Characterization,tissue distribution and grow th regulation of ghrs and igfbp1 in silver pomfret(Pampus argenteus)in hand ling stress
SUN Peng,YIN Fei,WANG Jian-jian,LI Yun-hang
(East China Sea Fisheries Research Institute,Chinese Academy of Fishery Sciences,Shanghai200090,China)
Characterization,tissue distribution and grow th regulation of ghrs and igfbp1 in silver pomfret(Pampus argenteus)in hand ling stress
SUN Peng,YIN Fei,WANG Jian-jian,LI Yun-hang
(East China Sea Fisheries Research Institute,Chinese Academy of Fishery Sciences,Shanghai200090,China)
Growth activities are mainly involved by growth hormone/insulin-like growth factors(GH/IGF)axis.GH and IGF are anabolic hormones,which increase muscle mass by stimulating protein synthesis and/or inhibiting protein breakdown.In fish,growth hormone receptor genes(ghrs)encode transmembrane receptor protein for GH.By binding GH,it can activate an intra-and inter cellular signal transduction pathway leading to growth.Type 1 insulin-like growth factor-binding protein is encoded byigfbp1gene,a member of igfbp family.Binding of this protein can prolong the half-life of IGFs and alter their interaction with cell surface receptors.Silver pomfret(Pampus argenteus)is an important commercial fish which has a wide distribution from the Arabian Gulf,the India Ocean and the East China Sea to Southeast Asia.Relevant artificial breeding techniques and P.argenteus reproductive biology have been studied in China and Kuwait since the 1980s.Due to its sensitivity to many kinds of stress factors,low growth rate and high mortality has been found inP.argenteusduring culture practices.However,little is known about its molecular aspects,including gene expression information of growth during stress phase.In the present study,characterization,distribution and expression pattern of growth-related gene(ghrsandigfbp1)responded to acute handling stress are examined.Our aim is to evaluate the effect of acute handling stress on growth in this species.Gene sequence analysis shows that the total length ofghr1,ghr2,andigfbp1ranges from 1280 to 3625 bp,and encodes amino acids from 64 to 627.Signal peptide sites and transmembrance helices are found in all three genes.GHR1 protein has three functional domains,including GHR binding domain,Erythropoietin receptor(EpoR)domain,and fibronectin type IIIdomain,while GHR2 protein has only two functional domains(GHR binding domain and EpoR domain).IGFBP-1protein has an insulin-like growth factor binding protein domain and a Tg I domain.Phylogenetic analysis reveals thatP.argenteusismore closely related to the Perciformes.ghr2andghr2genes share higher similarities withEpinephelus coioides.The results of quantitative real-time PCR show thatghrsandigfbp1are widely expressed in liver,heart,gonad,white muscle and kidney tissues,with relatively lower expression levels in kidney,gonad and heart.When treated with handling stress,different genes have different expression patterns.Expression ofghr2andigfbp1is down-regulated,while expression of ghr2 has no significant difference from those in control groups.Results from this study suggests the important role ofghr2andigfbp1in growth regulation during stress environment,and acute stress might affect growth activities by down-regulating growth related genes.Results of the present study will be helpful for the furtherstudy of fish growth and effects of environmental stress on it in silver pomfret and other marine fish as well.
fish;gene expression;growth hormone;handling stress;insulin-like
Growth is an important trait in economic species,while growth and metabolic profile are greatly determined by muscle growth and myofibril characteristics[1].Growth activities are mainly involved by growth hormone/insulin-like growth factors(GH/IGF)axis.GH exerts its action via specific cell membrane receptors such as growth hormone receptor(GHR)that belongs to the cytokine/hematopoietin receptor superfamily[2-3].In fish,GH and IGF are anabolic hormones,which increase muscle mass by stimulating protein synthesis and/or inhibiting protein breakdown[4].
In fish,ghrsencodes transmembrane receptor protein for GH.By binding GH,it can activate an intra-and intercellular signal transduction pathway leading to growth.Type 1 insulin-like growth factorbinding protein is encoded byigfbp1gene,a member of igfbp family.It has an IGFBP domain and a type-I thyroglobulin(Tg I)domain.Binding of this protein can prolong the half-life of the IGFs and alter their interaction with cell surface receptors[5-6].In recent years,tissue distribution and expression during different developmental stages of growth-related genes have been studied in several fishes,such as zebrafish(Danio rerio)[4],mangrove killifish(Kryptolebias marmoratus)[7],Mozambique tilapia(Oreochromismossambicus)[8],and blunt snout bream(Megalobrama amblycephala)[1]. However, study on transcriptional modulation of their mRNA by handling stress still needs to be explored in fish.
Silver pom fret(Pampus argenteus)is an important commercial fish which has a wide distribution from the Arabian Gulf,the India Ocean and the East China Sea to the Southeast Asia[9].Relevant artificial breeding techniques andP.argenteusreproductive biology have been studied in China and Kuwait since the 1980s[9].Due to its sensitivity to many kinds of stress factors,low growth rate and high mortality is found inP.argenteusduring culture practices.However,little is known about itsmolecular aspects,including gene expression information of growth during stress phase.In the present study,characterization,distribution and expression pattern of growth-related gene(ghrsandigfbp1)responded to acute handling stress are examined.Our aim is to evaluate the effect of acute handling stress on growth in this species.
Materials and Methods
Experimental fish
Captured wild juveniles ofP.argenteuswith body weight of 25.4±4.2 g(mean±SD)were temporarily maintained in 3000 L tanks.Fish were fed approximately 2%of body weight per day in five daily feedings.Water temperature was maintained at 23-25℃.Healthy fishes were divided into six groups(n=12).The first group was undisturbed fish used as the control,maintained under quiet and suitable conditions.The other five groups were subjected to handling stress by a20-second emersion from the water held within a dip net.In order to minimize the effect of density and repeated sampling from the same tank,four fish were sampled from each experimental tank at each time.Fish were ethically euthanized in sea water containing 100 mg ·L-1concentration of MS-222(Sigma).Tissue samples including the white muscle,heart,liver,kidney and gonad were immediately collected and frozen in liquid nitrogen and preserved at-80℃until further processing.
Sequence alignment and phylogenetic analysis
The full-length cDNA sequences of P.argenteus ghr1,ghr2andigfbp1were obtained by our previous transcriptome study.Proteins encoded by sequenced cDNA were tested for the presence of signal peptidase cleavage sites at the PSORTPrediction Server(Http://psort.nibb.ac.jp/).Putative transmembrane regions were predicted with HMM-TOP v 2.0(Http://www.enzim.hu/hmmtop)[10].Pfam 27.0 was used to predict functional domains(http://pfam.sanger.ac.uk/)[11].The putative amino acid sequences of GHR1,GHR2 and IGFBP1 were used with the neighbor-joining method to construct the phylogenetic tree by MEGA 4.0[12].The reliability of the estimated tree was evaluated by the bootstrap method with 1000 pseudu-replications.AndCryprinus carpiodwas used as the out-group.
Quantitative real-time PCRs(qRT-PCR)
Measurements of growth-related genes were made in both different tissues(including white muscle,heart,liver,kidney and gonad)and liver of treated fish by means of real-time PCR usingβactin as housekeeping gene.Primers used in the present study were shown in Table 1.Total RNA was isolated,and about1μg total RNA was reverse transcribed to a single-strand cDNA using Prime Script RT reagent kit(Takara,Japan)according to the manufacturer′s instructions.PCR was performed in a 20μL final volume containing 2 μL of10×LA Taq Buffer(20 mM Mg2+),1μL of the synthesized cDNA,200μM dNTP,2μM of a primer pair(Table 1),and 2 U of LA Taq hot start version(Takara,Japan).The reactions were carried out as follows:denaturation for 5 min at 94℃;30-40 cycles of amplification at 94℃for 30 s,30 s at57℃and 20 s at 72℃;and 10 min at 72℃.
Statistical analysis
The results of qRT-PCR were expressed as the standard error(S.E.)of the mean.Statistical analyses of differences among different tissues and treated groups were analyzed by SPSS 13.0.Differences were considered significant atP<0.05.
Results
Sequence and phylogenetic analysis of ghrs and igfbp1
The basic characterization of three genes was shown in Table2.The total length ofghr1,ghr2,andigfbp1ranged from 1280 to 3625 bp,and encoded amino acids from 64 to 627.Signal peptide sites and transmembrance helices were found in all three genes.GHR1 protein had three functional domains,including GHR binding domain,Erythropoietin receptor(EpoR)domain,and fibronectin typeⅢdomain,whileghr2protein had only two functional domains(GHR binding domain and EpoR domain).IGFBP-1 protein had an insulin-like growth factor binding protein domain and a Tg I domain(Figs.1-3).Phylogenetic analysis(Figs.4-6)revealed thatP.argenteuswas more closely related to the Perciformes.ghr1andghr2genes shared higher similarities withEpinephelus coioides.Due to a lack ofigfbp1information in some fish species in GenBank,igfbp1ofP.argenteusshowed higher similarity withTakifugu rubripes.
Expression of ghrs and igfbp1
The distribution ofghr1,ghr2andigfbp1inP.argenteuswas studied in juvenile individuals(Table 3).Three genes were expressed in all examined tissues,while significant differences(P<0.05)were found among different tissues.Ghr1andghr2had similar expression patterns among different tissues,with the highest expression in liver and relatively lower expression levels in kidney,gonad,and heart.Ghr2showed much higher expression thanghr1in each tissue.Although the highest expression ofigfbp1was also found in liver(P<0.05),expression of this gene in other tissues seemed to be much lower.

Fig.1 Nucleotides and deduced am ino acids sequence of P.argenteus ghr1

Fig.2 Nucleotides and deduced amino acids sequence of P.argenteus ghr2
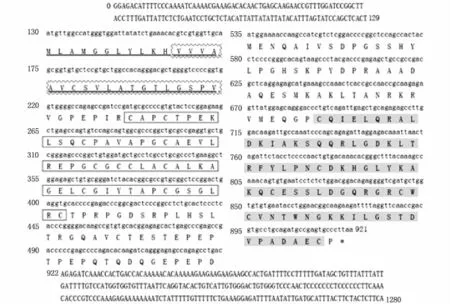
Fig.3 Nucleotides and deduced am ino acids sequence of P.argenteus igfbp-1

Fig.4 NJ phylogenetic tree based on the alignment of GHR1 amino acid sequences in P.argenteus

Fig.5 NJ phylogenetic tree based on the alignment of GHR2 am ino acid sequences in P.argenteus

Fig.6 NJ phylogenetic tree based on the alignment of igfbp1 amino acid sequences in P.argenteus
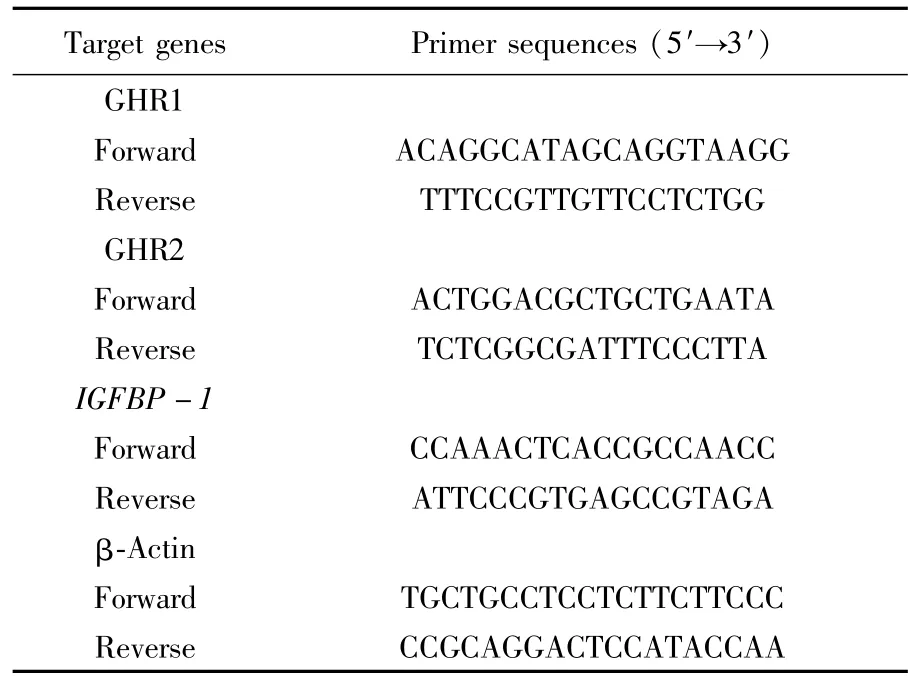
Tab.1 Four primer sequences used during gene expression process
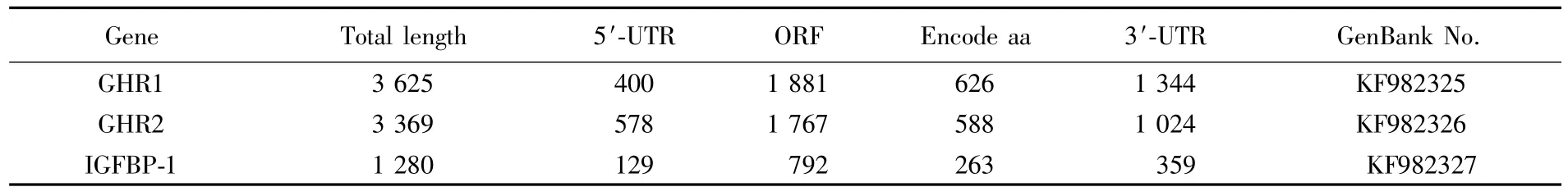
Tab.2 Comparison of basic characterization of GHR s and IGFBP-1 in Pampus argenteus
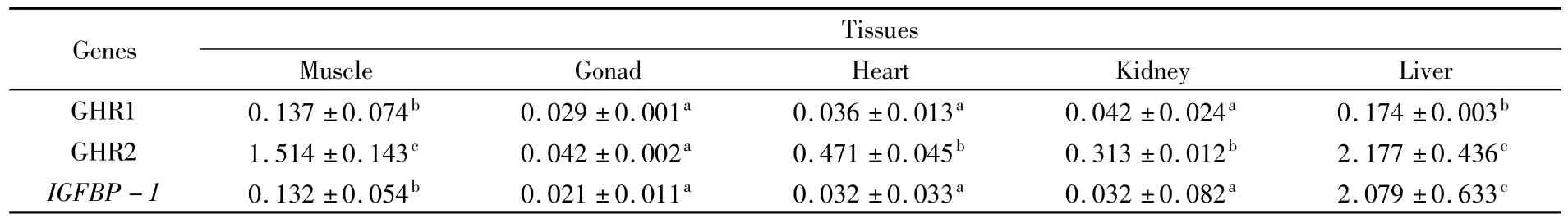
Tab.3 Comparison of expression levels of GHR s and IGFBP-1 in different tissues of P.argenteus(mean±S.E.)when subjected to stress
The expression ofghrsandigfbp1transcripts was detected in the liver ofP.argenteusindividuals that treated with acute handling stress.Significant differences of mRNA expression inghr2andigfbp1genes were found in treated groups(Fig.7).The expression of both genes dropped rapidly in the following day after stress treatment,which was significantly different from samples in the control groups(P<0.05).Then,the expression of two genes maintained at a fairly low level in the rest of the treated period.As forghr2,expression increased on the first day after stress treatment,and then decreased gradually,while no significant difference was found among expressions in different treated periods(P>0.05).

Fig.7 Expression of ghrs and igfbp1 in the liver of stressed P.argenteus(mean±S.E.)
Discussion
The present study provided an evaluation of key endocrine genes involved in the growth ofP.argenteusunder both normal and stressed environment.The amino acid sequences,domain structure were highly conserved and contained similar secondary and tertiary structure with other teleost species[13,7,1].In theP.argenteusgenome,two ghr genes were found,which was also proved in other fish species,such as blunt snout bream(Megalobrama amblycephala)[1],Mozambique tilapia(Oreochromis mossambicus)[8],Nile tilapia(Oreochromis niloticus)[14],black seabream(Acanthopagrus schlegeli)[15],zebrafish(Danio rerio)[16]and rainbow trout(Oncorhynchus mykiss)[17]etc.Both of the twoghrshad Erythropoietin receptor,ligand binding domain and growth hormone receptor binding domain.igfbp1had an insulin-like growth factor binding protein domain and type-I thyroglobulin domain.In addition,transmembrane regions were found in peptide sequences of all three genes,which were consistent with the structure of these sequences in other fish[4,13,17].
Growth hormone is a peptide hormone that stimulates growth,cell reproduction and regeneration.Its synthesis is stimulated by growth hormone releasing hormone and acts by interacting with GHR.Two putative ghr genes were reported in teleost fish species,and this was also proved in the present study.Expression pattern ofghrsandigfbp1in various tissues in P.argenteus suggested that these three genes were extensively expressed.The highest expression was found in muscle and liver,which indicated the importance of these two organs involved in function of GH/IGF axis.Liver plays an important role in the endocrine action of pituitary GH[2,18].Effects of GH on target tissues are mediated by the GHR,therefore,expression ofghr2andigfbp1in most tissues suggestes the important role they play during the regulation process of growth inP.argenteus.
Under common aquaculture practices fish may be stressed.After the perception of the stressor,an immediate cascade of hormone events through the hypothalamo-pituitary-interrenal axis and subsequent osmoregulatory and metabolic regulation will be activated[19].In fish,stress is found to have adverse effect on growth,immune function,and reproduction.Moreover,environmental stressors are linked with delayed mortality in fish populations through increased susceptibility to predators and disease[20].As an important commercial fish in China,the artificial breeding of thisP.argenteushas been developed in recent years.Due to its sensitivity to many kinds of stress factors,P.argenteusalso could be used as a typical model for stress research.By far,however,little has been known about its molecular aspects,including gene expression information of growth during stress period.In the present study,expression pattern ofghr2andigfbp1showed significant differences between normal and treated individuals under acute stress environment.When compared withghr2,the expression ofghr2presented much lower level and insignificant difference in stressedP.argenteusfish(Fig.7),which also indicated the significantly important role thatghr2played in the regulation of growth activity during stress phase in this species.In fish,there is evidence that GHR2 are functional receptors for GH,while the function of GHR1 is still controversial[13-14].For instance,the expression of GHR1 and GHR2 are differentially regulated by GH and insulin in tilapia[8].But,in gilthead sea bream,Sparus aurata,both ghr2 and ghr2 were down-regulated by fasting in liver and adipose.The present study suggested thatghrsin the liver ofP.argenteusalso have different functional patterns.
In addition,the dynamic transcriptional regulation and expression of teleost igfbps differs from species to species[6].Some studies have demonstrated that IGFBPs play a key role in early development and growth[21-22].IGFBPs bind both insulin-like growth factors(IGFs)I and II and circulates in the plasma.In zebrafish,muscularigfbp1expression increases during fasting and then rapidly returns to basal levels during refeeding[23].IGFBPs also bind to IGF-1 inside the liver to regulate its own level[5].In the present study,expression ofigfbp1down-regulated during acute handling stress,which also indicated a positive role in the regulation of growth inP.argenteus.
Conclusion
The present study for the first time evaluated the gene expression of three important growth-related genes in normal and stressed silve pomfret.The results from this study suggested the important role ofghr2andigfbp1in growth regulation during stress environment,and acute stress could affect growth activities by down-regulating growth related genes.The results will be helpful for the further study of fish growth and effects of environmental stress on it in silver pomfret and other marine fish as well.
Acknowledgements
This study is financially supported by a central Nonprofit Basic Scientifc Research Project for the Scientific Research Institutes of China(Project East 2014Y01)Natural Science Foundation of Shanghai City(Project NO.12ZR1455000)and the Natural Science Fundation of China(NO.41306170).The authors declare there has no conflict of interest.
[1] ZENG C,LIU X L,WANGW M,et al.Characterization of GHRs,IGFs and MSTNs,and analysis of their expression relationships in blunt snout bream,Megalobrama amblycephala[J].Gene,2014,535(2):239-249.
[2] REINECKE M.Influences of the environment on the endocrine and paracrine fish growth hormone-insulin-like growth factor-I system[J].Journal of Fish Biology,2010,76(6):1233-1254.
[3] REINDL K M,SHERIDAN M A.Peripheral regulation of the growth hormoneinsulin-like growth factor system in fish and other vertebrates[J].Comparative Biochemistry and Physiology A,2012,163(3-4):231-245.
[4] SCHLUETER P J,PENG G,LIY,et al.Insulinlike growth factor signaling regulates cell survival and cell cycle progression in developing zebrafish embryos[J].Developmental Biology 2006,295(1):360-360.
[5] CLEMMONSD R.Role of insulin-like growth factor binding proteins in the control of IGF actions[J].Molecular and Cellular Endocrinology,1998,140(1-2):19-24.
[6] FUENTESE N,VALDES JA,MOLINA A,et al.Regulation of skeletal muscle growth in fish by the growth hormone-Insulin-like growth factor system[J].General and Comparative Endocrinology,2013,192(1):136-148.
[7] RHEE J S,KIM B M,SEO J,et al.Cloning of growth hormone,somatolactin,and their receptor mRNAs,their expression in organs,during development,and on salinity stress in the hermaphroditic fish,Kryptolebias marmoratus[J].Comparative Biochemistry and Physiology A,2012,161(4):436-442.
[8] PIERCE A L,BREVES JP,MORIYAMA S,et al.Regulation of growth hormone(GH)receptor(GHR1 and GHR2)mRNA level by GH and metabolic hormones in primary cultured tilapia hepatocytes[J].General and Comparative Endocrinology,2012,179(1):22-29.
[9] HUANG X X,YIN Y Q,SHIZ,etal.Lipid content and fatty acid composition in wild-caught silver pomfret(Pampus argenteus)broodstocks:Effects on gonad development[J].Aquaculture,2010,310(1-2):192-199.
[10] TUSNADY G E,SIMON I.The HMMTOP transmembrane topology prediction server[J].Bioinformatics,2001,17(9):849-850.
[11] FINN R D,COGGILL P,EBERHARDTR Y,etal.The Pfam protein families database:toward a more sustainable future[J].Nuclei Acids Research,2016(44):279-285.
[12] TAMURA K,DUDLEY J,NEIM,et al.MEGA4:Molecular Evolutionary Genetics Analysis(MEGA) software version 4.0[J].Molecular Biology and Evolution,2007,24(8):1596-1599.
[13] SAERA-VILA A,CALDUCH-GINER JA,PEREZSANCHEZ J.Duplication of growth hormone receptor(GHR)in fish genome:gene organization and transcriptional regulation of GHR type I and II in gilthead sea bream(Sparus aurata)[J].General and Comparative Endocrinology,2005,142(1-2):193-203.
[14] MA X L,LIU X C,ZHANG Y,et al.Two growth hormone receptors in Nile tilapia(Oreochromis niloticus):molecular characterization,tissue distribution and expression profiles in the gonad during the reproductive cycle[J].Comparative Biochemisty and Physiology B,2007,147(2):325-339.
[15] TSE D L Y,TSE M C L,CHAN C B,et al.Seabream growth hormone receptor:molecular cloning and functional studies of the full-length cDNA,and tissue expression of two alternatively spliced forms[J].Biochemica et Biophysica Acta,2003,1625(1):64-76.
[16] DIPRINZIO CM,BOTTA PE,BARRIGA E H,et al.Growth hormone receptors in zebrafish(Daniorerio):adult and embryonic expression patterns[J].Gene Expression Patterns,2010,10(4-5):214-225.
[17] VERY N M,KITTILSON JD,NORBECK L A,etal.Isolation,characterization,and distribution of two cDNAs encoding for growth hormone receptor in rainbow trout(Oncorhynchus mykiss)[J].Comparative Biochemistry and Physiology B,2005,140(4):615-628.
[18] WOOD A W,DUAN C,BERN H A.Insulin-like growth factor signaling in fish[J].International Review of Cytology,2005,243(1):215-285.
[19] MOMODA T S,SCHWINDT A R,FEIST GW,et al.Gene expression in the liver of rainbow trout,Oncorhynchus mykiss,during the stress response[J].Comparative Biochemistry and Physiology D,2007,2(4):303-315.
[20] BOLASINA SN.Stress response of juvenile flounder(Paralichthys orbignyanus,Valenciennes 1839),to acute and chronic stressors[J].Aquaculture,2011,313(1-4):140-143.
[21] KAMEIH,LU L,JIAO S,et al.Duplication and diversification of the hypoxia-inducibleIGFBP-1gene in zebrafish[J].PLoS One,2008,3(8),e3091.
[22] WANG X,LU L,LI Y,et al.Molecular and functional characterization of two distinct IGF binding protein-6 genes in zebrafish[J].American Journal of Physiology,2009,296(5):1348-1357.
[23] AMARAL I P G,JOHNSTON I A.Insulin-like growth factor(IGF)signalling and genome-wide transcriptional regulation in fast muscle of zebrafish following a single-satiating meal[J].Journal of Experimental Biology,2011,214(13):2125-2139.
1004-2490(2016)05-0468-10
银鲳ghr和igfbp1基因表达特征、组织分布及操作胁迫对其的影响
孙 鹏,尹 飞,王建建,李云航
(中国水产科学研究院东海水产研究所,上海 200090)
为探讨急性操作胁迫对银鲳生长的影响,首次克隆了3种生长调控相关基因-生长激素受体1(ghr1)、生长激素受体2(ghr2)和胰岛素样受体1(igfbp1),并对3种基因在银鲳肝脏、心脏、性腺、肌肉和肾脏等不同组织中的表达进行了比较。定量表达结果表明3种基因均广泛分布于所研究的各个组织中,而其中肾脏、性腺和心脏中的表达量较其它组织低。在银鲳受到外界操作胁迫刺激后,3种基因呈现不同的表达模式:其中ghr2和igfbp1的表达下调,而ghr2的表达量与对照组无显著性差异。结果表明,ghr2和igfbp1在银鲳生长调节中起到重要的作用;在胁迫环境中,外界刺激可通过下调生长相关基因表达以影响鱼体的生长。
鱼类;基因表达;生长激素;操作胁迫;胰岛素样生长因子
Q 959.468
A
2015-10-23
中央级公益性科研院所基本科研业务费项目(东2014Y01);上海市自然基金项目(No.12ZR1455000);国家自然基金项目(No.41306170)
孙 鹏,男,山东枣庄人,博士,副研究员,主要从事海洋鱼类繁育与健康养殖研究。
Q 959.468
A
Received Date:2015-10-23
Foundation item:This study was finacially supported by Natural Science Foundation of Shanghai City(project No.12ZR1455000)and the Natural Science Fundation of China(No.4130617)
SUN Peng,male,PhD,associate professor,major in marine biology.E-mail:sunpeng1128@163.com
E-mail:sunpeng1128@163.com

