A Study on Phosphorus Use Efficiency of Wheat
, , , ,
Jinan Academy of Agricultural Sciences, Jinan 250316, China
1 Introduction
Phosphorus is one of 17 kinds of mineral elements necessary for plant growth, and it plays an important role in energy metabolism, sugar metabolism, enzymatic reaction and photosynthesis. Phosphorus is also a component of nucleic acid, plant hormone and lecithin, and it determines the yield and quality of crops to a large extent[1-2]. The usable phosphorus mainly exists in the form of phosphate, and phosphate can be readily combined with some metal ions in soil such as Ca2+, Fe3+and Mn2+to precipitate, resulting in scant available phosphorus in soil to meet the needs of the plant[3-4]. Even if the phosphorus fertilizer is applied, more than 80% of the phosphorus will be fixed in soil, and can not be used by crops[5-6]. The phosphorus, which is not absorbed and utilized by crops, will flow into rivers and lakes with the rain or irrigation water, causing eutrophication[7]. In addition, the phosphate ore, as the main source of phosphorus fertilizer, is a non-renewable resource. Experts predict that global phosphate reserves will be exhausted at the end of this century[8-12]. Wheat is China’s major grain crop, and the average annual consumption of phosphorus fertilizer for wheat is 103kg·ha, higher than that of rice (88 kg·ha) and maize (83 kg·ha)[13]. However, the utilization rate of phosphorus fertilizer is only 10% for wheat, lower than that of rice (14%) and maize (18%)[14]. Therefore, it is very necessary to improve the ability of wheat to absorb and utilize phosphorus, tap the genetic potential of wheat for efficiently using soil phosphorus, improve the genetic characteristics of phosphorus nutrition, and cultivate new varieties of high phosphorus efficiency, which is an effective way to solve the above problems, and of great significance to achieving sustainable development of agriculture[15-16].
2 Definition of phosphorus use efficiency
Phosphorus use efficiency is the biological yield or economic yield or fixed amount of CO2(g or mg) per unit of crop phosphorus concentration (%), namely plant phosphorus use efficiency=dry weight/phosphorus concentration. The plant phosphorus concentration is the ratio of phosphorus accumulation to dry weight, namely plant phosphorus concentration=phosphorus accumulation/dry weight. Therefore, the plant phosphorus use efficiency can be denoted by the amount of dry matter supported by per unit of phosphorus in crop, namely plant phosphorus use efficiency=dry weight of whole plant/phosphorus accumulation of whole plant[17-18]. High phosphorus use efficiency means that cells need low amount of biochemical phosphorus for various life activities, and phosphorus is transported rapidly in the plant, with high recycling efficiency, thus having particular importance to high efficiency phosphorus breeding[19]. How to improve phosphorus use efficiency while achieving increased crop yield, is a bottleneck on improving modern crop’s phosphorus use efficiency[20]. In addition, the phosphorus harvest index can be viewed as additional indicator to evaluate phosphorus use efficiency, in order to select the genotype with high yield and phosphorus use efficiency[21-22].
3 Mechanism of plant’s adaptation to low phosphorus stress
In the long process of evolution, plants have a series of strategies that adapt to low phosphorus environment, and they can be summarized as two simple mechanisms. One is the mechanism of improving root’s soil phosphorus absorption efficiency, and the other is the mechanism of rationally using phosphorus in plants and improving phosphorus use efficiency[23-24]. Root is the main organ of plants to absorb nutrients, and under low phosphorus stress, the phosphorus absorption and use efficiency can be improved to adapt to low phosphorus stress through a series of changes in morphology, such as changing metabolic pathways and secreting acidic substances[25-27]. Athikkattuvalasuetal.[28]use active tag T-DNA insertion method to obtain the mutantlpsiofArabidopsisthaliana, and this mutant can be used to detect the inhibiting effect on root growth and promoting effect on root hair differentiation under low phosphorus conditions. Through the study, Zhang Enheetal.[29]find that under phosphorus deficiency conditions, the activity of acid phosphatase in wheat Ganchun 20 increases by 41.6% and 101.9%, respectively, when compared with the treatments of moderate phosphorus supply and plentiful phosphorus supply, indicating that this genotype adapts to phosphorus-deficient environment through the release of phosphatase to rhizosphere. Yang Ruijietal. and Wu Zhaohuietal.[30-31]also do a similar study and find that under phosphorus stress, Ganchun 20 has high sensitivity to phosphorus, high acid phosphatase activity, as well as great root dry weight, shoot dry weight and strong yield potential, so it is a high phosphorus efficiency genotype. Phosphorus is also a component of some important substances involved in a variety of biochemical reactions inside the cell such as ATP, NADPH, RNA and DNA. Therefore, under low phosphorus conditions, many of the genes with a variety of metabolic pathways may respond to low phosphorus environment, including phosphorus transport and recycling, etc., in order to maintain the balance of intracellular phosphorus concentration[24].
4 Cloning of genes related to phosphorus use efficiency
4.1PlantphosphatetransportergeneInorganic phosphorus enters into the plants through the root membrane, which is regulated by a plurality of phosphorus transporter genes in the same or different family. People first cloned two phosphate transporter genes inArabidopsisthaliana, and also cloned a number of new encoded phosphate transporter genes in other higher plants such as rice, tomatoes, wheat, and potatoes[32-34]. So far, the plant phosphate transporter genes obtained by researchers generally belong to Pht1 and Pht2 families, and people have also cloned a few phosphate transporter genes belonging to Pht3, Pho1 and Pho2 families[35].
4.2EnzymegenesrelatedtophosphorususeefficiencyPlant has adaptive response to low phosphorus stress, and the phosphorus deficiency inducing the secretion of acid phosphatase in roots is a universal mechanism for higher plants to adapt to low phosphorus stress. Purple acid phosphatase, as a special kind of acid phosphatase, is involved in the plant’s use of soil organic phosphorus and reallocation of phosphorus in plants. The gene has been cloned in transgenicMedicagotruncatula,Arabidopsisthaliana,Aegilopssearsii, wild soybean and other plants[36-37]. Calcium-dependent protein kinase (CDPK) exists in the form of multi-gene family, and it is an important signal transmitter mediating abiotic stress signal transduction. In recent years, the CDPK genes have been cloned inArabidopsisthaliana, rice, wheat and other plants[38].
4.3TranscriptionfactorgenerelatedtophosphorususeefficiencyThe research of transcriptional regulatory mechanism under phosphorus starvation response is most detailed in yeast studies. The two transcription factors of yeast, namely PH02 and PH04, play an important regulatory function in the PHO system, and they belong to homeo and bHLH families, respectively. Currently, PTF1 gene in bHLH family has been cloned in rice, corn and other plants[39-40]. Some WRKY transcription factors in plant species play an important role in responding to and resisting low phosphorus abiotic stress, and some genes in this transcription factor family have been cloned in rice,Arabidopsisthalianaand wheat[41-42]. In addition, WER, a transcription factor controlling root hair initiation, has been cloned inArabidopsisthaliana, rape, cabbage and other plants[43].
5 Factors influencing phosphorus use efficiency of wheat
Phosphorus use efficiency is closely related to soil moisture conditions. When the soil is dry, the absolute amount of phosphorus dissolved in soil solution will be low, far from meeting the needs of crops, thus leading to phosphorus deficiency symptoms[44]. Therefore, people have studied the relationship between the phosphorus use efficiency of wheat and water use efficiency. Peng Changlianetal.[45]find that under water stress, compared with the wheat varieties with low phosphorus efficiency, the varieties with high phosphorus efficiency have high photosynthetic rate in leaves, and high water use efficiency, showing strong drought resistance. The study of Liu Guodongetal.[44]indicates that under field conditions, some genotypes with high water use efficiency such as Ji87-4617, Luofulin 10, have strong ability to endure low phosphorus. Wang Yuetal.[19]find that in the case of water shortage, high yield and water use efficiency can be obtained by applying 180 kg·ha of phosphorus and using 60 mm of water to irrigate in the Hluanghuai wheat growth area. The difference in some soil nutrients such as nitrogen, phosphorus and potassium, or different sources of phosphorus, will also affect the phosphorus use efficiency of wheat. The study of Zan Yaling[46]shows that with the increasing application of nitrogen fertilizer to wheat, plants’ phosphorus and potassium uptake, phosphorus partial productivity and phosphorus use efficiency will increase; likewise, wheat yield and plants’ nitrogen, phosphorus and potassium uptake will also significantly increase with the increasing application of phosphorus. Wu Yipoetal.[47]find that compared with the wheat varieties with low phosphorus efficiency, the varieties with high phosphorus efficiency have stronger ability to absorb and use organic phosphorus, and under the same treatment, both the dry matter amount and phosphorus accumulation for the wheat varieties with high phosphorus efficiency are higher than for the varieties with low phosphorus efficiency; a certain concentration of inorganic phosphorus can promote assimilation substance to be allocated to the roots, improve root growth, and induce the secretion of acid phosphatase in roots, while organic phosphorus has no such effect. There are significant differences in phosphorus use efficiency between different wheat genotypes. Li Yanetal.[48]divide 133 parent materials into three categories according to different nitrogen and phosphorus use efficiency, and select seven parents with high nitrogen and phosphorus use efficiency. Song Qingjieetal.[49]use different concentration of phosphorus to treat four cultivars of wheat in Heilongjiang Province, and find that they have different response to phosphorus. Wang Shuliangetal.[21]there are highly significant differences in phosphorus use efficiency and harvest index between different wheat varieties, and the average phosphorus use efficiency and phosphorus harvest index for all varieties under high fertility are 10.22% and 2.61% lower than under low fertility, respectively. Yang Xianbinetal.[50]find that there are great differences in phosphorus use efficiency between wheat varieties at tillering, jointing, flowering and maturity stages. He Wenshou[51]finds that regardless of phosphorus application, there are obvious differences in phosphate yield rate and phosphorus use efficiency between wheat varieties, especially more apparent under low phosphorus conditions. These studies lay foundation for selecting high phosphorus efficiency wheat germplasm, carrying out basic research of high phosphorus efficiency wheat, and breeding high phosphorus efficiency wheat varieties.
6 Evaluation and selection of high phosphorus efficiency wheat genotype
Currently, hydroponics and potted soil culture are generally used to primarily choose high phosphorus efficiency wheat genotypes at seedling stage, and then re-select these genotypes in the field at adult plant stage[24]. Based on nutrient solution formula of Hoaglandetal., hydroponics uses 0.02mmol L-1H2PO4-as the treatment standard for selecting high phosphorus efficiency wheat at seedling stage, but under other selection conditions, there is no additional phosphorus fertilizer to be added on the basis of original low phosphorus medium, and there are no specific low phosphorus treatment standards[52-53]. The screening indicators for high phosphorus efficiency are some traits significantly correlated with phosphorus efficiency, and the measuring method must be simple for these traits. Under low phosphorus, the phosphatase activity of wheat at the third-leaf stage is significantly correlated with phosphorus use efficiency (R=0.944,P<0.1), and it can be used as an indicator to select high phosphorus efficiency wheat genotype at seedling stage[54]. At adult stage of wheat, the higher the grain yield per unit of phosphorus or the lower the grain phosphorus content, the higher the phosphorus use efficiency of wheat, so it can be used as an indicator to select high phosphorus efficiency wheat genotype[55]. Under the same fertility conditions, spike number and grain number also can be used as indicators to select high phosphorus efficiency genotype[49]. In addition, Liu Guodongetal. use Ca3(PO4)2as the sole source of phosphorus, plus 0.75 mmol/L Ca2+, to establish the phosphorus source liquid controlled release system as the phosphorus nutrient buffer solution to screen different wheat genotypes within 40 d[56]. During the selection of high phosphorus efficiency wheat genotype, it should be based on one or more traits supplemented by many reference traits for comprehensive evaluation.
7 QTL analysis of phosphorus use efficiency of wheat
The development of molecular marker technology and the establishment of high-density genetic linkage map, make it possible to test QTL of phosphorus use efficiency of wheat in genome. Using different groups, researchers have successively located some QTLs closely linked with the phosphorus use efficiency and related traits (Table 1). Under normal supply of phosphorus and phosphorus stress, Cao Weidongetal.[57]use RIL population to detect five QTLs related to phosphorus use efficiency of shoot and whole plant, respectively, and they are distributed in 5 and 4 chromosomes. In addition, under two phosphorus supply conditions, the fragmentXfba354-Xfba69 in 7A chromosome can significantly affect phosphorus use efficiency of whole plant, and under normal supply of phosphorus, it interacts with other genes to affect phosphorus use efficiency of whole plant, and plays an important role in improving the phosphorus use efficiency of wheat. Under normal phosphorus, low phosphorus and phosphorus starvation conditions, Li Zhenxingetal.[58]also use RIL population to detect 30 (LOD>2.0) QTLs related to wheat root traits and phosphorus stress response, distributed in 14 chromosomes, and there are 5 QTLs with single locus contributing 10% to phenotype. Suetal.[59]use DH population to detect 20 QTLs under low phosphorus treatment and 19 QTLs under high phosphorus treatment, and these 39 QTLs are distributed in 21 chromosomes; there are 3 QTL clusters playing an important role in controlling low phosphorus tolerance, and two loci are closely linked with essential catalysis genesVRN-A1 (5A) andVRN-D1 (5D). Suetal.[60]use DH population to detect 7 QTLs controlling phosphorus uptake efficiency and 6 QTLs controlling phosphorus use efficiency, and detect 2 QTLs controlling both phosphorus use efficiency and phosphorus uptake efficiency under low phosphorus conditions, indicating that it is possible to improve phosphorus use efficiency and phosphorus uptake efficiency at the same time. They also detect many QTLs which control phosphorus uptake efficiency and tiller number at seedling stage, as well as spike number, biological yield and phosphorus uptake efficiency at adult plant stage. Pelegetal.[61]use RIL population to detect 82 QTLs related to 10 kinds of mineral elements, and 8 of these QTLs are related to grain phosphorus content and distributed in 7 chromosomes. Guoetal.[17]use RIL population to detect 171 QTLs linked with 12 wheat traits related to phosphorus use efficiency at seedling stage, 22 of which are linked with root phosphorus use efficiency, 9 of which are linked with shoot phosphorus use efficiency, and 13 of which are linked with whole plant phosphorus use efficiency.
Table1QTLanalysisofphosphorususeefficiencyandrelatedtraitsofwheat
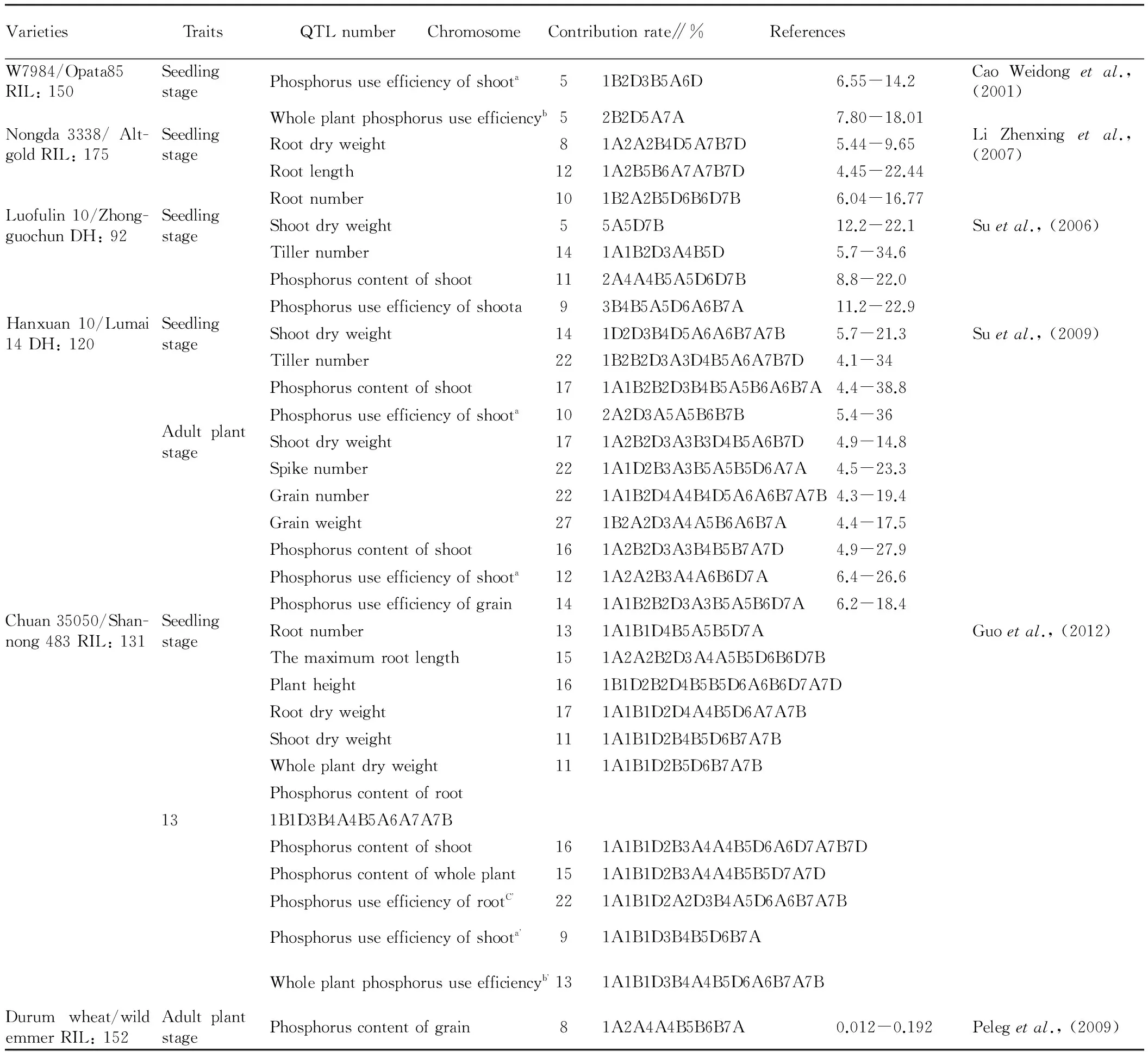
VarietiesTraitsQTLnumberChromosomeContributionrate∥%ReferencesW7984/Opata85RIL:150SeedlingstagePhosphorususeefficiencyofshoota51B2D3B5A6D6.55-14.2CaoWeidongetal.,(2001)Wholeplantphosphorususeefficiencyb52B2D5A7A7.80-18.01Nongda3338/Alt-goldRIL:175SeedlingstageRootdryweight81A2A2B4D5A7B7D5.44-9.65LiZhenxingetal.,(2007)Rootlength121A2B5B6A7A7B7D4.45-22.44Rootnumber101B2A2B5D6B6D7B6.04-16.77Luofulin10/Zhong-guochunDH:92SeedlingstageShootdryweight55A5D7B12.2-22.1Suetal.,(2006)Tillernumber141A1B2D3A4B5D5.7-34.6Phosphoruscontentofshoot112A4A4B5A5D6D7B8.8-22.0Phosphorususeefficiencyofshoota93B4B5A5D6A6B7A11.2-22.9Hanxuan10/Lumai14DH:120SeedlingstageShootdryweight141D2D3B4D5A6A6B7A7B5.7-21.3Suetal.,(2009)Tillernumber221B2B2D3A3D4B5A6A7B7D4.1-34Phosphoruscontentofshoot171A1B2B2D3B4B5A5B6A6B7A4.4-38.8Phosphorususeefficiencyofshoota102A2D3A5A5B6B7B5.4-36AdultplantstageShootdryweight171A2B2D3A3B3D4B5A6B7D4.9-14.8Spikenumber221A1D2B3A3B5A5B5D6A7A4.5-23.3Grainnumber221A1B2D4A4B4D5A6A6B7A7B4.3-19.4Grainweight271B2A2D3A4A5B6A6B7A4.4-17.5Phosphoruscontentofshoot161A2B2D3A3B4B5B7A7D4.9-27.9Phosphorususeefficiencyofshoota121A2A2B3A4A6B6D7A6.4-26.6Phosphorususeefficiencyofgrain141A1B2B2D3A3B5A5B6D7A6.2-18.4Chuan35050/Shan-nong483RIL:131SeedlingstageRootnumber131A1B1D4B5A5B5D7AGuoetal.,(2012)Themaximumrootlength151A2A2B2D3A4A5B5D6B6D7BPlantheight161B1D2B2D4B5B5D6A6B6D7A7DRootdryweight171A1B1D2D4A4B5D6A7A7BShootdryweight111A1B1D2B4B5D6B7A7BWholeplantdryweight111A1B1D2B5D6B7A7BPhosphoruscontentofroot131B1D3B4A4B5A6A7A7BPhosphoruscontentofshoot161A1B1D2B3A4A4B5D6A6D7A7B7DPhosphoruscontentofwholeplant151A1B1D2B3A4A4B5B5D7A7DPhosphorususeefficiencyofrootC221A1B1D2A2D3B4A5D6A6B7A7BPhosphorususeefficiencyofshoota91A1B1D3B4B5D6B7AWholeplantphosphorususeefficiencyb131A1B1D3B4A4B5D6A6B7A7BDurumwheat/wildemmerRIL:152AdultplantstagePhosphoruscontentofgrain81A2A4A4B5B6B7A0.012-0.192Pelegetal.,(2009)
Note: Phosphorus use efficiency of shoota=shoot dry weight/phosphorus content of shoot; phosphorus use efficiency of shoota’=shoot dry weight2/phosphorus content of shoot; whole plant phosphorus use efficiencyb=whole plant dry weight/phosphorus content of whole plant; whole plant phosphorus use efficiencyb’=whole plant dry weight2/phosphorus content of whole plant; phosphorus use efficiency of rootC’=root dry weight2/phosphorus content of root.
[1] YUN SJ, KAEPPLER SM. Induction of maize acid phosphatase activities under phosphorus starvation[J]. Plant and Soil, 2001, 237(1): 109-115.
[2] QIU H, MEI X, LIU C,etal. Fine mapping of quantitative trait loci for acid phosphatase activity in maize leaf under low phosphorus stress[J]. Molecular Breeding, 2013, 32(3): 629-639.
[3] SMIT AL, BINDRABAN PS, SCHRODER JJ,etal. Phosphorus in agriculture: global resources, trends and developments. Report to the Steering Committee Technology Assessment of the MiII. Phosphate fertilizer consumption and predicted demand[J]. Soils, 2004, 36(2): 113-116.
[4] SU JY, ZHENG Q, LI HW,etal. Detection of QTLs for phosphorus use efficiency in relation to agronomic performance of wheat grown under phosphorus sufficient and limited conditions[J]. Plant Science1, 2009, 76(6): 824-836.
[5] HOLFORD ICR. Soil phosphorus: its measurement, and its uptake by plants[J]. Australian Journal of Soil Research, 1997, 35(2): 227-240.
[6] SCHRODER JJ, SMIT AL, CORDELL D,etal. Improved phosphorus use efficiency in agriculture: a key requirement for its sustainable use[J]. Chemosphere, 2011, 84:822-832.
[7] YANG M, DING G, SHI L,etal. Detection of QTL for phosphorus efficiency at vegetative stage in Brassica napus[J]. Plant Soil, 2011, 339:97-111.
[8] YANG H, KNAPP J, KOIRALAI P,etal. Enhanced phosphorus nutrition in monocots and dicots over-expressing a phosphorus responsive type I H +-pyrophosphatase[J]. Plant Biotechnology Journal, 2007, 5:735-745.
[9] CORDELL D, DRANGERT JO, WHITE S. The story of phosphorus: global food security and food for thought[J]. Global Environmental Change , 2009, 19: 292-305.
[10] DAWSON CJ, HILTON J. Fertiliser availability in a resource-limited world: production and recycling of nitrogen and phosphorus[J]. Food Pol, 2011, 36: 514-522.
[11] JASINSKI SM. Phosphate rock[J]. Geological Survey Minerals Yearbook-2010, 2011, 56.1-56.10.
[12] MANSCHADI AM, KAUL HP, VOLLMANN J,etal. Developing phosphorus-efficient crop varieties-An interdisciplinary research framework[J]. Field Crops Research, 2014, 162: 87-98.
[13] MA WQ, ZHANG FS, ZHANG WF. Fertilizer production and consumption and the resources, environment, food security and sustainable development in China[J]. Resources Science, 2005, 27: 33-40.
[14] LU RK. Phosphorus resources and phosphate fertilizer production and consumption of China. sica napus[J]. Plant and Soil, 2011, 339(1-2): 97-111.
[15] LYNCH JP. Roots of the second green revolution[J]. Australian Journal of Botany, 2007, 55:493-512.
[16] MANSCHADI AM, MANSKE GGB, VLEK PLG. Root architecture and resource acquisition wheat as a model plant[J]. Plant Roots, 2013, 22.1-22.18.
[17] GUO Y, KONG F, XU Y,etal. QTL mapping for seedling traits in wheat grown under varying concentrations of N, P and K nutrients[J]. Theoretical and Applied Genetics, 2012, 124(5): 851-865.
[18] KONG FM, GUO Y, LIANG X,etal. Potassium (K) effects and QTL mapping for K efficiency traits at seedling and adult stages in wheat[J]. Plant and soil , 2013, 373(1-2): 877-892.
[19] WANG Y, NING TY, CHI SJ,etal. Effects of irrigation amount on water use characteristics and grain yield of wheat under different phosphorus application rates[J]. Journal of Soil and Water Conservation, 2012, 26(3): 232-237. (in Chinese).
[20] WANG XR, SHEN JB, LIAO H. Acquisition or utilization, which is more critical for enhancing phosphorus efficiency in modern crops[J]. Plant Science, 2010, 179:302-306.
[21] WANG SL, TIAN QZ, LI NN,etal. Differences of phosphorus utilization efficiency among different wheat varieties[J]. Journal of Triticeae Crops, 2008, 28(3): 476-483.(in Chinese).
[22] DING JF, YANG JF, ZI Y,etal. Accumulation, distribution and utilization characteristics of phosphorus in super-high yield wheat population under rice stubble in middle and lower reaches of the Yangtze River[J]. Journal of Triticeae Crops, 2013, 33(1): 129-136.(in Chinese).
[23] Vance C P, Uhde S C, Allan D L. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource[J]. New Phytologist, 2003, 157(3): 423-447.
[24] WANG RC, CHENG SH, CAO LY. Advancements in phosphorus deficiency tolerance study in rice(Oryza sativa L.)[J].Chinese Agricultural Science Bulletin, 2009, 25(06):77-83. (in Chinese).
[25]Benjamin P, Mathilde C, Laurent N,etal. Root developmental adaptation to phosphate starvation: better safe than sorry[J]. Cell, 2011, 16(8): 442-450.
[26] Jeannette S B J, Marín G V, Verónica A P D,etal. Genotypic variation for root traits of maize (Zea mays L.) from the Purhepecha Plateau under contrasting phosphorus availability[J]. Field Crops Research, 2011, 121: 350-362.
[27]Zhang Y K, Chen F J, Chen X C,etal. Genetic improvement of root growth contributes to efficient phosphorus acquisition in maize (Zea mays L.)[J]. Journal of Integrative Agriculture, 2013, 12(6): 1098-1111.
[28] Athikkattuvalasu S K, Ajay J, Vinay K N,etal. Arabidopsis thaliana mutant lpsi reveals impairment in the root responses to local phosphate availability[J]. Plant Physiology and Biochemistry, 2014, 77: 60-72.
[29] ZHANG EH, WANG HZ, YAN QJ. Adaptable effects of phosphorus stress on different genotypes of spring wheat[J]. Journal of Triticeae Crops, 2006, 26(2): 117-120.(in Chinese).
[30] YANG RJ, ZHANG XH, WANG HL,etal. Adaptabilities of different genotypes of spring wheat to phosphorous deficiency[J]. Acta Botanica Boreali-Occidentalia Sinica, 2006, 25(11): 2314-2318.(in Chinese).
[31] WU ZH, HE LY, ZHANG LM,etal. Research progress in screening germplasm resources of crops with high phosphorus efficiency[J]. Journal of Mountain Agriculture & Biology, 2008, 27(1): 61-68.(in Chinese).
[32] ZHOU JQ, TAN XF, YUAN J,etal. Isolation and expression analysis of CoPhtl; 1 from oil tea[J].Journal of Plant Genetic Resources, 2013, 14 ( 3 ): 512-517.(in Chinese).
[33] SU SZ, WU FK, LIU D,etal. Cloning and characterization of a phosphate transporter gene of Pht1 family in maize[J]. Acta Agriculturae Nucleatae Sinica, 2013, 27( 7) : 0885-0894.(in Chinese).
[34] WU ZY. The screen of Aegilopstauschii and Triticumaestivum and its clone and sequence analysis of Pht1 gene[D]. Kaifeng: He’nan University, 2012.(in Chinese).
[35] WANG CR. Functional analysis of Na~+/Pi transporter gene from T.halophila in transgenic tobacco[D]. Ji’nan: Shandong University, 2008.(in Chinese).
[36] HOU LJ, NIU N, MA SC,etal. Cloning and sequence analysis of purple acid phosphotase PAP1 gene from Aegilops tauschii[J]. Acta Agriculturae Boreali-occidentalis Sinica, 2014, 23(10):105-111. (in Chinese).
[37] WANG JW, HOU LJ, LI D,etal. Cloning and sequence analysis of purple acid phosphotase PAPI gene in wild soybean[J].Soybean Science, 2013, 32(5):596-600.(in Chinese).
[38] LONG SX. The molecular characterization, expression patterns of wheat CDPKs and genetic transformation of TaCDPK1[D]. Baoding: Agricultural University of Hebei Province, 2009.(in Chinese).
[39] WU ZC. OsPTF1: A novel phosphorus-starvation induced transcription factor involved in tolerance for phosphorus starvation in rice (Oryza sativa L.)[D]. Hangzhou:Zhejiang University, 2004.(in Chinese).
[40] LI ZX, GAO Q, LIU YZ,etal. Cloning of ZmPTF1 from zea mays and its overexpression analysis[J].Journal of Hunan Agricultural University, 2007, 33(S1):92-96.(in Chinese).
[41] MIAO HY, ZHAO JF, LI XJ,etal. Cloning and expression of wheat transcription factor gene TaWRKY72b-1 and its effect on phosphorus use efficiency in transgenic tobacco plants[J]. Acta Agronomica Sinica, 2009, 35(11): 2029-2036.(in Chinese).
[42]DING CH ,SUN SH, LI XJ,etal. Cloning and expression profiles of TaWRKY46,a WRKY type transcription factor gene in wheat(Triticum aestivum L.)[J]. Acta Agriculturae Boreali-Sinica, 2012, 27 (5): 65-71. (in Chinese).
[43] NING HC. Expression of WER, a transcription factor controlling root hair initiation in arabidopsis and brassica napus and their association with phosphate uptake[D]. Wuhan: Huazhong Agricultural University, 2010.(in Chinese).
[44] LIU GD, LI JY, LI ZS. On the differences of genotype of overground part of wheat under low phosphorus stress[J]. Acta Pedologica Sinica, 1998, 35(2): 235-242.(in Chinese).
[45] PENG CL, LIN ZF, LIN GZ. Photosynthesis and water use efficiency in wheat varieties differing in phosphate use efficiency[J]. Acta Agronomica Sinica, 2000, 26(5): 543-548.(in Chinese).
[46] ZAN YL. Effect of nitrogen and phosphorus fertilizer rate on yield, nutrient utilization and grain mineral nutrient quality of wheat in dryland[D]. Yangling: North West Agriculture and Forestry University, 2012.(in Chinese).
[47] WU XB, ZHANG XZ, LI TX,etal. Difference in P utilization from organic phosphate between two wheat varieties and its relations with acid phosphatase activity[J]. Acta Agriculturae Nucleatae Sinica, 2013, 27(3): 351-357.(in Chinese).
[48] LI Y, DONG ZD, CUI DQ,etal. Cluster analysis of nitrogen and phosphorus utilization efficiency in 133 wheat parent materials[J]. Chinese Agricultural Science Bulletin, 2005, 21(1):76-78.(in Chinese).
[49]SONG QJ, GUO YL, ZHANG CL,etal. Studies on phosphorus use efficiency of different genotypes of wheat varieties[J]. Bulletin of Agricultural Science and Technology, 2010(12): 52-56.(in Chinese).
[50] YANG XB, ZHANG XZ, LI TX,etal. Differences in phosphorus utilization efficiency among wheat cultivars[J]. Chinese Journal of Applied Ecology, 2012, 23(1): 60-66.(in Chinese).
[51] HE WS. Genotypic differences in phosphorus utilization efficiency among spring wheat varieties in Ningxia[J]. Agricultural Research in the Arid Areas, 2003, 21(2): 1-6.(in Chinese).
[52] LI DH, XIANG CL, JIANG YQ,etal. Physiological characteristic of roots of different rice variety under the stress of low phosphorus[J]. Journal of Huazhong Agricultural University, 2006, 25(6): 626-629.(in Chinese).
[53] SHIMIZU A, YANAGIHARA S, KAWASAKI S,etal. Phosphorus deficiency-induced root elongation and its QTL in rice (Oryza sativa L.)[J]. Theor Appl Genet, 2004, 109:1361-1368.
[54] QIU HJ, HE MR, CHANG X,etal. The differential leaf acid phosphatase(APase) activities in different genotypes of winter wheat[J]. Acta Agronomica Sinica, 2004, 30(8): 739-744.(in Chinese).
[55] QIU HJ, XU XM, LENG SC,etal. Difference of phosphorus metabolization in winter wheats[J]. Journal of Shandong Agricultural University, 2004, 35(2): 169-172.(in Chinese).
[56] LIU GD, LI ZSW, JI JY. Principles and methods to establish controlled release system of P source in liquid phase for screening wheat genotypes for efficient utilization of phosphate[J]. Scientia Agricultura Sinica, 1997, 30(2): 70-76.(in Chinese).
[57] CAO WD, JIA JZ, JIN JY. Identification and interaction analysis of QTL for phosphorus use efficiency in wheat[J]. Plant Nutrition and Fertilizer Science, 2001, 7(3): 285-292.(in Chinese).
[58] LI ZX, NI ZF, PENG HR,etal. The QTL location of wheat root characteristics to phosphorus stress[J]. Progress in Natural Science, 2007, 17(10): 1352-1360.(in Chinese).
[59] SU J, XIAO Y, LI M,etal. Mapping QTLs for phosphorus-deficiency tolerance at wheat seedling stage[J]. Plant and Soil, 2006, 281(1-2): 25-36.
[60] SU JY, ZHENG Q, LI HW,etal. Detection of QTLs for phosphorus use efficiency in relation to agronomic performance of wheat grown under phosphorus sufficient and limited conditions[J]. Plant Science, 2009, 176(6): 824-836.
[61] PELEG Z, CAKMAK I, OZTURK L,etal. Quantitative trait loci conferring grain mineral nutrient concentrations in durum wheat 3 wild emmer wheat RIL population[J]. Theoretical and Applied Genetics, 2009, 119:353-369.
 Asian Agricultural Research2016年5期
Asian Agricultural Research2016年5期
- Asian Agricultural Research的其它文章
- Is It Worthwhile for Farmers to Grow Grain?—A Study of Farmers’ Behavior of Growing Grain
- Establishment of Chinese Agricultural Brand Value Scale and Study of Its Reliability and Validity Based on Customer Value
- How to Develop Chinese Rural Tourism in the Context of New Urbanization?
- Chinese Customers’ WTP for Legal Digital Music Downloading
- High Standard Capital Farmland Construction Based on Grain Security
- Research on the Brand Construction of Agritourism Enterprise in Chongqing
