Morphogenesis of Chinese Water Chestnut Pistil and Pollen Tube during Germination
, , , , , , CN, ,
Biotechnology Research Institute, Guangxi Academy of Agricultural Sciences, Nanning 530007, China
1 Introduction
The Chinese water chestnut (Eleocharisdulcis) is a grass-like sedge native to southern China and India, and it is widely grown in many countries for its edible corms, mostly in the tropics and subtropics. It is also grown in Korea, Japan, Vietnam, and the United States[1]. In China, it is mainly distributed in the Yangtze River basin and the southern provinces[2], and Guangxi has the largest sown area, reaching nearly 20000 ha, accounting for half of the national sown area of water chestnut. With the industrial development of Chinese water chestnut, the market places a greater demand on the varieties of Chinese water chestnut, but the current breeding of Chinese water chestnut is still based on asexual reproduction, which is difficult to adapt to market changes. In recent years, sedge seed plants have started to receive people’s constant attention, and researchers conduct many studies on Chinese water chestnut’s flower season[3], traits of blossoming and having seeds[4]and seed germination[5], and explore the possibilities in crossbreeding of Chinese water chestnut. Based on the above research, we carry out observation and artificial pollination experiments on Chinese water chestnut stigma, and study the pollination process in order to reveal the Chinese water chestnut pollination characteristics and pollen tube forms, as well as the crossbreeding phenomenon of Chinese water chestnut.
2 Materials and methods
2.1MaterialsThe experimental material is "Guiti 2" Chinese water chestnut variety planted in germplasm nursery of Biotechnology Research Institute, Guangxi Academy of Agricultural Sciences[6], and the flower spikes were selected from robust and disease-free flowers, stems and leaves.
2.2MethodsThe full-bloom stage of Chinese water chestnut is August-September[4], and the flower spikes of pistil at full-bloom stage was selected. The imbricate scales of flower spikes were peeled off with tweezers, and the whole pistil within scales was extracted for measuring and observation. In December when Chinese water chestnut seeds were mature, the flower spikes were picked from robust flowers, stems and leaves, and the complete pistil was taken after the removal of scales, for observation of structure. During August-September, 9: 00-11: 00 is the best time for cross-pollination of Chinese water chestnut. The flowers, stems and leaves were selected from stamen and pistil in full blossom respectively as the male parent and female parent for hybridized artificial pollination, bagging was conducted immediately after pollination, and flower spikes were taken after about 2 h for sample preparation and observation[7]. Scissors were used to cut the filaments of flower spikes, and the filaments were observed and photographed with optical microscope after carefully placing them on the glass slide wetted with distilled water. After fixation with fixative, the flower spikes went through dehydration, displacement, critical point drying, and spraying for observation. The homemade container was used to wrap around the Chinese water chestnut flower spikes which were placed in fixative to be fixed for electron microscope observation. The ethanol with dehydrated ethanol concentration of 50%, 70%, 80% and 100% was used for dehydration[8], and the electron microscope was used to observe.
3 Results and analysis
3.1PistilobservationwithopticalmicroscopeThe pistil stigmas of Chinese water chestnut flower spikes showed white filament forms, and most of them had three stigmas (Fig. 1a) while few of them had two and four stigmas (Fig. 1b). The split point of stigma was not entirely in the same position, and some stigmas may continue to be split into two sub-stigmas in the growth process (Fig .1c). Some of the flower spike scales protruding from each stigma were 5-12 mm long, and each stigma was magnified 40 times by the optical microscope to see that it had vascular bundle form and was crystal clear. Countless branched hairs extended outwardly from the vascular bundle surface (Fig. 1d), and the same vessel could extend a plurality of branched hairs by keeping at a certain distance away. Since stigma grew quickly, there was a time difference in the extension of branched hairs on the surface, and the length of the extension was not the same. The mature branched hairs were about 80 μm long, they would develop later and be shorter if they got closer to the tip of the stigma, and the shortest length was about 10 μm. It was also observed that the flower spike surface gradually turned yellowish after the seeds were ripe, the scales were always tightly packed, and all pistils and stamens protruding from scales completely withered. After ripping scales, it was found that about 1 mm long stigma was still wrapped inside scales, and was preserved intact with the entire pistil style. The pistil style was about 3 mm long, and flat cylindrical. It quickly inflated at about one fourth of style near the ovary, and was attached to the ovary. The hardness gradually increased, and obvious node was clearly seen at the junction of pistil style and ovary. After the seeds were ripe, the style was brownish-black and naturally curved after peeling the scales, and it was found that a black tube was in the middle of style after being magnified by 40 times with optical microscope. The pistil ovary showed an inverted heart and oval shape, and it was light green and translucent at initial growth stage. After its development, the seed pericarp was formed, and it was brown, about 3 mm long, 2.2 mm wide and 1.3 mm thick. After peeling the thick seed pericarp, the Chinese water chestnut seeds could be seen. Some seeds were very full, closely stuck to the inner wall of rind, and the seed pericarp was shiny; some seeds were not full, and there were many wrinkles in seed pericarp. Eight jagged bristles grew in lower ovary, and grew upward symmetrically against the outer wall of the ovary. They wrapped seeds, and each bristle was about 4 mm long (Fig. 1c).

Fig.1 Pistil observation with optical microscope
3.2ObservationwithopticalmicroscopeafterpistilpollinationAfter artificial pollination of pistil stigma, pollen grains were densely distributed around pistil stigma (Fig. 2a), and by magnifying the pollen grains 40 times with optical microscope, it was observed that pollen was oval and attached to the surface of stigma, and was between branched hairs. 2 h after pollination, it was observed that pollen and pollen tube showed different states. Some of the pollen grains were closely integrated with branched hairs through the recognition, and pollen tube was at the germination stage; some of pollen tubes entered into branched hairs, the materials inside pollen cell were concentrated at one end of germinal aperture, and part of materials entered into pollen tube; some of pollen tubes entered stigma vessel, and the materials inside pollen were transported outward; after the transfer of some pollen materials was completed, it left an empty shell in branched hairs (Fig. 2b, c). It was also observed that during the growth process of pollen tube, the matter inside pollen continued to concentrate at one end of germinal aperture, moved in the direction of pollen tube growth through germinal aperture, and continued to advance with the pollen tube growth. Nevertheless, in the course of transmission, the inner substance of pollen did not make one-way movement in pollen tube, and there was substance reflux phenomenon; the outflow rate was significantly higher than the reflux rate, but all the materials would eventually flow into the stigma vessel. It took about 5-10 min for pollen substance to enter into all vessels. It was observed that pollen tube was combined with branched hairs and penetrated into branched hairs; there was no pollen tube penetrating into branched hairs directly through vascular bundle; the penetration position was not fixed, and it could be anywhere. It was also observed that there were very few pollen grains combined with branched hairs to generate pollen tube, but pollen tube found no suitable entry point, so it was close to the surface of branched hairs to grow continuously toward base (Fig. 2d) until it could find a penetration point.
3.3ObservationwithelectronmicroscopeThrough scanning electron microscope (2500-3000 times), the stigma, pollen and pollination forms of Chinese water chestnut could be clearly observed, and similar to the observation results under optical microscope, the Chinese water chestnut stigma showed a vascular bundle shape, and vessel extended with branched hairs outwardly; pollen grains were between branched hairs, and tightly attached to branched hairs; the pollen tube penetrated into branched hairs at the top (Fig. 3a) or in the middle (Fig. 3b) of branched hairs. Under the scanning electron microscope, it was not observed that pollen tube directly entered into vessel for substance transfer. It was clearly observed that the pollen of Chinese water chestnut had a granular surface and a two-layer structure, with thin membrane in inner wall and granular matter structure in outer wall. The germinal aperture surface of pollen tube was lacerate after pollen tube germination, indicating that the pollen tube was formed by the inner wall of pollen grain, and the thin membrane in inner wall broke through outer wall of pollen via germinal aperture to outwardly project with thin tube to form a tubular structure, and entered directly into branched hairs after recognition. As shown in Fig. 3, the germination of pollen tube was located at both ends of oval pollen tube, so it could be inferred that the germinal aperture of Chinese water chestnut pollen was near the both ends. The scanning electron microscopy scale was used to measure the size of pollen grain and pollen tube. The polar axis length was about 32 μm; the equatorial axis length was about 28 μm; the pollen tube diameter was about 4 μm.
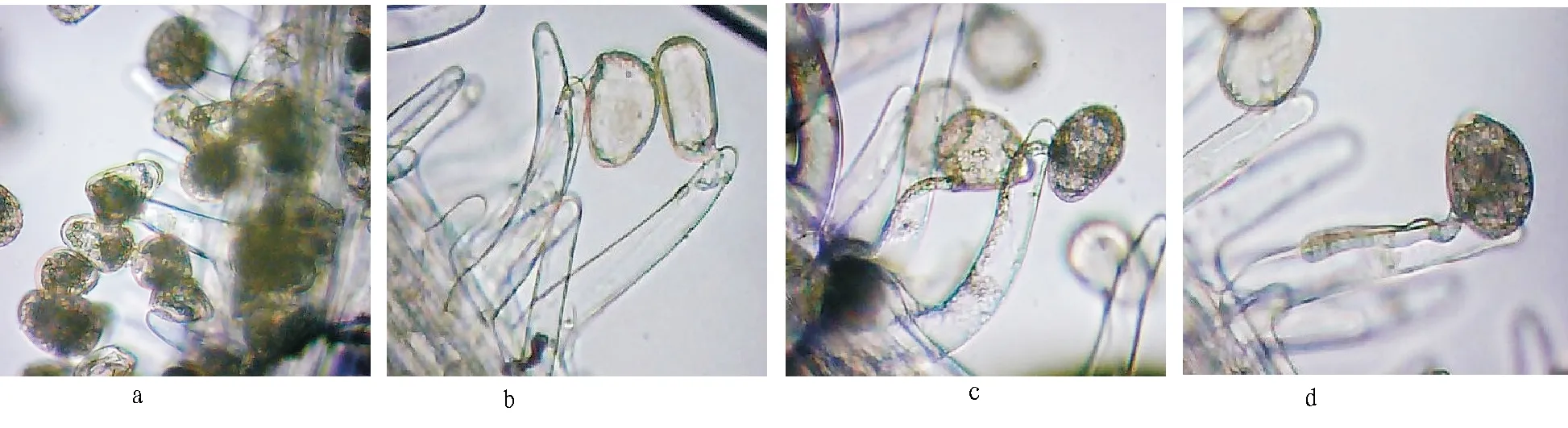
Fig.2 Observation with optical microscope after pistil pollination
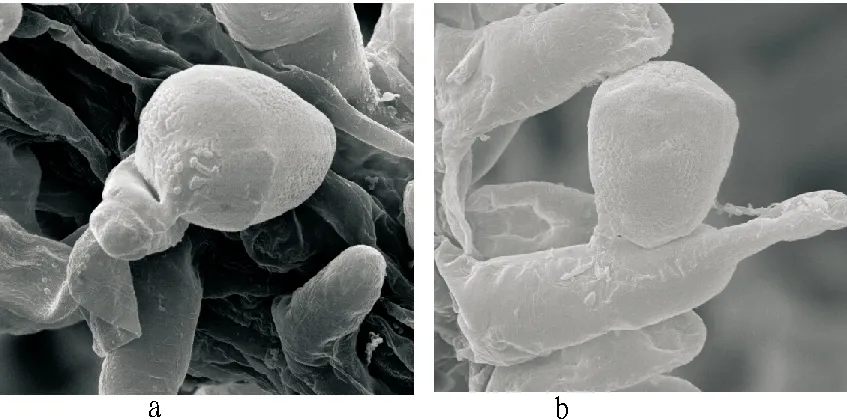
Fig.3 Observation with electron microscope
4 Discussions
(i) In this study, we conduct the optical and scanning microscopy observation on the forms of Chinese water chestnut pollen grain and pollen tube during stigma germination, demonstrate the vessel structure and pollen grain form of Chinese water chestnut pistil stigma as well as germination form of pollen tube 2 h after pollination from the microscopic point of view, reveal the transfer path of genetic material within pollen during Chinese water chestnut pollination, and fundamentally confirm the theory that Chinese water chestnut is fully available for cross-pollination. It provides a reliable theoretical basis and practical reference for crossbreeding of Chinese water chestnut, and opens up a new research way for further carrying out crossbreeding of Chinese water chestnut. (ii) Through the experimental observation, it is found that pollen grain is randomly combined with stigma, but not all pollen grains can germinate; only when the both ends of pollen grains touch and recognize branched hairs can the pollen tube be successfully germinated. In addition, the branched hairs protruding from stigma vessel and densely distributed around stigma, play a crucial role in pollination of Chinese water chestnut. Firstly, the pollen grain bearing structure constructed on the stigma surface can hinder the continuous top-down fall of mature pollen grains and effectively expand the stigma pollination area. Secondly, only by combining pollen with branched hairs can the genetic materials of the pollen grains observed lead to germination of pollen tube. After entering into branched hairs, pollen tube continues to grow, and genetic materials are eventually transferred to stigma vessel with the continuous growth of pollen tube, and the relevant binding mechanism needs further study. In the study, it is found that most pistils of Chinese water chestnut have 3 stigmas, and sometimes 2-4 stigmas, different from the report of related literature[3]. Each stigma has hundreds of branched hairs, and all the genetic materials obtained after considerable branched hairs are in contact with pollen, directly enter into the corresponding vessel, but it needs to be further proved about the pollen tube growth stop, genetic material delivery and pistil’s transport and regulation mechanisms. The sedge research is seldom reported, and in recent years, some scholars have carried out the investigation and research of sedges in Hainan, Shandong, Guizhou and other places[9-10], but it is still at the resource collection stage; some researchers study the sedge seed germination[11]. Chinese water chestnut is one of the sedge plants to be studied most, and also a cash crop that can be cultivated on a large scale, so the research of Chinese water chestnut can provide a theoretical reference for the study of sedges.
[1] LI F, LIU YM, LI MH,etal. Production and research of Chinese water chestnut in the United States[J]. Journal of Changjiang Vegetables, 2009(8):19-22. (in Chinese).
[2] LI F, KE WD, LIU YM. 2006. Advances in research of common spikesege [J]. Journal of Changjiang Vegetables, 2006(8):39-43. (in Chinese).
[3] XU XS, SHI GX. Observations on the inflorescence and the floral morphology of Eleocharis tubelosa(Roxb.) Roem et Schult [J]. Journal of Nanjing Normal University, 1985 (2):67-72. (in Chinese).
[4] LI SM, KE WD, LIU YM,etal. Observation of flowering habit in Chinese water chestnut [Eleocharis dulcis (Burm. f.) Trin.ex Hensch][J]. Journal of Changjiang Vegetables,2009(16):56-57. (in Chinese).
[5] LI SM, KE WD, LI F,etal. Effects of different treatments on seeds germination of waternut [J]. China Vegetables, 2011(8):55-59. (in Chinese).
[6] CHEN LJ, CAI BH, JIANG W,etal. A new water chestnut(Eleocharis tuberose)variety -Guiti No.2[J]. China Vegetables, 2011(14):96-98. (in Chinese).
[7] YANG QH, YANG HS, LI JQ. Categories and adaptive evolution of plant self-pollination[J]. Journal of Jiaying University, 2011, 29(8):55-64. (in Chinese).
[8] LIU HX, REN SF, ZHANG CH,etal. Observation of pollination and fertilization process in Forsythia suspensa [J]. Scientia Silvae Sinicae, 2010, 46(9)45-49. (in Chinese).
[9] YANG HB, WANG QL, BAI CJ,etal. New exploration of Cyperaceae resource in Hai’nan [J].Chinese Journal of Tropical Crops, 2013, 34(11):2150-2157. (in Chinese).
[10] DONG SX, GUO CY, ZHOU GM,etal. Eleocharis pellucida,a newly recorded plant of the family Cyperaceae from Shandong Province[J]. Guihaia,2013, 33 (2) : 279-282. (in Chinese).
[11] ZHOU ZQ, LI TS, HU XW. Seed dormancy and germination characteristics four cyperaceae species[J]. Acta Botanica Boreali-Occidentalia Sinica, 2013, 33(9):1885-1890. (in Chinese).
 Asian Agricultural Research2016年5期
Asian Agricultural Research2016年5期
- Asian Agricultural Research的其它文章
- Is It Worthwhile for Farmers to Grow Grain?—A Study of Farmers’ Behavior of Growing Grain
- Establishment of Chinese Agricultural Brand Value Scale and Study of Its Reliability and Validity Based on Customer Value
- How to Develop Chinese Rural Tourism in the Context of New Urbanization?
- Chinese Customers’ WTP for Legal Digital Music Downloading
- High Standard Capital Farmland Construction Based on Grain Security
- Research on the Brand Construction of Agritourism Enterprise in Chongqing
