A Review of Pteridophyta Potential in Phytoremediation of Heavy Metal Contaminated Environments
,
1. School of Environmental Science and Engineering, Southwest Forestry University, Kunming 650224, China; 2. School of Ecology and Environmental Science, Yunnan University, Kunming 650091, China; 3. Research Institute of Gardening and Landscape of Kunming, Kunming 650224, China
1 Introduction
Pteridophyta can be traced back to the late Silurian Period as early as 400 million years ago[1]. Pteridophyta are main terrestrial plants during the Paleozoic Era and the Mesozoic Era, lie between bryophyta and spermatophyte in system evolution, and are most primitive and earliest vascular plants. Most existing pteridophyta are herbaceous plants and varieties are numerous[2], and ecological types are rich[3], propagation and adaptation methods are diversified[4], and pteridophyta are essential parts of plant diversity. Some varieties have high adaptation to extreme hostile environment. For example,Hymenophyllumanguinolentumhas high resistance to drought, and can survive several days of -40 Mpa osmotic stress[5].Trichomanesspeciosumis widely distributed in western Europe, and it is highly barren and weak light resistant, and it even can survive in volcanic ashes[6]. There are also varieties that can survive in barren soil[7], metal abandoned mine[8], and seriously polluted areas[9]. Many pteridophyta may be used as indicator of environmental changes due to special adaptation to living environment[10-13]. In recent years, it has proved that Pteris has function of accumulating heavy metals, especially for arsenic (As) and stibium (Sb), and it has huge potential of ecological remediation.
2 Application of plants in ecological remediation of heavy metal contamination
In this paper, ecological remediation refers to phytoremediation. Since its introduction, phytoremediation has been receiving wide concern in control of heavy metal contamination. In recent years, it has been reported that there are more than 450 varieties of hyperaccumulators for heavy metals, belonging to 45 families respectively[14,15], most of them are hyperaccumulators for nickel (Ni), up to 318 varieties[16], and there are several tens of pteridophyta hyperaccumulators, mainly are As, Sb hyperaccumulators and most arePteridaceae.
2.1ResourceandperformanceofpteridophytathatcanaccumulateAsArsenic is a chemical metalloid element known to cause cancer[17]. Its physical and chemical properties and environment behavior are much similar to heavy metals, so it is often included when discussing heavy metals[18]. In normal soil, ordinary plants contain As generally not higher than 3 mg/kg[19], while some pteridophyta can absorb excessive As and still can normally grow.
PterisvittataL. is the first As hyperaccumulator found[20-22]. The As content accumulated in its above-ground part is up to 7526 mg/kg, accounting for 2.3% of biomass (dry weight) of above-ground part, and the content is even higher than phosphorus in the plant. Another As hyperaccumulator Pteris cretica has average As content in the above-ground part up to 418 mg/kg (dry weight) and the maximum As content is up to 694 mg/kg. The average As content in under-ground part (root) is up to 293 mg/kg, the maximum As content is 552 mg/kg, the bioaccumulation factor is 1.3-4.8, and the translocation factor (ratio of As content in above-ground part to under-ground part) is 1-2.6[23], and the As absorbed is mainly accumulated in mesophyll tissue of fronds[24,25]. Later, many As hyperaccumulators were found[26-30], and many of them arePteridaceae, and some are screened fromHemionitedaceae. Visoottivisethetal.[31]found that fronds ofPityrogrammacalomelanoscan accumulate As as high as 8350 mg /kg (dry weight). Xu Weihongetal.[32]measured the As content in above-ground part ofP.calomelanosvar.austroamericana up to 2 438.33 mg/ kg (dry weight). By now, domestic and foreign countries have found more than 20 varieties (including variants) of As hyperaccumulators, as listed in Table 1.
Table1Ashyperaccumulatorpteridophyta
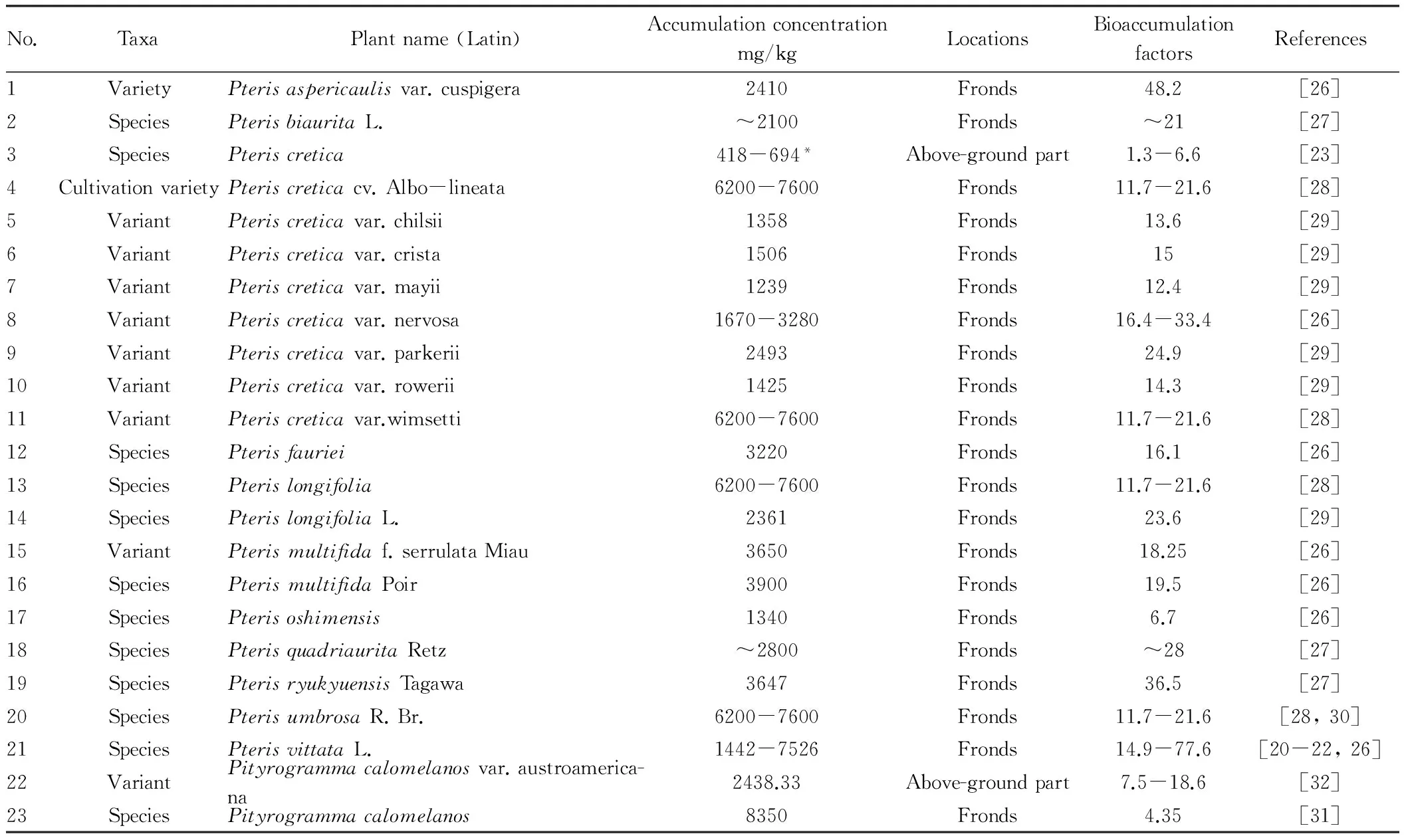
No.TaxaPlantname(Latin)Accumulationconcentrationmg/kgLocationsBioaccumulationfactorsReferences1VarietyPterisaspericaulisvar.cuspigera2410Fronds48.2[26]2SpeciesPterisbiauritaL.~2100Fronds~21[27]3SpeciesPteriscretica418-694*Above-groundpart1.3-6.6[23]4CultivationvarietyPteriscreticacv.Albo-lineata6200-7600Fronds11.7-21.6[28]5VariantPteriscreticavar.chilsii1358Fronds13.6[29]6VariantPteriscreticavar.crista1506Fronds15[29]7VariantPteriscreticavar.mayii1239Fronds12.4[29]8VariantPteriscreticavar.nervosa1670-3280Fronds16.4-33.4[26]9VariantPteriscreticavar.parkerii2493Fronds24.9[29]10VariantPteriscreticavar.rowerii1425Fronds14.3[29]11VariantPteriscreticavar.wimsetti6200-7600Fronds11.7-21.6[28]12SpeciesPterisfauriei3220Fronds16.1[26]13SpeciesPterislongifolia6200-7600Fronds11.7-21.6[28]14SpeciesPterislongifoliaL.2361Fronds23.6[29]15VariantPterismultifidaf.serrulataMiau3650Fronds18.25[26]16SpeciesPterismultifidaPoir3900Fronds19.5[26]17SpeciesPterisoshimensis1340Fronds6.7[26]18SpeciesPterisquadriauritaRetz~2800Fronds~28[27]19SpeciesPterisryukyuensisTagawa3647Fronds36.5[27]20SpeciesPterisumbrosaR.Br.6200-7600Fronds11.7-21.6[28,30]21SpeciesPterisvittataL.1442-7526Fronds14.9-77.6[20-22,26]22VariantPityrogrammacalomelanosvar.austroamerica-na2438.33Above-groundpart7.5-18.6[32]23SpeciesPityrogrammacalomelanos8350Fronds4.35[31]
Note: data with*were inferred according to block diagram in original document.
2.2ResourceandperformanceofpteridophytathatcanaccumulateSbAntimony (Sb) is a trace element in the Earth’s crust[33]. In plant body, 5-10 mg/kg Sb can cause toxicity[19]. At present, there are only several potential Sb hyperaccumulators[34]. Because Sb and As have similar chemical properties, while most As hyperaccumulators belong to pteridophyta, researchers screened Sb hyperaccumulators from pteridophyta. Tisarumetal.[35]tookPterisvittataL. of the United States of America, China, and Brazil as materials, measured accumulation concentration of Sb 4192-12000 mg/kg, and found thatPterisvittataL. mainly absorbed trivalent Sb. Another As hyperaccumulatorPteriscreticahas high endurance to high concentration Sb[36]. The cultivation variantPteriscreticacv. Albo-lineata can accumulate as high as 6405 mg/kg Sb[37]. However, Sb is mainly accumulated in roots, especially forPterisvittataL., more than 99% of absorbed total Sb is accumulated in roots[35](Tisarumetal., 2014), and the transfer factor is extremely low. However, few researches about transfer factor have exact evidences and most are based on inference. Fengetal.[38]held that Sb can be transferred in many ways: trivalent Sb can be transferred through the transfer pathway of trivalent As, while the transfer pathway is still not found for pentavalent Sb, the possible transfer pathway is phosphate transfer system[35]. In angiosperm, there are also Sb accumulators, such asAchilleaageratum,Plantagolanceolata, andSilenevulgarishave Sb accumulation concentration up to 1367 mg/kg (basal leaves), 1150 mg/kg (roots), and 1164 mg/kg (stems)[39]. In recent years, Affholderetal.[40]also found thatRosmarinusofficinaliscan be cultivated in heavy metal contaminated soil, and the roots can accumulate 309 mg/kg Sb. These plants can effectively accumulate Sb, but the accumulation concentration is lower than pteridophyta. Although pteridophyta have low transfer factor of Sb, the potential accumulation of Sb is high.
2.3ResourceandperformanceofpteridophytathatcanaccumulateotherheavymetalsandrareearthelementsExisting data indicate that pteridophyta also have high resistance and accumulation to other heavy metals apart from As. When screening hyperaccumulators and tolerant plants, Kolleretal.[41]found thatPterisvittataL. can absorb much As apart from absorbing heavy metals such as Pb and Zn. Roccotiello[42]also found thatPterisvittataL. andPolypodiumcambricumcan absorb Zn. Li Yingetal.[43, 44]studied Cu absorption and transfer byEquisetumramosissimumandPterisvittataL., the results indicate thatEquisetumramosissimumandPterisvittataL. have high resistance and accumulation to Cu, and the total Cu accumulation is up to 1439.47 mg/kg and 398.62 mg/kg, and the accumulation factor of root system is greater than 1, so they can be used pioneer plants to remediate Cu contaminated soil. Pteridophyta also can tolerate combined pollution of many heavy metals.Salvinianatanscan accumulate Cr, Ni, Fe and Cd in Cr accumulated waste water[45], but it is relatively sensitive to Cd, and the semi-effect concentration is only 2.41 mg/L[46]. Although it has certain purification effect on Cd, it is extremely vulnerable to damage. In combined pollution of Cd, Pb, Mn, Cu, and Zn, roots have highest accumulation of Cd, and the accumulation factor is up to 2.3[47]. In combined pollution of Cr and Ni,Pteridiumaquilinumcan absorb higher Cr and Ni than single treatment, in other words, showing a cooperative phenomenon[12]. In addition,Dicranopterislinearisin rare-earth mining areas has rare earth content as high as 3263.8 mg/kg[48].Dicranopterisdichotomahas high accumulation ability of rare earth elements La, Ce, and Nd, which is 100-1000 times higher thanPinusmassonianaLamb. living in the same gold mine environment, so this feature can be used for remediation of rare earth contaminated areas.
3 Accumulation and tolerance mechanism of pteridophyta to heavy metals
3.1ExcretionmechanismSome pteridophyta can excrete heavy metals or accumulate heavy metals in old leaves and discharge excessive heavy metals through falling leaves. Tu[50](2002) found that whenP.vittataL. grows in soil with As content higher than 0.5 mg/kg, As will accumulate in old leaves and then be discharged with leaf falling.
3.2BindingdeactivationandcompartmentalizationCell wall or cell membrane or vacuole contains binding holder for binding toxic matters such as heavy metals. For example, polysaccharide aldehydic acid in pectin substance of cell wall and carboxylic acid and aldehyde group in cellulose molecules can bind heavy metals, to reduce transfer of heavy metals to cytoplasm, so as to detoxify. Besides, heavy metals entered into cytoplasm can bind organic acids (such as citric acid and malic acid), amino acid (like histidine), or metallothionein, and phytochelatin. Nishizonoetal.[51]analyzed function of root cell wall ofAthyriumyokoscensein detoxication of heavy metals, and results indicate that 70%-90% of total Cu, Zn, and Cd entering into the plant body remain on cell wall, larger portion remains in the form of ion or bind to structural substances of cell wall, such as cellulose and lignin. Webbetal.[52]studied distribution of As inP.vittataL. through X ray absorption spectrometry, and results indicate that phytochelatin may play an active role in accumulation of As. Chen Tongbinetal.[24]found that 78% As in fronds is distributed in cell sap, and such compartmentalization[25]is an essential reason for detoxication ofPterisvittataL.
3.3Anti-oxidantfunctionAfter metals and metalloid elements enter into plant body, they may generate some reactive oxygen species (ROS). Excessive ROS may poison plants. To remove ROS, plants will synthesize some enzymes and non-enzyme anti-oxidant substances. The anti-oxidant substances will promote normal plant electron to smoothly transfer. According to studies of Feng Renweietal.[53], under Se stress, the GSH and GR enzyme content in leaves ofP.vittataL. have significant higher activity because GSH and GR enzyme play the role of adjusting O2-; in comparison, POD, APX, and CAT remove H2O2only in low concentration Se treatment. Through comparing anti-oxidant system ofP.multifidaandP.vittataL., Zhangetal.[54]found that with rise of As and Pb concentration, the activity of SOD and POD rises, GSH content increases, and thus mitigating metal toxicity.
3.4CombinedactionofmicroorganismsRhizospheric microorganisms can promote plants to absorb nutritional elements and heavy metals in soil, or strengthen adaptation ability to contaminated environment through secreting growth regulator and antibiotic, bacteriostatic agent or phytochelatins. In pteridophyta, researches about microorganisms strengthening absorption of contaminants focus on As, and experimental microorganisms include bacteria, fungi, and actinomyces. Rhizospheric microorganismPseudomonasaeruginosacan increase absorption ofPteridiumaquilinumto As[55], possibly because it can secrete siderophores to soil in iron deficient environment, and combine with As to form Fe and As compound, which is easily be absorbed by root system and then transferred to leaves and branches for accumulation. Other studies[56]found that with addition of allogenic bacteria Ts37, the As content of above-ground part ofP.vittataL. is up to 837 mg/kg; with addition of actinomyce shf2, the As content of under-ground part is 427 mg/kg, which are 206% and 88% higher than the control group respectively. Liuetal.[57]found that after inoculation ofGlomusmosseae, the As content ofP.vittataL. is up to 300 mg/kg, with As accumulation increase about 43%. In the long term of evolution, pteridophyta constantly adapt to external environment, including contaminated environment. In this process, it may develop various adaptation strategies. Once under stress of contaminated environment, generally several mechanisms act jointly.
4 Strengths of pteridophyta in phytoremediation
4.1AncientoriginandhighadaptationPteridophyta are ancient plants. With long time of natural selection and evolution, pteridophyta have developed wide adaptation and strong vitality[6].P.vittataL. has strong As tolerance and can normally grow in slags with As content of 23400 mg/kg[21]; in vegetation remediation of tropical and subtropical areas, pteridophyta often become dominant species of herbaceous layer[58,59]. Thus, they can play an important role in remediation of contaminated land.
4.2HavinguniquemetalloidAsresistinggenesPteridophyta are characterized by barren resistance, rapid growth, high breeding ability, and high resistance. They have high resistance to stressed environment and can make up for certain drawbacks and weaknesses of existing remediation plants. In recent years, there are extensive researches about As resisting genes of As hyperaccumulator pteridophyta[60-62], to find out reasons for hyperaccumulation of As from molecular mechanism. Indrioloetal.[63]found thatPterisvittataL. has gene ACR3 for encoding arsenious acid transferring protein, the expression of sporophyte root system and gametophyte is subject to adjustment of As, and it is positioned on vacuole membrane. It is interesting that this gene is lost in angiosperm. Therefore, in remediation of As contaminated soil, pteridophyta have inborn strength.
4.3VariousandpoorselectionoforganicpollutantsaccumulatedbypteridophytaPteridophyta can accumulate various organic pollutants and the selection is poor[64]. Some pteridophyta have high ability of pollution tolerance and can accumulate many kinds of heavy metals.Azollapinnatavar.imbricatacan grow in sewage, and its ability of resisting acid and alkali, salt and fertilizer, pH tolerance limit is 2.8 and 12.6, and the acid and alkali resistant range is pH 3.5-11.7, and the N and P tolerance limit is 175 mg/L and 800 mg/L[65].A.filiculoideshas obvious accumulation function of Cd, As, Zn[66], Pb, and Hg[67]in polluted river, can obsorb much N and P[68,69], and the removal rate of ammonium nitrogen, nitrate nitrogen and phosphorus is up to 94.04%, 95.46%, and 99.0%[70]. At the same time of accumulating Cd,P.vittataL. can absorb Pb, Mn, Cu, and Zn[47];A.imbircatacan absorb radioactive uranium, in hydroponic condition, the removal rate of uranium is up to 92%-97%[71];Pteridiumaquilinumcan accumulate Cr and Ni[12]. Such poor selection feature may be correlated with its evolution degree, but from the perspective of phytoremediation, such broad spectrum activity has high application value.
5 Prospects
In the process of formation and evolution of land ecosystem, pteridophyte is the pioneer population conquering land ecological environment. In modern plants, pteridophyta are diminishing in both the variety and individual quantity. Compared with spermatophyte, pteridophyte is still a small population, but as an essential member of plant kindom, pteridophyta still undertake a great role in keeping diversity of land ecosystem and ecological balance. Pteridophyta have unique biological and ecological characteristics, and have huge potential in remediation of contaminated ecosystem. In recent years, there have been extensive researches about phytoremediation mechanism of pteridophyta. In future, it may be further developed in following aspects: (i) the interaction and influencing rules of absorption and accumulation of As and Sb by pteridophyta in combined pollution condition; (ii) cultivation of new varieties easy for cultivation and remediation of heavy metal contaminated environment based on clone and characterization of existing genes; (iii) biological degradation of pteridophyta to organic pesticide; Nesterenko-Malkovskaya[72]once reported thatEichhorniacrassipescan remove naphthalene in waste water without assistance of rhizosphere microorganisms, indicating that plant makes great contribution to degradation of organic pollutants. However, it is still not clear whether pteridophyta have such ability. (iv) study of rapid breeding technology for pteridophyta with remediation potential. Pteridophyta are not reproduced through seeds, thus, high efficient breeding of pteridophyte seedlings may be a bottleneck in remediation. Although there have been some breeding methods[73,74], the survival rate is limited and it is difficult to satisfy demands of large field planting. (v) research and development of final risk control and recovery and treatment technologies for accumulators. After pteridophyte accumulating heavy metals, if control is not taken, it may pose ecological risks. Mathewsetal.[75]and Jeongetal.[76]found that in planting areas of As accumulating pteridophyta, herbivores contain higher As in body than the control group, even up to wounding effect. If effective control is not taken, it probably leads to ecological imbalance. If treating by traditional methods such as landfill or burning, it may lead to secondary pollution. Therefore, research and development of high efficient and safe resource based utilization will have broad prospects.
[1] LI XX, ZHOU ZY, GUO SX. On the development and evolution of plant kindom[M]. Beijing: Science Press, 1981: 50-51.(in Chinese).
[2] LU SG. Chinese pteridophyta flora[M]. WU ZY, CHEN XQ. Flora of China.Beijing: Science Press,2004: 78-94.(in Chinese).
[3] LU SG, CHEN F. On the pteridophyte ecological types[J]. Journal of Yunnan University(Natural Sciences),2013,35(3) : 407-415. (in Chinese).
[4] WU ZH, QIN RC. Chinese fern, family and genus[M]. Beijing: Science Press, 1991:4. (in Chinese).
[5] PROCTOR MCF. Light and desiccation responses of some Hymenophyllaceae (filmy ferns) from Trinidad, Venezuela and New Zealand: poikilohydry in a light-limited but low evaporation ecological niche[J]. Annals of Botany, 2012, 109(5): 1019-1026.
[6] JOHNSON GN, RUMSEY FJ, HEADLEY AD,etal. Adaptations to extreme low light in the fern Trichomanes speciosum[J]. New Phytologist, 2000, 148(3) : 423-431.
[7] PAGE CN. Ecological strategies in fern evolution: a neopteridological overview[J]. Review of Palaeobotany and Palynology, 2002, 119(1): 1- 33.
[8] LI Y, CHEN ML. Effects of the inhabitation by Hippochaete ramosissimum on heavy metal speciations and enzyme activities in copper mine tailing soil[J]. Acta Ecologica Sinica, 2010, 30(21) : 5949-5957. (in Chinese).
[9] OLIVER MJ, TUBA Z, MISHLER BD. The evolution of vegetative desiccation tolerance in land plants[J]. Plant Ecology, 2000, 151 (1):85-100.
[10] GUPTA M, DEVI S. Uptake and toxicity of cadmium in aquatic ferns[J]. Journal of Environmental Biology, 1995, 16(2) : 131-136.
[11] CHANG JS, YOON IH, KIM KW. Heavy metal and arsenic accumulating fern species as potential ecological indicators in As contaminated abandoned mines[J]. Ecological Indicators, 2009, 9(6):1275-1279.
[12] KAMILA K, ALEKSANDRA SC, KRZYSZTOF K,etal. Chromium and nickel inPteridiumaquilinumfrom environments with various levels of these metals[J]. Environmental Science and Pollution Research, 2015, 22(1):527-534.
[13] YAN YH, ZHANG XC, MA KP. The diversity and geographical distribution of Chinese Pteridophyta[M]. Beijing: Science Press, 2013:17-26. (in Chinese).
[14] PRASAD MNV, FREITAS H, FRAENZLE S,etal. Knowledge explosion in phytotechnologies for environmental solutions[J]. Environmental Pollution, 2010,158(1):18-23.
[15] SEBASTIAN A, PRASAD MNV. Cadmium minimization in rice[J]. Agronomy for Sustainable Development,2014,34(1):155-173.
[16] KRAMER U, COTTER-HOWELLS JD, CHARNOCK JM,etal. Free histidine as a metal chelator in plants that accumulate nickel[J]. Nature, 1996, 379(6566):634-738.
[17] NRIAGU JO. Arsenic in the environment. Part Ⅱ: Human health and ecosystem effects[M]. John Wiley& Sons , New York, 1994: 1-5.
[18] CHEN HM. The behavior and environmental quality of chemical substances in the soil[M].Beijing: Science Press,2002: 79.(in Chinese).
[19] KABATA-PENDIAS A, PENDIAS H. Trace elements in soils and plants (3nd)[M]. Boca Raton, FL, USA: CRC Press, 2001:3-20.
[20] MA LQ, KOMAR KM , TU C ,etal. A fern that hyperaccumulates arsenic: a hardy, versatile, fast-growing plant helps to remove arsenic from contaminated soils[J]. Nature. 2001, 409(6820): 579.
[21] CHEN TB, WEI CY, HUANG ZC,etal. Arsenic hyperaccumulatorPterisvittataL. and its enrichment characteristic of arsenic[J]. Chinese Science Bulletin, 2002, 47 (3) : 207-210. (in Chinese).
[22] CHEN TB, WEI CY, HUANG ZC,etal. Arsenic hyperaccumulatorPterisVittataL. and its arsenic accumulation[J].Chinese Science Bulletin, 2002,47(11):902-905.
[23] WEI CY, CHEN TB, HUANG ZC,etal. Cretan brake (Pteris cretica L.): an arsenic-accumulating plant[J]. Acta Ecologica Sinica, 2002,22 (5) : 777-778. (in Chinese).
[24] CHEN TB, HUANG ZC, HUANG YY,etal. The micro area distribution of elements in arsenic hyperaccumulation plantsand its relationship with arsenic enrichment[J]. Chinese Science Bulletin, 2003, 45(11): 1163-1168. (in Chinese).
[25] CHEN TB, YAN XL, LIAO XY,etal. The subcellular distribution and segmentation of arsenic inPterisvittataL.[J]. Chinese Science Bulletin,2005,50(24):2739-2744. (in Chinese).
[26] WANG HB , WONG MH , LAN CY ,etal. Uptake and accumulation of arsenic by 11Pteristaxafrom southern China[J]. Environmental Pollution, 2007, 145 (1) : 225-233.
[27] SRIVASTAVA M, MA LQ, SANTOS JAG. Three new arsenic hyperaccumulating ferns[J]. Science of the Total Environment, 2006, 364(1): 24-31.
[28] ZHAO FJ, DUNHAM SJ, MCGRATH SP. Arsenic hyperaccumulation by different fern species[J]. New Phytologist, 2002,156 (1) : 27-31.
[29] MEHARG AA. Variation in arsenic accumulation-hyperaccumulation in ferns and their allies[J]. New Phytologist, 2003, 157(1):25-31.
[30] KOLLER CE , PATRICK JW , ROSE RJ ,etal.PterisumbrosaR. Br. as an arsenic hyperaccumulator: accumulation, partitioning and comparison with the established As hyperaccumulatorPterisvittata[J]. Chemosphere, 2007, 66 (7): 1256-1263.
[31] VISOOTTIVISETH P , FRANCESCONI K, SRIDOKCHAN W. The potential of Thai indigenous plant species for the phytoremediation of arsenic contaminated land[J]. Environmental Pollution, 2002 , 118(3):453-461.
[32] XU WH,ANTHONY GK, BALWANT S. Arsenic-hyperaccumulatorPityrogrammacalomelanos(L.) Link var.austroamericana(Domin) Farw and its uptake and accumulation of arsenic[J]. Journal of Soil and Water Conservation, 2009, 23(2): 173-177. (in Chinese).
[33] HE M, WANG X, WU F,etal. Antimony pollution in China[J]. Science of the Total Environment, 2012,421-422:41-50.
[34] FENG RW, WEI CY, TU SX. Research advances in uptake, metabolism and toxicity of antimony in plants[J]. Chinese Bulletin of Botany, 2012, 47 (3): 302-308. (in Chinese).
[35] TISARUM R, LESSL JT, DONG X,etal. Antimony uptake, efflux and speciation in arsenic hyperaccumulatorPterisvittata[J]. Environmental Pollution, 2014, 186:110-114.
[36] FENG RW, WEI CY, TU SX,etal. Simultaneous hyperaccumulation of arsenic and antimony in Cretan brake fern: evidence of plant uptake and subcellular distributions[J]. Microchemical Journal, 2011, 97(1): 38-43.
[37] FENG R, WANG X, WEI C,etal. The accumulation and subcellular distribution of arsenic and antimony in four fern plants[J]. International Journal of Phytoremediation, 2015, 17(4):348-354.
[38] FENG RW, WEI CY, TU SX,etal. The uptake and detoxification of antimony by plants: a review[J]. Environmental and Experimental Botany, 2013, 96:28-34.
[39] BARONI F, BOSCAGLI A, PROTANO G,etal. Antimony accumulation inAchilleaageratum,PlantagolanceolataandSilenevulgarisgrowing in an old Sb-mining area[J]. Environmental Pollution, 2000, 109(2), 347-352.
[40] AFFHOLDER MC, PRUDENT P, MASOTTI V,etal. Transfer of metals and metalloids from soil to shoots in wild rosemary (RosmarinusofficinalisL.) growing on a former lead smelter site: human exposure risk[J]. Science of the Total Environment, 2013, 454:219-229.
[41] KOLLER CE , PATRICK JW , ROSE RJ ,etal. Arsenic and heavy metal accumulation byPterisvittataL. andP.umbrosaR. Br[J]. Bulletin of Environmental Contamination and Toxicology, 2008, 80(2):128-133.
[42] ROCCOTIELLO E, MANDREDI A, DRAVA G,etal. Zinc tolerance and accumulation in the fernsPolypodiumcambricumL. andPterisvittataL.[J]. Ecotoxicology and Environmental Safety, 2010,73(6):1264-1271.
[43] LI Y, CHU L. The uptake and accumulation of Cu in Hippochaete ramosissimum[J]. Acta Ecologica Sinica,2008, 28 (4) : 1565-1572.(in Chinese).
[44]LI Y, WANG YB. Research on Cu uptake and tolerance of four pteridophyta plants[J]. Acta Prataculturae Sinica, 2010b, 19(3): 191- 197. (in Chinese).
[45] DHIR B,SHARMILA P, PARDHA SP,etal. Physiological and antioxidant responses ofSalvinianatansexposed to chromium-rich wastewater[J]. Ecotoxicology and Environmental Safety, 2009, 72(6):1790-1797.
[46] XU QS, JI WD, YANG HY,etal. Cadmium accumulation and phytotoxicity in an aquatic fern,Salvinianatans( Linn. )[J]. Acta Ecologica Sinica, 2009,29(6):3019-3027. (in Chinese).
[47] HE ZJ, XUE H. Determination of enrichment of heavy metal of Cd, Pb inPterisvittataat Mianyang[J]. Journal of Mianyang Normal University, 2011, 30(11):130-134. (in Chinese).
[48] LI FQ, MAO ZW. Study on the distribution of rare earth element inDicranopterisdichotoma[J]. Chinese Rare Earths, 1992, 13(5) : 16-19. (in Chinese).
[49] MIAO L, XU RS, MA YL,etal. Characteristics of distribution, accumulation and transportation of rare earth elements in soft-plant system of the Hetai goldfield[J]. Ecology and Environmnet, 2008, 17(1): 350-356. (in Chinese).
[50] TU C, MA LQ. Effects of arsenic concentrations and forms on arsenic uptake by the hyperaccumulator ladder brake[J]. Journal of Environmental Quality, 2002, 31(2): 641-647.
[51] NISHIZONO H, MINEMURA H, SUZUKI S. An inducible copper-thiolate in the fern, Athyrium yokoscense : involvement in copper tolerance of the fern[J]. Plant & Cell Physiology, 1988, 29(8): 1345- 1351.
[52] WEBB SM, GAILLARD JF, MA LQ,etal. XAS speciation of arsenic in a hyperaccumulating fern[J]. Environmental Science & Technology, 2003, 37 (4) : 754-760.
[53] FENG RW. Mechanisms for the accumulation of and the resistance to arsenic, selenium and antimony in plants[D]. Wuhan:Huazhong Agricultural University,2009. (in Chinese).
[54] ZHANG KM, DENG T, FANG YM,etal. Influence of co-contamination of As and Pb on the frond physiology and ultrastructure ofPterisvittataL.[J]. Fresenius Environmental Bulletin, 2011, 21(8a):2215-2223.
[55] SEULKI J, HEE SM, KYOUNGPHILE N. Enhanced uptake and translocation of arsenic in Cretan brake fern (PteriscreticaL.) through siderophorearsenic complex formation with an aid of rhizospheric bacterial activity[J]. Journal of Hazardous Materials, 2014, 280:536-543.
[56] ZHAO GC, LIAO XY, YAN XL,etal. Enhancement of As-accumulation byPterisvittataL. affected by microorganisms[J]. Chinese Journal of Environmental Science, 2010, 31(2):431-436. (in Chinese).
[57] LIU Y, ZHU YG, CHEN BD,etal. Influence of the arbuscular mycorrhizal fungusGlomusmosseaeon uptake of arsenate by the As hyperaccumulator femPterisvittataL.[J]. Mycorrhiza, 2005, 15(3):187-192.
[58] HU LL, LIU Q, YAN BQ,etal. Composition and structure of plant communities in Qianyanzhou, Jiangxi Province under ecological restoration[J]. Forest Research,2006, 19(6):807-812.(in Chinese).
[59] GAO L, LIU HM. Restoration of tropical rainforest after removing amomum villosum in Xishuangbanna[J]. Acta Phytoecologica Sinica, 2003, 27 (3) :366-372. (in Chinese).
[60] SUNDARAM S, WU S, MA LQ,etal. Expression of aPterisvittataglutaredoxin PvGRX5 in transgenicArabidopsisthalianaincreases plant arsenic tolerance and decreases arsenic accumulation in the leaves[J]. Plant, Cell and Environment, 2009, 32(7): 851-858.
[61] HE ZY, MA M, XU WZ,etal. The protein related to plant arsenic and encoding gene as well as its application[P]. China,ZL201010116397.2. ,2013. 5.1. (in Chinese).
[62] SMITA K, RAMA SD, RUDRA DT,etal. Omics and biotechnology of arsenic stress and detoxification in plants: current updates and prospective[J]. Environment International, 2015, 74 (6): 221-230.
[63] INDRIOLO E, NA GN, ELLIS D,etal. A Vacuolar arsenite transporter necessary for arsenic tolerance in the arsenic hyperaccumulating fernPterisvittatais missing in flowering plants[J]. The Plant Cell, 2010, 22(6): 2045-2057.
[64] ZHENG JM, TANG SR, CHEN ZY,etal. Uptake of inorganic contaminants by pteridophyte[J]. Acta Agriculturae Nucleatae Sinica, 2005, 19(2) :155- 159. (in Chinese).
[65] LANG YG, ZHAO HQ, LIU ZY,etal. Study on the sewage treatment of azolla[C]. Fuzhou: Fujian Provincial Academy of Agricultural Sciences, 1985. (in Chinese).
[66] QI ES, LIU QS, YANG GF,etal. A preliminary study on sewage purification effect of aquatic plant on irrigation heavy metals[J]. Chinese Journal of Ecology, 1984, 9(1): 14-18. (in Chinese).
[67] REN AZ, TANG TG. The clean-up effect ofAzollaimbricata(Roxb.)Nakai on lead, mercury and its biological effect[J]. Acta Scientiarum Naturalium University Nankaiensis, 1996, 29(1): 74-79. (in Chinese).
[68] FORNI C, CHEN J, TANCIONI L,etal. Evaluation of the fern azolla for growth, nitrogen and phosphorus removal from wastewater[J]. Water Research, 2001, 35 (6) : 1592-1598.
[69] YI HY, WU AP, WANG H. The nitrogen absorption effect ofAzollaimbricataunder different ratios of Nitrogen source[J].Journal of Mountain Agriculture and Biology, 2013,32(2) : 138-142. (in Chinese).
[70] XIONG JB, CHANG HQ, HE ZL,etal. Effects ofAzollafiliculoideslam.on nitrogen and phosphorus from surface water under low temperature[J]. Journal of Soil and Water Conservation, 2007,21(6):96-99. (in Chinese).
[71] HU N, DING DX, LI GY,etal. Uranium removal from water by five aquatic plants[J]. Acta Scientiae Circumstantiae,2012, 32(7):1637-1645. (in Chinese).
[72] NESTERENKO-MALKOVSKAYA A,KIRZHNER F, ZIMMELS Y,etal.Eichhorniacrassipescapability to remove naphthalene from wastewater in the absence of bacteria[J]. Chemosphere, 2012,87 (10): 1186-1191.
[73] LI RX. Several ferns asexual reproduction technology research[J]. Territory & Natural Resources Study, 2011(2):55-56. (in Chinese).
[74] HUANG BG, CHEN QS, LIN WX. Investigation of habitat in farmland and artificial propagation of aquatic fernCeratopteristhalictroides[J]. Chinese Journal of Eco-Agriculture, 2012,20(6):810-812. (in Chinese).
[75] MATHEWS S, MA L Q, BALA R,etal. Arsenic reduced scale-insect infestation on arsenic hyperaccumulatorPterisvittataL.[J]. Environmental and Experimental Botany, 2009, 65(2/3):282-286.
[76] SEULKI J, HEE SM, KYOUNGPHILE N. Increased ecological risk due to the hyperaccumulation of As inPteriscreticaduring the phytoremediation of an As-contaminated site[J]. Chemosphere, 2015, 122: 1-7.
 Asian Agricultural Research2016年11期
Asian Agricultural Research2016年11期
- Asian Agricultural Research的其它文章
- Feasibility Analysis of Agricultural Product Price Index Insurance Based on Pilot Cases
- Forecast on Price of Agricultural Futures in China Based on ARIMA Model
- Model Building for Community Participating in Rural Tourism and Game Analysis of Core Stakeholders
- Embedded Programmable Single Point Multiple Output Intelligent Data Acquisition and Transmission System
- A Study of the Factors that Affect Farmers’ Willingness to Transfer Land in the Central Regions Based on a Survey of 180 Farmers in Suizhou City
- Comparative Study of Cotton Plant Height Difference in the Arid Areas Based on LandSat8 OLI Data
