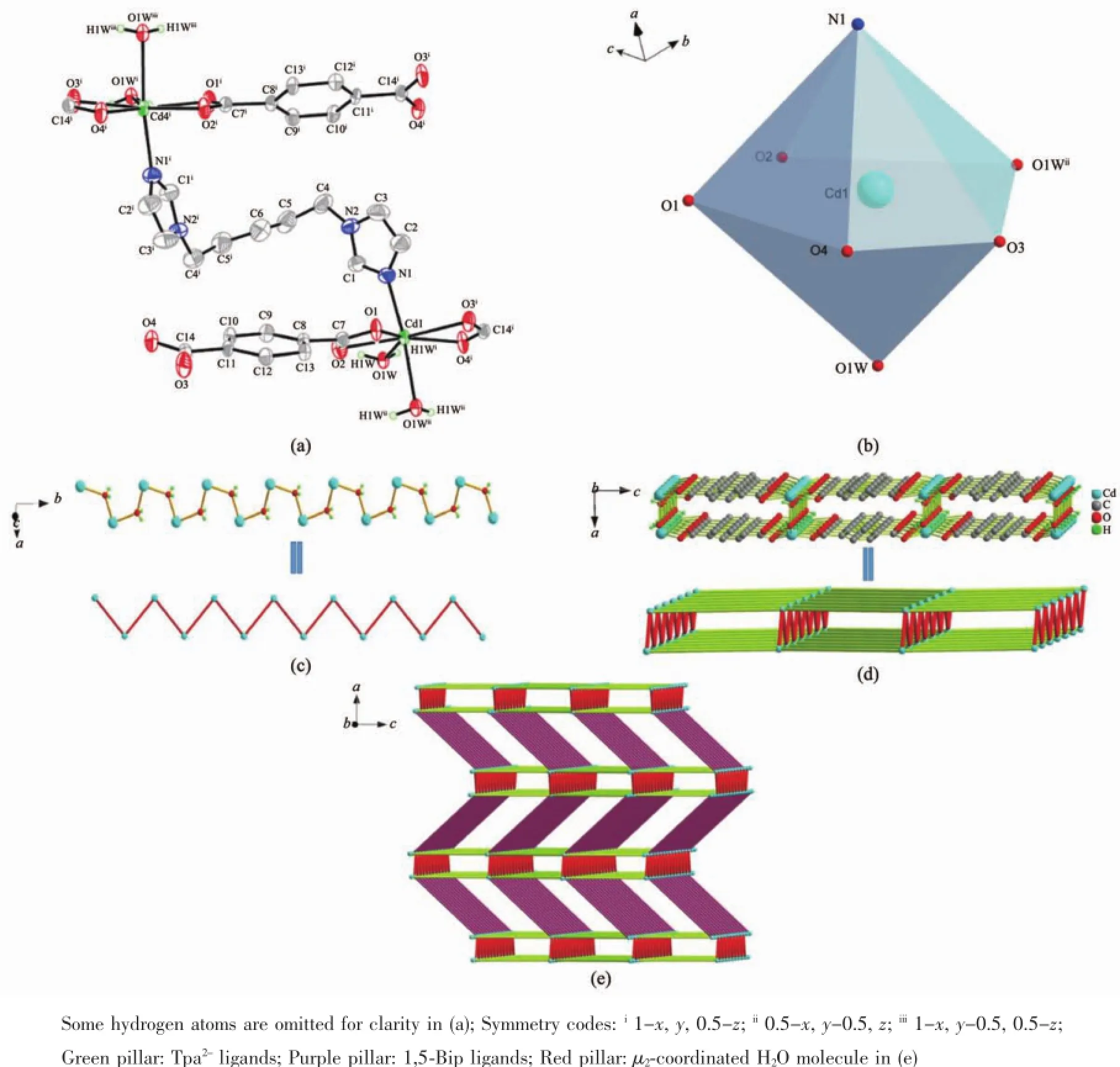
Fig.1(a)Structure of complex 1 with thermal ellipsoid at 50%probability levels;(b)View of distorted coordination geometry of Cd(Ⅱ)ion;(c)View of a 1D zigzag chain constructed by Cd(Ⅱ)ions and H2O molecule;(d)View of a 2D layer constructed by Cd(Ⅱ)ions,μ2-coordinated H2O and Tpa2-anions;(e)View of 3D uninodal 5-connected net of(46.64)topology in complex 1
From the topological perspective[21],the Cd(Ⅱ)ions can be considered as a 5-connected uninodal node, the 1,5-Bip,Tpa2-ligands and μ2-coordinated H2O molecule as a μ2-bridged linker with a Schlfli symbol of(46.64).
2.1.2 [Cd(1,5-Bip)(Oba)]n(2)
Single-crystal X-ray diffraction analysis showed that complex 2 exhibits a 2D(4,4)grid structure.The asymmetric unit contains one Cd(Ⅱ)ion on the inversion center,one 1,5-Bip ligand and one Oba2-anion.As depicted in Fig.2a,the Cd(Ⅱ)ion is in a CdN2O2tetrahedral geometry,completed by two N atoms from two 1,5-Bip ligands,two O atoms from two Oba2-anions(Cd-O 0.190 7 nm,Cd-N 0.199 5 nm). The bond angles for Cd are in the range of 103.98°~ 114.49°.The Bip and Oba2-ligands adopt a bidentate bridging mode to extend the Cd(Ⅱ)atoms to the resulting 2D(4,4)sql network(Fig.2b)incorporating a [Cd4(1,5-Bip)2(Oba)2]window of 1.453 8 nm×1.335 9 nm based on the Cd…Cd distances.
Although the interpenetration often occurs in 44-sql networks with large windows[22-23],no interpenetration between adjacent sheets is observed in 2,which may be due to the occupancy of the part of the twisting of flexible 1,5-Bip and Oba2-ligands.The adjacent 2D sheet are packed into the resultant 3D supramolecular framework(Fig.2c)by CBip-H…OOba(0.264 5 nm)and CBip-H…COba(0.269 7 nm)hydrogen bonds.
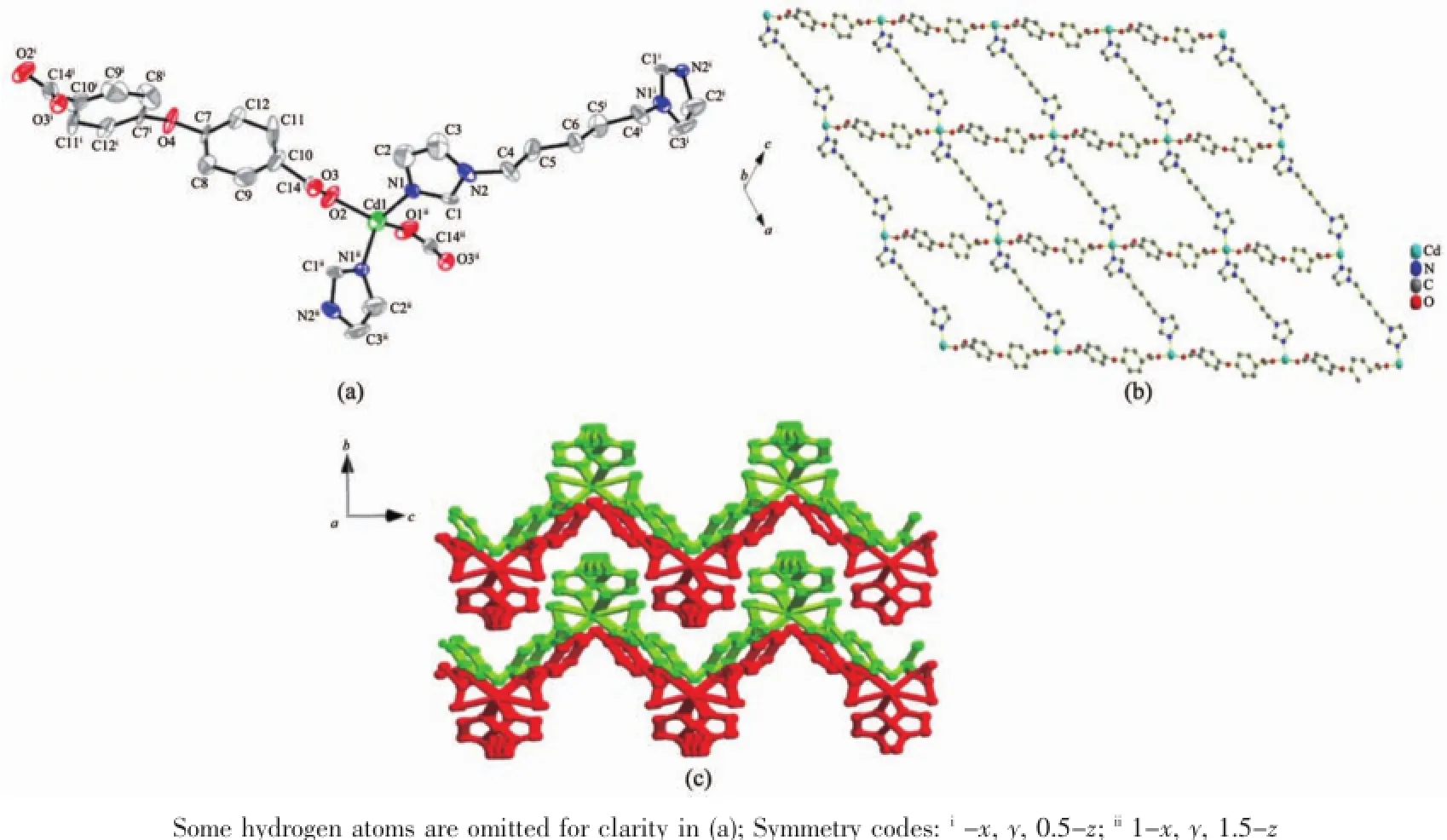
Fig.2(a)Structure of complex 2 with thermal ellipsoid at 40%probability levels;(b)2D(4,4)grid structure of 2 along b axis;(c)Packing of 2D nets in the crystal with an-ABAB-fashion
2.2 FT-IR spectra and X-ray diffraction
As shown in Fig.3,The strong band at 3 440 cm-1for 1 may be attributed to the stretching vibration of μ2-coordinated H2O molecule.The strong absorptions at 1 615,1 420 cm-1for 1,and 1 611,1 512 cm-1for 2,respectively,are characteristic of asymmetric and symmetric stretching bands of carboxylate groups (COO-),and the difference in the value between them is less than 200 cm-1,which indicates that the carboxylate groups behave as the chelate coordination modes[24].
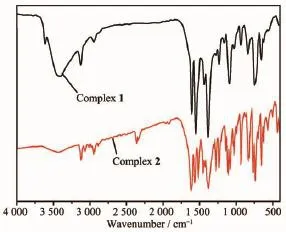
Fig.3IR spectra of complexes 1 and 2
The phase purities of complexes 1 and 2 were confirmed by powder X-ray diffraction measurements. As shown in Fig.4,the experimental patterns of 1 and 2 are consistent with the simulated ones based on the single crystal X-ray diffraction data.
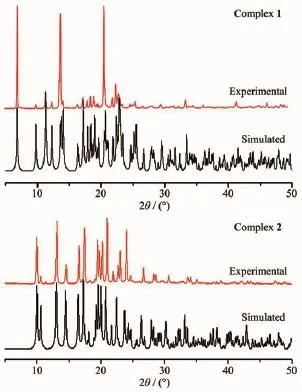
Fig.4Powder XRD patterns of complexes 1 and 2
2.3 Thermal behaviors
To have insight into the changes occurring during heat treatment of the prepared complexes,thermal gravimetric analysis(TGA)of the sample were carried out from 30 to 800℃at a heating rate of 10℃·min-1. The TGA curve of 1 displays a weight loss of 4.2% (Calcd.4.5%)from room temperature to 250℃,which corresponds to complete loss of two μ2-coordinated water molecule per unit cell.Its framework is stable up to 380℃and then the framework begins to collapse, accompanied by the release of Bip and Tpa2-ligands. After decomposition,the final residues is 17.3%, which can be attribute to CdO(Calcd.16.2%).For 2, the framework is stable up to 360℃and then the framework begins to collapse,accompanying the release of coordinated Bip and Oba2-ligands,the final residues of 2 is 21.9%corresponding to CdO(Calcd.22.4%).
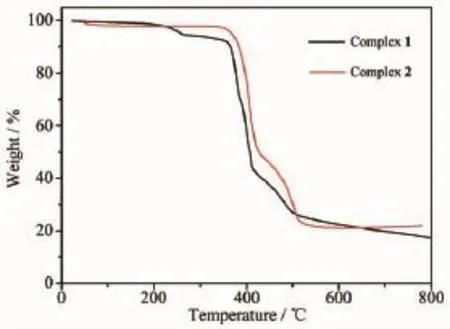
Fig.5TGA curves for complexes 1 and 2
2.4 Fluorescent properties
MCPs with d10metal centers have many potential applications such as in chemical sensors,photochemistry,and electroluminescent display[25-27].Therefore,in the present work,the photoluminescent properties of 1,5-Bip and complexes 1 and 2 have been investigated in the solid state at room temperature.The photoluminescence spectra of 1,5-Bip ligand and complexes 1 and 2 are shown in Fig.5.The free ligand Bip displays photoluminescence with emission maximum at 405 nm(λex=322 nm,).It can be presumed that this peak originate from the π*-π transition.While the emission of dicarboxylate belongs to π*-n transitions which is very weak compared to that of the π*-π transition of the Bip,so the dicarboxylates almost have no contribution to the fluorescent emission of assynthesized complexes[28].Upon an excitation band at 322 nm,intense emissions are observed at 427 nm for 1 and 432 nm for 2,respectively.In comparison to the free Bip ligands,the emission maximums of complexes 1 and 2 have changed and show blue or red shifts.This inequality in emission behavior may be a combination of several factors together,such asdifferent dicarboxylate co-ligand,coordinated water molecule,and the role of structural complexity and framework robustness cannot be ignored.Meanwhile, the Cd(Ⅱ)ion is difficult to oxidize or to reduce due to the d10configuration,so the emission of this compound is neither MLCT nor LMCT in nature.As a result,the emission can be assigned to intraligand transitions.
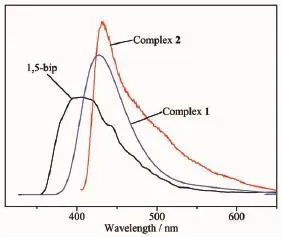
Fig.6Fluorescent emission spectra of complexes 1,2 and 1,5-Bip ligand in solid state at room temperature
3 Conclusions
In conclusion,we presented two novel Cd(Ⅱ) coordination polymers constructed from dicarboxylate and bis(imidazole)mixed ligands.Complex 1 is a 3D frameworkwitha5-connected(46.64)topology.Complex 2 shows a 2D layer with a(4,4)topology.In addition, photoluminescence of complexes 1 and 2 were studied inthesolidstateatroomtemperature.Further experiments exploring the structural effects of the spacer length of bis(imidazole)ligands on coordination polymers,and any resulting changes in physicochemical properties,are underway in our laboratory.
[1]Eddaoudi M,Moler D B,Li H L,et al.Acc.Chem.Res., 2001,34:319-330
[2]Halder G J,Kepert C J,Moubaraki B,et al.Science,2002, 98:1762-1765
[3]Dybtsev D N,Chun H,Yoon S H,et al.J.Am.Chem.Soc., 2004,126:32-33
[4]Kesanli B,Cui Y,Smith M R,et al.Angew.Chem.Int.Ed., 2005,44:72-75
[5]Ghosh A K,Jana A D,Ghoshal D,et al.Cryst Growth Des., 2006,6:701-707
[6]Anokhina E V,Vougo-Zanda M,Wang X Q,et al.J.Am. Chem.Soc.,2005,127:15000-15001
[7]Pan L,Parker B,Huang X Y,et al.J.Am.Chem.Soc., 2006,128:4180-4181
[8]Xu B,Xie J,Hu H M,et al.Cryst.Growth Des.,2014,14: 1629-1641
[9]Yin P X,Zhang J,Li Z J,et al.Crys.Growth Des.,2009,9: 4884-4892
[10]Wang R H,Hong M C,Luo J H,et al.Chem.Commun., 2003,8:1018-1022
[11]Zhang X M,Tong M L,Gong M L,et al.Chem.Eur.J., 2002,8:3187-3191
[12]MontneyMR,SupkowskiRM,LaDucaRL.CrystEngCommun, 2008,10:111-116
[13]Wen L L,Li Y Z,Lu Z D,et al.Crys.Growth Des.,2006,6: 530-534
[14]Fan J,Slebodnick C,Angel R,et al.Inorg.Chem.,2005,44: 552-558
[15]Lu J F,Liu J,Ge H G,et al.Chin.J.Struct.Chem.,2015, 34:284-252
[16]Lu J F,Min S T,Ge H G.J.Res.Chem.,2014,38:726-730
[17]Lu J F,Ge H G,Shi J.Chin.J.Struct.Chem.,2015,34: 1259-1264
[18]SMART&SAINT,Software Reference Manuals,Version 6.22,Madison(WI,USA),Bruker AXS Analytic X-ray Systems Inc.,2000.
[19]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structures,University of Göttingen,Germany,1997.
[20]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[21]Blatov V A.Struct.Chem.,2012,23:955-963
[22]Lu J F,Li L Q,Qiao L J,et al.J.Mol.Struct.,2015,1081: 79-84
[23]Lu J F,Wang M Z,Liu Z H.J.Mol.Struct.,2015,1098:41-46
[24]Chen W J,Wang Y,Chen C,et al.Inorg.Chem.,2003,42: 944-946
[25]Wu Q,Esteghamatian M,Hu N X,et al.Chem.Mater., 2000,12:79-85
[26]McGarrah J E,Kim Y J,Hissler M,et al.Inorg.Chem., 2001,40:4510-4518
[27]Santis G D,Fabbrizzi L,Licchelli M,et al.Angew.Chem., Int.Ed.Engl.,1996,35:202-209
[28]Hao H J,Liu F J,Su H F,et al.CrystEngComm,2012,14: 6726-6731
基于一种柔性双咪唑和不同双羧基配体构筑的两例镉(Ⅱ)的配位聚合物
卢久富*赵蔡斌靳玲侠史娟李利华葛红光*
(陕西理工学院化学与环境科学学院,陕西省催化基础与应用重点实验室,汉中723001)
在水热条件下合成了2个新颖的镉(Ⅱ)配位聚合物:[Cd2(μ2-H2O)(1,5-Bip)(Tpa)2]n(1)和[Cd(1,5-Bip)(Oba)]n(2)(H2Oba=4,4′-二羧酸二苯甲醚,H2Tpa=对苯二甲酸,1,5-Bip=1,5-二(咪唑基)戊烷)。并通过X射线单晶衍射,粉末XRD、红外光谱、元素分析以及热重分析对其结构进行表征。单晶解析结果表明:配位聚合物1是一个单节点5连接(46.64)拓扑三维空间网络结构,配位聚合物2是一个二维的(4,4)网格层状结构。另外,研究了2个配位聚合物在室温下的热稳定和荧光性能。
水热合成;配位聚合物;拓扑;荧光性质
O614.24+2
A
1001-4861(2016)06-0961-07
2015-12-29。收修改稿日期:2016-04-22。
国家自然科学基金(No.21373132)、陕西省教育厅项目(No.15JK114)、陕西理工学院博士启动项目(No.SLGQD14-10,SLGKYQD2-13)和陕西理工学院校级项目(No.SLGKY15-36)资助。
*通信联系人。E-mail:jiufulu@163.com,gehg@snut.edu.cn;会员登记号:S06N3217S1305。
10.11862/CJIC.2016.141

