Role of calcium-activated potassium channels in neuronal pacemaker activity
Nancy DONG,Zhong-ping FENG
(Department of Physiology,University of Toronto,1 King′s College Circle, Toronto,Ontario,Canada M5S 1A8)
·REVIEW·
Role of calcium-activated potassium channels in neuronal pacemaker activity
Nancy DONG,Zhong-ping FENG
(Department of Physiology,University of Toronto,1 King′s College Circle, Toronto,Ontario,Canada M5S 1A8)
Spontaneous rhythmic activity of pacemaker neurons in the central nervous system underlies fundamental neurological processes such as locomotion,cognition and circadian rhythm. Among the wide range of ion channels required for its generation,the Ca2+-activated K+(KCa)channels play a prominent role in maintaining physiologically-relevant frequency and pattern of pacemaker activity. Much of our understanding of the functions of KCachannels in pacemaker neurons have been derived from pharmacological studies using channel modulators,such as iberiotoxin and apamin. Despite the significant advances made,recent studies have painted an increasingly complex picture of the effects of widely used KCachannel modulators on unintended targets that may confound our under⁃standing of their functions.In this review,we discussed the utility and shortcomings of the KCachannel modulators,and highlighted the significance of these findings,because the KCachannel modulators have been used in early clinical trials to treat disorders ranging from Parkinson disease to alcoholism.
potassium channels,calcium-activated;pacemaker neurons;rhythmic activity;channel modulators
Pacemaker neurons are capable of generating rhythmic activity in the absence of external inputs[1].They play a key role in many neurological functions,including locomotion,cognition and circadian rhythm[2].The computational advantage of spontaneous firing lies in that both excitatory and inhibitory synaptic inputs can be coded in a timely fashion in changes in the frequency and/or pattern of rhythmic activity.Therefore,the ability to maintain stable and precise rhythmic activity is fundamental to the physiological functions of pacemaker neurons.
Pacemaker activity arises from an intricate interplay between the complement of ion channels present on the pacemaker neuron membrane, including hyperpolarization cyclic nucleotide(HCN)-activatedchannels[3],Na+leakchannels(NALCN)[4], voltage-gated Ca2+and Na+channels[5],and Ca2+-activated K+(KCa) channels[6]. KCachannels contribute to the repolarization and after-hyperpo⁃larization(AHP)phases of the action potential, thereby providing the negative feedback mechanism that stabilizes rhythmic activity in pacemaker neurons.Pharmacologicalmodulators ofKCachannels,such as iberiotoxin(IbTX)and periciazine (apamin),have been instrumental in elucidating the physiological functions of the large-conduc⁃tance(BK) and small-conductance(SK) KCachannels as regulators of pacemaker neuron activity[6]and as potential therapeutic targets in the treatment of neurological disorders[7-8].The goal of this article is to discuss the utility and potential caveats of commonly used pharmaco⁃logical agents in investigating the physiological function and therapeutic potential of KCachannels as regulators of pacemaker neuron activity.
1 LARGE-CONDUCTANCE KCaCHANNELS
1.1Structure and biophysical properties of BK channels
BK channels are composed of both a tetra⁃meric α pore-forming subunit[9]and modulatory β subunits[10].Each monomer of the α-subunit, encodedbytheSlo1gene,consistsofan N-terminal transmembrane pore forming region (S0-S6)and a C-terminal cytoplasmic regulatory domain(S7-S10).The S0 domain is required for interaction with β-subunits,which greatly diversify the channel properties.The S1-S4 domains make up the voltage sensor,by virtue of charged amino acid residues in the S2-S4 domains[11-13]. The P-loop is likewise found in between the S5 and S6 domains,and together with the other three subunits to form the pore region that con⁃tains the signature K+selectivity GYG motif and binding sites for the pore blockers charybdotoxin (ChTX)and IbTX[14-15].The C-terminal cytoplasmic domain can be divided into two regulator of K+conductance domains(RCK1 and RCK2)that each contain one low affinity Ca2+binding site[16-17]. The C-terminaldomains of all four subunits come together to form a″gating ring″[18]that makes Ca2+-dependent activation of BK channels possible.Multiple promoters and splicing variants of the subunits have been identified to give rise to diversity in the structure and properties of the pore forming domain[19].
The properties of a BK channel can be wideranging depending on the β-subunits associated with it.Currently,four β-subunits have been cloned[20-23]:β1is found in the smooth muscles,β2and β3specifically in neurons,and β4in the brain. The β-subunits associate in equal stoichiometric ratio with monomers of the α-subunits via the S0 segment to significantly modify the gating[24-25], pharmacological[26],and kinetic properties of the channel[27].For example,whereas BK channels containing the α-subunit alone or associated with the β1or β4-subunit do not inactivate,those with β2and β3-subunit show rapid inactivation[22-23].There⁃fore,careful biochemical characterization of the BK channels under examination is imperative before proceeding onto pharmacological studies.
BK channels activate rapidly in the coincident presence of membrane depolarization and an intracellular Ca2+concentration>10 μmol·L-1[28]. The ensuing large K+current rapidly repolarizes the action potential and hyperpolarizes the membrane potential by mediating the fast component of the AHP(fAHP).The feedback loop is terminated with equal rapidity when BK channels are deactivated by membrane hyperpolarization and deactivation of voltage-gated Ca2+channels.
1.2 Pharmacological modulators of BK channels
As the structure and pharmacological prop⁃erties of BK channel modulators have been extensively reviewed elsewhere[29-30],only the most commonly used compounds in studying pacemaker neurons will be briefly introduced here.The earliest generation of BK channel blocker is ChTX,a potent yet largely non-selective K+channel blocker that is derived from the venom of the scorpionLeiurus quinquestriatus[31].It is now rarely used due to its additional activity inhibiting the voltage-gated K channels and inter⁃mediate-conductance KCa(IK)channels.
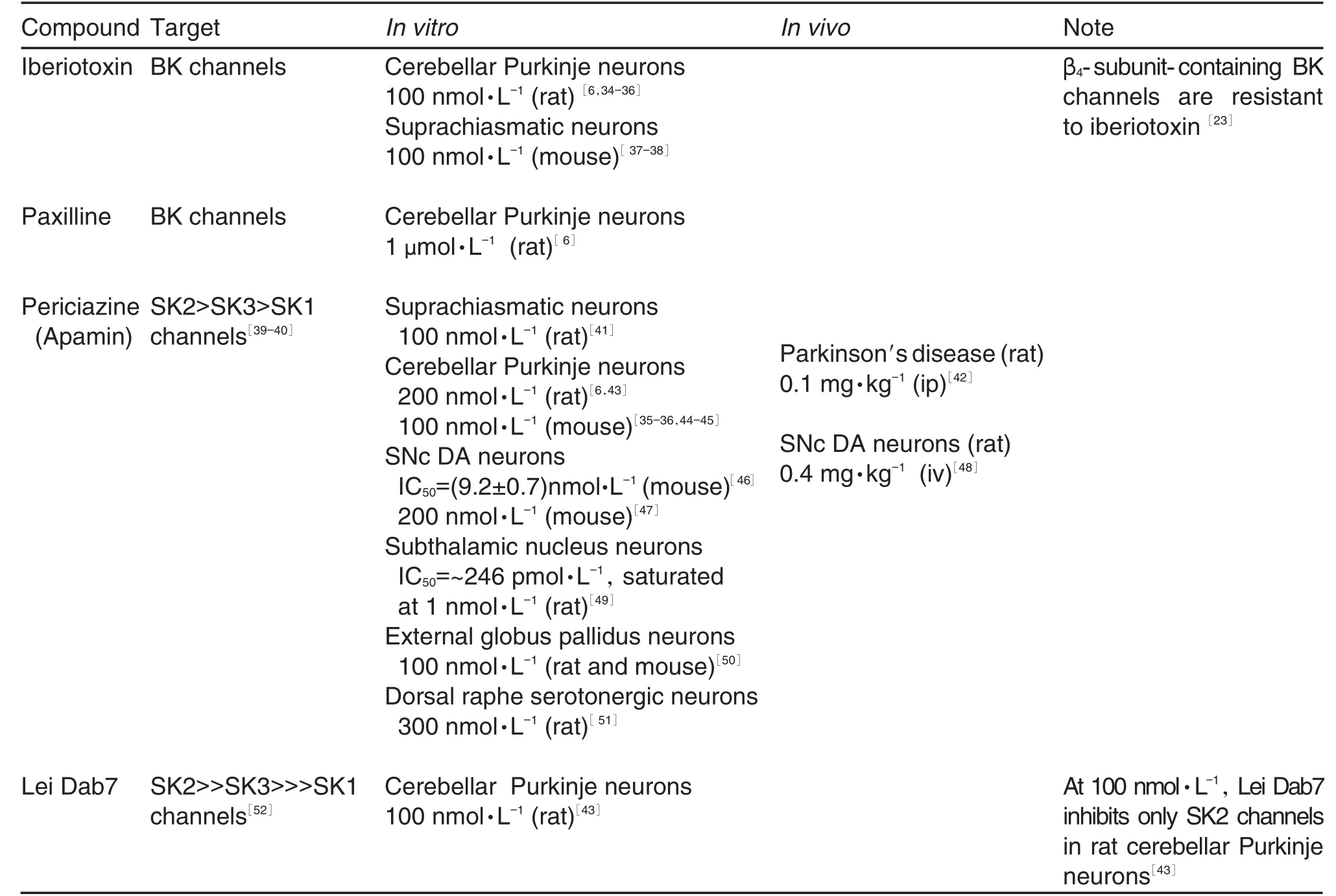
Currently two BK channel blockers have been used extensively(Tab.1).IbTX,the most commonly used BK channel blocker,was later isolated from the venom of another species of scorpionsButhus tamulus[32].Like ChTX,IbTX acts by binding to the external opening of BK channels.However,the latter exhibits higher affinity and selectivity for BK channels than the former,possibly due to slight differences in the amino acid make-up and resultant charge[32-33]. One potentially serious pitfall of IbTX is that β4-subunit-containing BK channels are shown to be resistant to it.
The tremorgenic indole alkaloid paxilline, produced byPenicillium paxilli,is the most widely used non-peptide BK channel blocker,due to itshigh potency,selectivity and reversibility of action[53]. Unlike ChTX and IbTX,paxilline acts on the αsubunit from the cytoplasmic side[54].The recently
Tab.1 Pharmacology of KCachannel blockers commonly used in studies of central pacemaker neurons in vitro and in vivo
isolated non-peptidergic BK channel blocker 1-〔1-hexyl-6-(methyloxy)-1H-indazol-3-yl〕-2-methyl-1-propanone(HMIMP)holds considerable promise, as it inhibits both the IbTX-sensitive and-resistant BK channel isoforms at nanomolar range and does not appear to affect human voltage-gated Na+,Ca2+and K+channels[55].
While activators of BK channels have long been identified,such as the benzoimidazolones NS004 and NS1619,their usefulness is limited because of their poor potency and selectivity[56-57]. Furthermore,these compounds are designed to act at membrane potentials~50-100 mV,which are more positive than the physiological range[58]. To address these shortcomings,the Hollywood group[59]synthesized a family of anthraquinone analogues,named GoSlo-SR,that are capable ofactivating BK channels atphysiological membrane potentials.
1.3 BK channels regulate central neuronal activities
As BK channel activity is time-locked to the action potential,itis particularly suited to shaping the spike profile.Indeed,in excised patch recordings of cerebellar Purkinje neurons, a significant amount of BK current is activated during action potential-like waveforms[34].IbTX is the most widely used tool to study the role of BK channels in regulating rhythmic firing frequency in several central pacemaker neuron populations, though with varied results.Currently,the best understood system is arguably the suprachias⁃matic nucleus(SCN),which is the central circadian pacemaker of the mammalian brain.Spontaneous firing frequency of the SCN neurons is modulated in a diurnal fashion to mark the day/night cycle,i.e.high during the subjective day and low during the subjective night[60].Downstream of the circadian clock genePer1[37],BK channel expression andactivity in SCN neurons peak during the middle of the night phase in order to suppress the frequency of spontaneous firing[38].This circadian pattern of rhythmic activity is blunted when BK channel activity is inhibited by IbTX[38],a result that is replicated in a global BK channel knock⁃out model[61].
A more complex picture is seen in the cerebellarPurkinje neurons,which highlights potential caveats of IbTX that should be taken into consideration when interpreting findings.In slice recordings,these neurons exhibit high frequency spontaneous firing(~40-50 Hz)[34].As the sole output neurons of the cerebellar cortex,the Purkinje neurons integrate a myriad of sensory, cortical and vestibular information needed to guide and coordinate voluntary motor behaviour[62]and encode them with high fidelity in the rate and precision of their firing activity.The discharge frequency is found to alternate over a wide range between rates higher and lower than the resting frequency in a consistent temporal relationship with the movement cycle[63].Indeed,recent studies have shown that the firing frequency is tightly controlled on the scale of milliseconds[64].Consistent with the role of BK channels in contributing to the fAHP,in vitrorecordings from acute cerebellar slices show that IbTX reduced the AHP[41]and increased the spontaneous firing activity[34,44].
Paradoxically,Purkinje neurons of global BK channel knockout animals exhibit reduced spon⁃taneous activity[65].While developmental compen⁃sation in the knockout animals is a possibility,it may be prudent to employ multiple BK channel blockers to control for potential off-target effects. Interestingly,the abnormal Purkinje cell firing pattern and ataxic behaviour in BK channel knockout mice were reproduced in wildtype mice byin vivomicroinjection of paxilline into the vermis[66],suggesting that paxilline may be an effective alternative to IbTX.On a related note, an IbTX-resistant but 1-ethyl-2-benzimidazolinone (1-EBIO)-sensitive BK current has recently been identified in Purkinje neurons[67].IbTX-and ChTX-resistant BK channels contain the β4-subunit, whose extracellular loop results in a~1000 fold decrease in toxin association,rendering them insensitive to nanomolar concentrations of these blockers that are normally sufficient to inhibit BK channels[23].The β4-subunit mRNA is abundant in the mammalian brain[23],suggesting that effects of IbTX may not be entirely attributable to inhibition ofBK channels.Furtherinvestigations are required to provide additional evidence.
1.4 Therapeutic potential of BK channels
From the evidences summarized above,BK channels are potential drug targets in the treatment of motor and circadian rhythm disorders,such as ataxia,narcolepsy and insomnia.However,rescue of BK channel function in disease states is hindered by the lack of effective channel activators, for the efficacy of GoSlo-SR compounds have yet to be testedin vivo.Conversely,the toxin BK channel blockers are poor drug candidates,for in addition to potential off-target effects,the pepti⁃dergic nature of ChTX and IbTX means that they are not active orally,have short half-lives and poor blood-brain permeability[29].Nevertheless, paxilline may be a promising target for future studies,as no major side-effects were detected in a study examining its efficacy as a systemicallyadministered anti-convulsant in rodents[68].
2 SMALL-CONDUCTANCE KCaCHANNELS
2.1Structure and biophysical properties of SK channels
Comparatively much more similar to voltagegated K+channels than BK channels,all three members of the SK family,SK1,SK2 and SK3, are homomeric tetramers of 6-transmembrane domain subunits[39].Thus it is the more surprising that SK channels are entirely voltage-independent, for the S4 segment contains only three positively charged residues as compared to many in most voltage-gated channels[69-70]. Instead,they are activated by submicromolar elevation in intracellular Ca2+by virtue of the constitutively bound calmodulin at the C-terminal calmodulin-binding domain (CaMBD)of each subunit[71-72].While the exactmechanism of gating is not yet clear,it is currently believed that Ca2+binding to calmodulin induces a conformational change in the arrangement of the tetramer that is translated into a mechanical force that opens the activation gate[73].This indirect coupling of Ca2+binding to channel opening results in slower activation kinetics as compared to BK channels,so that SK channels are fully open~5 ms after the action potential to mediate the medium AHP(mAHP)that lasts over hundreds of milliseconds[19].
2.2 Pharmacological modulators of SK channels
Excellent discussions of SK channel modulators can be found in two recent reviews[74-75],so only those most commonly used in studies of pacemaker neurons will be summarized here(Tab.1,channel blockers and Tab.2,channel activators).
The isolation of apamin,from the honeybee venom,as a selective blocker of SK2 and SK3 channels has been of immeasurable significance in elucidating the physiological function of SK channels[69,86].Initially believed to be a simple pore blocker based on the finding that several critical amino acid residues in the outer pore region are critical to sensitivity to apamin[40],there is recent evidence that apamin binding to the outer pore causes a conformational change in the selectivity pore that prevents K+conductance[87]. However,SK3 channels have been shown to be less sensitive than SK2 channels to blockade by apamin[88].It may therefore be advisable to prop⁃erly characterize the biochemical profile of the SK channel under examination and employ multiple pore blockers to control for theeffects of subtype specificity.To more selectively manipulate SK2 channels,the synthetic peptide toxin Lei Dab7 may be employed[43,52].

Tab.2 Pharmacology of KCachannel activators commonly used in studies of central pacemaker neurons in vitro and in vivo
Both 1-EBIO and chlorzoxazone(CZX)potentiate SK channel activity by increasing channel open probability[76].Interestingly,CZX is already an FDA-approved central muscle relaxant,making it an attractive drug candidate in modulation of SK channels in pacemaker neurons.Nevertheless, the lack ofspecificity/selectivity remains a challenge as 1-EBIO activates SK1,SK2,and SK3 channels equally[89]and both 1-EBIO and CZX activate IK channels,which are not expressed in neurons,in a concentration-dependent manner[76]. The most recently discovered activator,CyPPA, is ineffective against IK channels but cannot differentiate between SK2 and SK3 subtypes[80].
A recent study reported that the transient receptor potential melastatin member 7(TRPM7) channel is inhibited by SK channel activators derived from plant alkaloid quinine,including CyPPA,NS8593,SKA31 and UCL1684[81]. TRPM7channelsareubiquitouslyexpressed and implicated in a wide range of physiological functions including synaptic neurotransmission, intestinal peristalsis,regulation of vascular tone, bone growth and embryonic development[90],thus the effects of the plant alkaloid SK channel activators on related functions should be interpreted with caution.There is considerable promise in the CyPPA structural derivative NS13001,which is shown to be highly selective of the SK3 subtype and exhibit none of the off-target effects of CyP⁃PA in the micromolarconcentration range[43](Tab.2).
2.3 SK channels regulate pacemaker neuronal activities
In comparison with BK channels,the greater variety and selectivity of the SK channel modulators have allowed for better characterization of the role of SK channels in regulating pacemaker neuron activity.Through its contribution to the mAHP,SK channels control the firing threshold and thus regulate single spike in pacemaker cells.Its role is critical as a consistent mAHP after each spike ensures both that voltage-gated ion channels recover during each cycle and that cellular excitability remains low enough so random synaptic noises would not alter firing pattern.In addition to the frequency of firing,the precision/ regularity of rhythmic activity is another important component of the physiological functions of pace⁃maker neurons.
In cerebellar Purkinje neurons,precise rhythmic firing is required to accurately reflect the strength and timing of excitatory and inhibitory synaptic inputs that shape the final output of the cerebellum. In addition to BK channels,SK channels are highly expressed in cerebellar Purkinje neurons[91]. Pharmacological blockade of SK channels by apamin[6,35]or UCL1684[92]both resulted in decreased regularity of tonic spiking in Purkinje neurons.Enhancement of SK channel activity using 1-EBIO,riluzole or CZX restores regular Purkinje neuron firing in mice with either SK channel knockout[93]or with mutations in P/Q-type voltage-gated Ca2+channels[78],to which SK channels are closely coupled.
Spontaneously active dopaminergic(DA) neurons found in the substantia nigra pars com⁃pacta(SNc)and ventral tegmental area(VTA) exhibit two possible modes of firing,regular simple spiking and bursting.Changes in the firing patterns of these DA neurons impact the spatiotemporal profile of dopamine release in different regions of the brain,thereby affecting motor, motivation and memory processes in both physi⁃ological and pathological conditions,such as schizophrenia and Parkinson′s disease.Inhibition of SK channels in these neurons by apamin not only disrupts the precision of tonic firing but also causes transition to burst firing;and the effects of SK channel blockers were reversed by SK channel activators[46,48,94-95].Multiple subtypes of SK channels are expressed in these neurons,with SK3 channels being the most abundant[96].Deignan,et al[47]demonstrated SK2 and SK3 channels contribute differently to the pacemaker activity of SNc DA neurons,where the former is found primarily in the distal dendrites and regulates theprecision of spike firing,and the latter is mainly somatic and modulates the frequency of firing. While specific blockers to the channel subtypes are needed to elucidate the subtype-specific function of SK channels,it would be of interest to see if NS13001 can be used to selectively modulate SK3 channels in midbrain DA neurons.
Membrane depolarization and irregular firing due blockade of SK channels have also been reported in several other pacemaker neuron populations,such as the subthalamic nucleus[49], dorsalraphe nuclei[51]and externalglobus pallidus[50],suggesting that SK channels may play a conserved role as regulatorofpacemaker activity in the central nervous system.
2.4 Therapeutic potential of SK channels
Several SK channel modulators are currently showing promise as drug candidates.In the cerebellum,abnormal Purkinje neuron pacemaker activity and thus loss of its information encoding ability results in ataxia,a feature of many conditions characterized by gaitdisturbances,postural instability and degraded fine motor coordination. In rodent models of spinocerebellar ataxia types 2[43]and 3[97],episodic ataxia type 2[78,82],and ataxia caused by CACNA1A mutation[79],SK channel activators are shown to be able to restore regular Purkinje neuron pacemaker activity and improve motor performancein vivo.Most recently,a randomized,double-blind,placebo-controlled trial found that riluzole,another FDA-approved muscle relaxant and SK channel activator,can alleviate motor symptoms in some human patients of spinocerebellar ataxia or Friedreich′s ataxia with few side effects[85].
The basal ganglia are also critically involved in motor control,such that their neurodegeneration results in Parkinson′s disease,a progressive disorderthataffects generation ofvoluntary movements.Whereas neurons in both the SNc and subthalamic nucleus exhibit spontaneous rhythmic,single-spike activity in healthy subjects, burst-firing activity predominates in patients with Parkinson′s disease[98-99].Reduction in SK channel activity is hypothesized to underlie such a change,as they play a fundamental role in main⁃taining regular spiking behaviour and oppose the transition to burst firing[48-49].Consistent with such an idea,it has recently been shown that apamin alleviates some motor deficits in a rat model of Parkinson′s disease[42].
In addition to motor processes,midbrain DA neurons also play an important role in cognitive functions such as memory,reward and motivation. The KCNN3 gene,which encodes the SK3 channel,contains a polymorphic CAG repeat in the amino-terminal cording region whose length has been implicated in schizophrenia[100-101]and anorexia nervosa[102].SK channels may also be a drug target in the treatment of addiction,for it has recently been shown that the systemic administration of the FDA-approved muscle relaxant CZX reduces excessive alcohol intake in rats[7].
Nevertheless,the potential for widespread side-effects is a concern for systemically adminis⁃tered SK channel modulators.All three subtypes of SK channels are widely expressed throughout the brain[103-104]and the body,including vascular endothelium,skeletal muscle,smooth muscle, and cardiac myocytes[105].In addition,as mentioned above,the SK channel activators 1-EBIO and CZX also potentiate IK channel activity due to their structural similarities[76-77].While IK channels are not expressed in neurons,they are widely expressed in peripheral tissues,such as the colon,placenta,lungs and pancreas.Indeed, reported side effects of orally administered CZX and riluzole are mild but wide ranging,including nausea,diarrhea,hypertension,and somno⁃lence[106].Memory impairments are also possible, given studies showing that transgenic animals over-expressing SK2 channels exhibitimpaired performance in hippocampal-dependent memory tasks[107-108]and the systemic administration of CyPPA impairs encoding of object recognition memory in mice[109].
3 CONCLUSION
Pharmacologicalactivators and blockershave been instrumental in advancing our under⁃standing of the role of KCachannels as regulators of pacemaker neuron activity in the central nervous system.Nevertheless,as with all experimental approaches,knowledge of their limitations is necessary for proper interpretation of the findings. This is all the more important now as several of these compounds are being tested in human clinical trials.The discovery of increasingly more potent and selective KCachannel modulators holds great promise for unraveling the mechanism and physiological functions of pacemaker neuron activity in future studies.
REFERENCES:
[1] HäusserM,RamanIM,OtisT,SmithSL,Nelson A,du Lac S,et al.The beat goes on:spontaneous firing in mammalian neuronal microcircuits[J].J Neurosci,2004,24(42):9215-9219.
[2] Ramirez JM,Tryba AK,Pena F.Pacemaker neurons and neuronal networks:an integrative view[J].Curr Opin Neurobiol,2004,14(6):665-674.
[3] Atkinson SE,Maywood ES, Chesham JE,Colwell CS,Hastings MH,Williams SR.Cyclic AMP signaling control of action potential firing rate and molecular circadian pacemaking in the suprachiasmatic nucleus[J].J Biol Rhythms,2011,26(3):210-220.
[4] Lu TZ,Feng ZP.A Sodium leak current regu⁃lates pacemaker activity of adult central pattern generator neurons in Lymnaea stagnalis[J]. PLoS One,2011,6(4):384-384.
[5] Jackson AC,Yao GL,Bean BP.Mechanism of spontaneous firing in dorsomedial suprachias⁃matic nucleus neurons[J].J Neurosci,2004,24(37):7985-7998.
[6] Edgerton JR,Reinhart PH.Distinct contributions of small and large conductance Ca2+-activated K+channels to rat Purkinje neuron function[J].J Physiol-London,2003,548(1):53-69.
[7] Hopf FW, Simms JA, Chang SJ,Seif T,Bartlett SE,Bonci A.Chlorzoxazone,an SK-type potassium channel activator used in humans,reduces excessive alcohol intake in rats[J].Biol Psychiatry,2011,69(7):618-624.
[8] Ristori G,Romano S,Visconti A,Cannoni S,Spadaro M,FrontaliM,etal.Riluzolein cerebellar ataxia:a randomized,double-blind,placebo-controlled pilot trial[J].Neurology,2010,74(10):839-845.
[9] Atkinson NS,Robertson GA,Ganetzky B.A component of calcium-activated potassium channels encoded by the Drosophila-slo locus[J].Science,1991,253(519):551-555.
[10] Shen KZ,Lagrutta A,Davies NW,Standen NB,Adelman JP,North RA.Tetraethylammonium block of slowpoke calcium-activated potassium channels expressed in Xenopus oocytes:evidence for tetrameric channel formation[J]. Pflugers Arch,1994,426(5):440-445.
[11] Papazian DM,Timpe LC,Jan YN,Jan LY. Alteration ofvoltage-dependence ofshaker potassium channelbymutationsin the S4 sequence[J].Nature,1991,349(6307):305-310.
[12] Papazian DM,Shao XM,Seoh SA,Mock AF,Huang YA.Electrostatic interactions of S4 voltage sensor in shaker K+channel[J].Neuron,1995,14(6):1293-1301.
[13] Seoh SA,Sigg D,Papazian DM,Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the shaker K+channel[J].Neuron,1996,16(6):1159-1167.
[14] Mullmann TJ, MunujosP, Garcia ML,Giangiacomo KM.Electrostatic mutations in iberiotoxin as a unique tool for probing the electrostatic structure of the maxi-K channel outer vestibule[J].Biochemistry,1999,38(8):2395-2402.
[15] Vergara C,Moczydlowski E,Latorre R.Conduc⁃tion,blockade and gating in a Ca2+-activated K+channel incorporated into planar lipid bilayers[J].Biophys J,1984,45(1):73-76.
[16] Schreiber M,Salkoff L.A novel calcium-sensing domain in the BK channel[J].Biophys J,1997,73(3):1355-1363.
[17] Xia XM,Zeng XH,CJ L.Multiple regulatory sites in large-conductance calcium-activated potassium channels[J].Nature,2002,418(6900):880-884.
[18] Yuan P,Leonetti MD,Pico AR,Hsiung Y,Mackinnon R.Structure of the human BK channel Ca2+-activation apparatus at 3.0 angström reso⁃lution[J].Science,2010,329(5988):182-186.
[19] Lancaster B,Nicoll RA.Properties of two calciumactivated hyperpolarizations in rat hippocampal neurones[J].J Physiol,1987,389(1987):187-203.
[20] KnausHG,FolanderK,Garcia-CalvoM,Garcia ML,Kaczorowski GJ,Smith M,et al. Primary sequence and immunological character⁃ization of beta-subunit of high conductance Ca2+-activated K+channel from smooth muscle[J].J Biol Chem,1994,269(25):17274-17278.
[21] Brenner R,Chen QH,Vilaythong A,Toney GM,Noebels JL,Aldrich RW.BK Channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures[J].Nat Neurosci,2005,8(12):1752-1759.
[22] Wallner M,Meera P,Toro L.Molecular basis of fast inactivation in voltage and Ca2+-activated K+channels:A transmembrane beta-subunit homolog[J].Proc Natl Acad Sci USA,1999,96(7):4137-4142.
[23] Meera P,Wallner M,Toro L.A neuronal beta subunit(KCNMB4)makes the large conduc⁃tance,voltage-and Ca2+-activated K+channel resistant to charybdotoxin and iberiotoxin[J].P Natl Acad Sci Proc USA,2000,97(10):5562-5567.
[24] Cox DH,Cui J,Aldrich RW.Allosteric gating of a large conductance Ca-activated K+channel[J].J Gen Physiol,1997,110(3):257-281.
[25] Wallner M,Meera P,Ottolia M,Kaczorowski GJ,Latorre R,Garcia ML,et al.Characterization of and modulation by a beta-subunit of a human maxi KCachannel cloned from myometrium[J]. Receptors Channels,1995,3(3):185-199.
[26] Meera P,Jiang ZTL,Wallner M.A calcium switch for the functional coupling between alpha(hslo)and beta subunits(Kv,cabeta)of maxi K channels[J].Febs Lett,1996,382(1-2):84-88.
[27] Sah P,Faber ES.Channels underlying neuronal calcium-activated potassium currents[J].Prog Neurobiol,2002,66(5):345-353.
[28] Pallotta BS.Calcium-activated potassium channels in rat muscle inactivate from a short-duration open state[J].J Physiol,1985,363(1):501-516.
[29] Nardi A,Olesen SP.BK Channel modulators:a comprehensive overview[J].Curr Med Chem,2008,15(11):1126-1146.
[30] Yu M,Liu SL,Sun PB,Pan H,Tian CL,Zhang LH.Peptide toxins and small-molecule blockers of BK channels[J].Acta Pharmacol Sin,2016,37(1):56-66.
[31] Miller C,Moczydlowski E,Latorre R,Phillips M. Charybdotoxin,a protein inhibitor of single Ca2+-activated K+channels from mammalian skeletal muscle[J].Nature,1985,313(6000):316-318.
[32] Galvez A,Gimenez-Gallego G,Reuben J P, Roy-Contancin L,Feigenbaum P,Kaczorowski GJ,etal.Purification and characterization ofa unique,potent,peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus[J]. J Biol Chem,1990,265(19):11083-11090.
[33] Candia S,Garcia ML,Latorre R.Mode of action of iberiotoxin,a potent blocker of the large conductance Ca2+-activated K+channel[J]. Biophys J,1992,63(2):583-590.
[34] Womack MD,Khodakhah K.Characterization of large conductance Ca2+-activated K+channels in cerebellar Purkinje neurons[J].Eur J Neurosci,2002,16(7):1214-1222.
[35] Womack MD,Khodakhah K.Dendritic control of spontaneous bursting in cerebellar Purkinje cells[J].J Neurosci,2004,24(14):3511-3521.
[36] Womack MD,Carolyn C,Kamran K.Calciumactivated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons[J].J Neurosci,2004,24(40):8818-8822.
[37] Takashi K,Block GD,Colwell CS.The circadian clock GenePeriod1 connects the molecular clock to neural activity in the suprachiasmatic nucleus[J].ASN Neuro,2015,7(6):e103309.
[38] Pitts GR,Ohta H,Mcmahon DG.Daily rhythmicity of large-conductance Ca2+-activated K+currents in suprachiasmatic nucleus neurons[J].Brain Res,2006,1071(1):54-62.
[39] Köhler M,Hirschberg B,Bond CT,Kinzie JM,Marrion NV,Maylie J,et al.Small-conductance,calcium-activated potassium channels from mammalian brain[J].Science,1996,273(5282):1709-1714.
[40] Ishii TM,Maylie J,Adelman JP.Determinants of Apamin and d-tubocurarine block in SK potassium channels[J].J Biol Chem,1997,272(37):23195-23200.
[41] Teshima K,Kim SH,Allen CN.Characterization of an apamin-sensitive potassium currentin suprachiasmatic nucleus neurons[J].Neuroscience,2003,120(1):65-73.
[42] Maurice N,Deltheil T,Melon C,Degos B,Mourre C,Amalric M,et al.Bee venom alleviates motor deficits and modulates the transfer of cortical information through the basal ganglia in rat models of Parkinson′s disease[J].PLoS One,2015,10(11):e0142838.
[43] Kasumu AW,Hougaard C,Rode F,Jacobsen TA,Sabatier JM,Eriksen BL,et al.Selective positive modulator of calcium-activated potassium channels exerts beneficial effects in a mouse model of spinocerebellar ataxia type 2[J].Chem Biol,2012,19(10):1340-1353.
[44] Womack MD,Hoang C,Khodakhah K.Large conductance calcium-activated potassium channels affect both spontaneous firing and intracellular calcium concentration in cerebellarPurkinje neurons[J].Neuroscience,2009,162(4):989-1000.
[45] Karina A, Kamran K.Selective regulation of spontaneous activity of neurons of the deep cerebellar nuclei by N-type calcium channels in juvenile rats[J].J Physiol,2008,586(10):2523-2538.
[46] Wolfart J,Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance,calcium-activated potassium channelSK3 is critical for pacemaker control in dopaminergic midbrain neurons[J].J Neurosci,2001,21(10):3443-3456.
[47] Deignan J,LujánR,BondC,RiegelA,Watanabe M,Williams JT,et al.SK2 and SK3 expression differentially affect firing frequency and precision in dopamine neurons[J].Neurosci⁃ence,2012,217(2012):67-76.
[48] Ji H,Shepard PD.SK Ca2+-activated K+Channel ligands alter the firing pattern of dopaminecontaining neurons in vivo[J].Neuroscience,2006,140(2):623-633.
[49] Hallworth NE,Wilson CJ,Bevan MD.Apaminsensitive small conductance calcium-activated potassium channels, through theirselective coupling to voltage-gated calcium channels,are critical determinants of the precision,pace,and pattern ofaction potentialgeneration in rat subthalamic nucleus neurons in vitro[J].J Neurosci,2003,23(23):7525-7542.
[50] Deister CA,Chan CD.Calcium-activated SK channels influence voltage-gated ion channels to determine the precision of firing in globus pallidus neurons[J].J Neurosci,2009,29(26):8452-8461.
[51] Rouchet N,Waroux O,Lamy C,Massotte L,Scuvée-Moreau J,Liégeois JF,et al.SK channel blockade promotes burst firing in dorsal raphe serotonergic neurons[J].Eur J Neurosci,2008,28(6):1108-1115.
[52] Shakkottai VG,Regaya I,Wulff H,Fajloun Z,Tomita H,Fathallah M,et al.Design and characterization of a highly selective peptide inhibitor ofthe smallconductance calciumactivated K+channel,SkCa2[J].J Biol Chem,2001,276(46):43145-43151.
[53] KnausHG, McmanusOB, Lee SH,Schmalhofer WA,Garcia-Calvo M,Helms LM,et al.Tremorgenic indole alkaloids potently inhib⁃it smooth muscle high-conductance calcium-acti⁃vated potassium channels[J].Biochemistry,1994,33(19):5819-5828.
[54] DefariasFP, Carvalho MF, Lee SH,Kaczorowski GJ,Suarez-Kurtz G.Effects of the K+channel blockers paspalitrem-C and paxilline on mammalian smooth muscle[J].Eur J Pharmacol,1996,314(1-2):123-128.
[55] Zeng H,Gordon EZ,Lozinskaya I,Willette R,Xu X.1-〔1-Hexyl-6-(methyloxy)-1H-indazol-3-yl〕-2-methyl-1-propanone,a potent and highly selective small molecule blocker of the largeconductance voltage-gated and calcium-depen⁃dent K+channel[J].J Pharmacol Exp Ther,2008,327(1):168-177.
[56] Gribkoff VK,Lum-Ragan JT,Boissard CG,Post-Munson DJ,Meanwell NA,Starrett JE,et al.Effects of channel modulators on cloned large-conductance calcium-activated potassium channels[J].Mol Pharmacol,1996,50(1):206-217.
[57] Dérand R,Bulteau-Pignoux L,Becq F.Compar⁃ative pharmacology of the activity of wild-type and G551D mutated CFTR chloride channel:effect of the benzimidazolone derivative NS004[J].J Membr Biol,2003,194(2):109-117.
[58] Bentzen BH,Nardi A,Calloe K,Madsen LS,Olesen SP,Grunnet M.The small molecule NS11021 is a potent and specific activator of Ca2+-activated big-conductance K+channels[J]. Mol Pharmacol,2007,72(4):1033-1044.
[59] Roy S,Morayo Akande A,Large RJ,Webb TI,Camarasu C,Sergeant GP,et al.Structureactivity relationships of a novel group of largeconductance Ca2+-activated K+(BK)channel modulators:the GoSlo-SR family[J].Chem Med Chem,2012,7(10):1763-1769.
[60] Schwartz WJ.Further evaluation of the tetrodo⁃toxin-resistant circadian pacemaker in the supra⁃chiasmatic nuclei[J].J Biol Rhythms,1991,6(2):149-158.
[61] Kent J,Meredith AL.BK channels regulate spon⁃taneous action potential rhythmicity in the supra⁃chiasmatic nucleus[J].PLoS One,2008,3(12):e3884.
[62] Ito M.The modifiable neuronal network of the cerebellum[J].Jpn J Physiol,1984,34(5):781-792.
[63] Thach WT.Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey[J].J Neurophysiol,1968,31(5):785-797.
[64] Person AL,Raman IM.Synchrony and neural coding in cerebellar circuits[J].Front Neural Circuits,2012,6(50):97.
[65] Sausbier M,Hu H,Arntz C,Feil S,Kamm S,Adelsberger H,et al.Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+channel deficiency[J].Proc Natl Acad Sci USA,2004,101(25):9474-9478.
[66] Cheron G,Sausbier M,Sausbier U,Neuhuber W,Ruth P,Dan B,et al.BK channels control cerebellar Purkinje and Golgi cell rhythmicity in vivo[J].PLoS One,2009,4(11):e7991.
[67] Benton MD,Lewis AH,Bant JS,Raman IM. Iberiotoxin-sensitive and-insensitive BK currents in Purkinje neuron somata[J].J Neurophysiol,2013,109(10):2528-2541.
[68] Sheehan JJ,Benedetti BL,Barth AL.Anticon⁃vulsant effects of the BK-channel antagonist paxilline[J].Epilepsia,2009,50(4):711-720.
[69] Blatz AL,Magleby KL.Single apamin-blocked Ca-activated K+channels of small conductance in cultured rat skeletal muscle[J].Nature,1986,323(690):718-720.
[70] Hirschberg B,Maylie J,Adelman JP,Marrion NV.Gating of recombinant small-conductance Ca-activated K+channels by calcium[J].J Gen Physiol,1998,111(4):565-581.
[71] Xia XM,Fakler B,Rivard A,Wayman G,Johnson-Pais T,Keen JE,et al.Mechanism of calcium gating in small-conductance calciumactivated potassium channels[J].Nature,1998,395(671):503-507.
[72] Keen JE,Khawaled R,Farrens DL,Neelands T,Rivard A,Bond CT,et al.Domains responsible for constitutive and Ca2+-dependent interactions between calmodulin and small conductance Ca2+-activated potassium channels[J].J Neurosci,1999,19(20):8830-8838.
[73] Schumacher MA,Crum M,Miller MC.Crystal structures of apocalmodulin and an apocalmodulin/ SK potassium channel gating domain complex[J].Structure,2004,12(5):849-860.
[74] Christophersen P,Wulff H.Pharmacological gating modulation of small-and intermediate-conduc⁃tance Ca2+-activated K+channels(KCa2.x and KCa3.1)[J].Channels,2015,9(6):336-343.
[75] Cui M,Qin GR,Yu KQ,Bowers M.Targeting the small-and intermediate-conductance Ca2+-activated potassium channels:the drug-binding pocket at the channel/calmodulin interface[J]. Neurosignals,2014,22(2):65-78.
[76] Syme CA,Gerlach AC,Singh AK,Devor DC. Pharmacological activation of cloned intermediateand small-conductance Ca2+-activated K+channels[J].Am J Physiol Cell Physiol,2000,278(3):C570-C581.
[77] Lappin SC,Dale TJ,Brown JT,Trezise DJ,Davies CH.Activation of SK channels inhibits epileptiform bursting in hippocampal CA3 neurons[J].Brain Res,2005,1065(1065):37-46.
[78] Walter JT,Alvina K,Womack MD,Chevez C,Khodakhah K.Decreases in the precision of Purkinje cellpacemaking cause cerebellar dysfunction and ataxia[J].Nat Neurosci,2006,9(3):389-397.
[79] Gao Z,Todorov B,Barrett CF,Van Dorp S,Ferrari MD,Van Den Maagdenberg AM,et al. Cerebellarataxia by enhanced Ca(V)2.1 currents is alleviated by Ca2+-dependent K+-channel activators in Cacna1a(S218L)mutant mice[J]. J Neurosci,2012,32(44):15533-15546.
[80] Hougaard C, Eriksen BL, Jørgensen S,Johansen TH,Dyhring T,Madsen LS,et al. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+channels[J].Br J Pharmacol,2007,151(5):655-665.
[81] ChubanovV,MederosYSchnitzlerM,MeißnerM,Schäfer S,Abstiens K,Hofmann T,et al.Natural and synthetic modulators of SK(Kca2)potassium channels inhibit magnesium-dependent activity ofthe kinase-coupled cation channel TRPM7[J].Br J Pharmacol,2012,166(4):1357-1376.
[82] Alviña K,Khodakhah K.KCachannels as thera⁃peutic targets in episodic ataxia type-2[J].J Neurosci,2010,30(21):7249-7257.
[83] Strøbaek D,Teuber L,Jørgensen TD,Ahring PK,Kjaer K,Hansen RS,et al.Activation of human IK and SK Ca2+-activated K+channels by NS309(6,7-dichloro-1H-indole-2,3-dione 3-oxime)[J].Biochim Biophys Acta,2004,1665(1-2):1-5.
[84] Cao YJ,Dreixler JC,Couey JJ,Houamed KM. Modulation of recombinant and native neuronalSK channels by the neuroprotective drug riluzole[J].Eur J Pharmacol,2002,449(1-2):47-54.
[85] Romano S,Coarelli G,Marcotulli C,Leonardi L,Piccolo F,Spadaro M,et al.Riluzole in patients with hereditary cerebellar ataxia:a randomised,double-blind,placebo-controlled trial[J].Lancet Neurol,2015,14(10):985-991.
[86] Habermann E.Apamin[J].Pharmacol Ther,1984,25(2):255-270.
[87] Lamy C,Goodchild SJ,Weatherall KL,Jane DE,Liégeois JF,Seutin V,et al.Allosteric block of KCa2 channels by apamin[J].J Biol Chem,2010,285(35):27067-27077.
[88] Pedarzani P,Stocker M.Molecular and cellular basis of small-and intermediate-conductance,calcium-activated potassium channel function in the brain[J].Cell Mol Life Sci,2008,65(20):3196-3217.
[89] PedarzaniP, Mccutcheon JE, RoggeG,Jensen BS,Christophersen P,Hougaard C,et al. Specific enhancement of SK channel activity selectively potentiates the afterhyperpolarizing currentⅠ(AHP)and modulates the firing properties of hippocampal pyramidal neurons[J].J Biol Chem,2005,280(50):41404-41411.
[90] Yee NS,Kazi AA,Yee RK.Cellular and devel⁃opmentalbiologyof TRPM7 channel-kinase:implicated roles in cancer[J].Cells,2014,3(3):751-777.
[91] Hosy E,Piochon C,Teuling E,Rinaldo L,Hansel C.SK2 channel expression and function in cerebellar Purkinje cells[J].J Physiol,2011,589(Pt14):3433-3440.
[92] Kaffashian M,Shabani M,Goudarzi I,Behzadi G,Zali A,Janahmadi M.Profound alterations in the intrinsic excitability of cerebellar Purkinje neurons following neurotoxin 3-acetylpyridine(3-AP)-induced ataxia in rat:new insights into the role of small conductance K+channels[J]. Physiol Res,2011,60(2):355-365.
[93] Shakkottai VG,Chou CH,Oddo S,Sailer CA,Knaus HG,Gutman GA,et al.Enhanced neuronal excitability in the absence of neurodegeneration induces cerebellar ataxia[J].J Clin Invest,2004,113(4):582-590.
[94] Wolfart J,Roeper J.Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons[J].J Neurosci,2002,22(9):3404-3413.
[95] Waroux O,Massotte L,Alleva L,Graulich A,Thomas E,Liégeois JF,et al.SK channels control the firing pattern of midbrain dopaminergic neurons in vivo[J].Eur J Neurosci,2005,22(12):3111-3121.
[96] Tacconi S,Carletti R,Bunnemann B,Plumpton C,Merlo Pich E,Terstappen GC.Distribution of the messenger RNA for the small conductance calciumactivated potassium channel SK3 in the adult rat brain and correlation with immunoreactivity[J]. Neuroscience,2001,102(1):209-215.
[97] Shakkottai VG,do Carmo Costa M,Dell′Orco JM,Sankaranarayanan A,Wulff H,Paulson HL. Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3[J].J Neurosci,2011,31(36):13002-13014.
[98] Schiemann J,Schlaudraff F,Klose V,Bingmer M,Seino S,Magill PJ,et al.K-ATP channels in dopamine substantia nigra neurons control bursting and novelty-induced exploration[J].Nat Neurosci,2012,15(9):1272-1280.
[99] Zaghloul KA,BlancoJA,WeidemannCT,Mcgill K,Jaggi JL,Baltuch GH,et al.Human substantia nigra neurons encode unexpected financial rewards[J].Science,2009,323(5920):1496-1499.
[100] Ivkovic′M,Rankovic′V,Tarasjev A,Orolicki S,Damjanovic′A,Paunovic′VR,et al.Schizophrenia and polymorphic CAG repeats array of calciumactivated potassium channel(KCNN3)gene in Serbian population[J].Int J Neurosci,2006,116(2):157-164.
[101] Grube S,Gerchen MF,Adamcio B,Pardo LA,Martin S,Malzahn D,et al.A CAG repeat poly⁃morphism of KCNN3 predicts SK3 channel function and cognitive performance in schizophrenia[J]. EMBO Mol Med,2011,3(6):309-319.
[102] Koronyo-Hamaoui M,Gak E,Stein D,Frisch A,Danziger Y,Leor S,et al.CAG repeat polymor⁃phism within the KCNN3 gene is a significant contributor to susceptibility to anorexia nervosa:a case-control study of female patients and several ethnic groups in the Israeli Jewish population[J].Am J Med Genet B Neuropsychiatr Genet,2004,131B(1):76-80.
[103] Sailer CA,Hu H,Kaufmann WA,Trieb M,Schwarzer C,Storm JF,et al.Regional differ⁃ences in distribution and functional expression of small-conductance Ca2+-activated K+channels in ratbrain[J].J Neurosci,2002,22(22):9698-9707.
[104] SailerCA,KaufmannWA,MarksteinerJ,Knaus HG.Comparative immunohistochemical distribution ofthree small-conductance Ca2+-activated potassium channel subunits,SK1,SK2,and SK3 in mouse brain[J].Mol Cell Neurosci,2004,26(3):458-469.
[105] Ledoux J,Werner ME,Brayden JE,Nelson MT.Calcium-activated potassium channels and the regulation of vascular tone[J].Physiology,2006,21(1):69-78.
[106] Bensimon G,Doble A.The tolerability of riluzole in the treatment of patients with amyotrophic lateral sclerosis[J].Expert Opin Drug Saf,2004,3(6):525-534.
[107] HammondRS,BondCT,StrassmaierT, Ngo-Anh TJ, Adelman JP, Maylie J, et al. Small-conductance Ca2+-activated K+channel type 2(SK2)modulates hippocampal learning,memory,and synaptic plasticity[J].J Neurosci,2006,26(6):1844-1853.
[108] Stackman RW,Bond CT,Adelman JP.Contex⁃tual memory deficits observed in mice overex⁃pressing smallconductance Ca2+-activated K+type 2(KCa2.2,SK2)channels are caused by an encoding deficit[J].Learn Mem,2008,15(4):208-213.
[109] Vick KA,Michael G,Stackman RW.In vivo pharmacological manipulation of small conduc⁃tance Ca2+-activated K+channels influences motor behavior,object memory and fear conditioning[J].Neuropharmacology,2010,58(3):650-659.
钙离子激活钾通道对起搏神经元活性的作用
Nancy DONG,冯中平
(Department of Physiology,University of Toronto,1 King′s College Circle,Toronto,Ontario,Canada M5S 1A8)
中枢起搏神经元的自发节律活动是神经功能的基础,这些神经功能例如体位移动,昼夜节律和认识知觉。神经元的自发节律活动的生成涉及多种离子通道,钙离子激活钾通道(KCa通道)在维持生理性起博频率和规律中起着的突出的作用。根据对iberiotoxin和蜂毒明肽类的KCa通道调节药物的药理研究,我们对KCa通道在起搏神经元功能中的作用的认识获得很大的提高。尽管近年的研究取得了显著的进步,钙激活钾通道调节剂的广泛使用增加了非特异性药物作用意想不到的复杂性。由于KCa通道调节剂已经用于帕金森病和乙醇中毒等多种疾病的早期临床试验治疗,本文强调了这些药物作用和不足在使用中的重要性。
钾通道钙激活;自发起搏神经元;节律活动;通道调节剂
冯中平,E-mail:zp.feng@utoronto.ca,Tel:+1(416)946-0671
2016-03-16 接受日期:2016-06-13)
R966
:A
:1000-3002-(2016)06-0627-13
10.3867/j.issn.1000-3002.2016.06.003
Biography:Nancy DONG,female,HBSc,graduate student, main research field is neuroscience,E-mail:nancy.dong@ mail.utoronto.ca
Zhong-ping FENG,Tel:+1(416) 946-0671,E-mail:zp.feng@utoronto.ca
(本文编辑:乔 虹)
- 中国药理学与毒理学杂志的其它文章
- Brief Introduction of the Executive Committee of the Chinese Pharmacological Society North America Chapter(CPSNAC) and the Specialists in This Issue
- Developing high quality chemical probes targeting ubiquitin-specific proteases
- Reversal effects of desipramine on resistance of U251/TR cells to temozolomide
- Effects of calcium channel blockers on growth cone and their clinical implications
- Pathological role of transient receptor potential melastatin member 2 channel in neurodegenerative diseases and Alzheimer disease
- Drug reward memory:implication from drug-induced conditioned place preference model

