Relative Explosive Strength of Some Explosive Mixtures Containing Urea and/or Peroxides
Relative Explosive Strength of Some Explosive Mixtures Containing Urea and/or Peroxides
Ahmed K. HUSSEIN1, Svatopluk ZEMAN1, Muhamed SUCESKA2, Marcela JUNGOVA1
(1. Institute of Energetic Materials, Faculty of Chemical Technology, University of Pardubice, Czech Republic;2. Brodarski Institute-Marine Research and Special Technologies, Zagreb, Croatia)
Several mixtures, based on urea derivatives and some inorganic oxidants, including also alumina, were studied by means of ballistic mortar techniques with TNT as the
tandard. The detonation pressure(P), detonation velocity(D), detonation energy(Q), and volume of gaseous product at standard temperature and pressure (STP), V, were calculated using EXPLO5 V6.3 thermochemical code. The performance of the mixtures studied was discussed in relation to their thermal reactivity, determined by means of differential thermal analysis (DTA). It is shown that the presence of hydrogen peroxide in the form of its complex with urea (i.e. as UHP) has a positive influence on the explosive strength of the corresponding mixtures which is linked to the hydroxy-radical formation in the mixtures during their initiation reaction. These radicals might initiate (at least partially) powdered aluminum into oxidation in the CJ plane of the detonation wave. Mixtures containing UHP and magnesium are dangerous because of potential auto-ignition.
ballistic mortar;TNT;DTA; peroxides; perchlorates; nitrates; urea
Introduction
Mixtures of compounds based on ammonium nitrate (AN) and urea (U) are used as liquid nitrogen fertilizers, referred to as UAN[1]with melting points between -18℃ and -5℃ (depending on the water content) and as intermolecular castable industrial explosives, commonly known as Carbatols[2-3], with relatively high density, detonation velocity and resistance to initiation[2]. Another subject of practical interest concerning mixtures based on urea nitrate (UN) is their use for criminal purposes[4-5], unfortunately increasingly commonplace[6-8]. The globally commercially available urea hydrogen peroxide (UHP)[9]has, in light of its explosive risk, only been described recently[10]; its behavior when mixed with some inorganic salts has not been studied until now, and thus this paper focuses on it in comparison with several previously studied other mixtures with peroxides[11-13]and/or ammonium nitrate content.
1 Experimental
1.1 Materials
Among the substances used, i.e. ammonium nitrate (AN), sodium nitrate (SN), urea (U), urea hydrogen peroxide complex (UHP), ammonium perchlorate (AP), the urea hydrogen peroxide complex was not studied in any more detail from the point of view of its use in explosive compositions. UHP has a density of 1.4 g/cm3, and is a white crystalline substance that decomposes on melting at 80-90℃. A kinetic study of UHP′s thermal decomposition gave an activation energy of 113kJ/mol with frequency factor of 10-13s-1[14]. Its heat of formation is 65.1kJ/mol and its experimental detonation velocity was 3470-3600 m/s[10]. Aluminum (Al) quality AP-4[2]was used with specific surface area of grains 1000-1100 cm2/g and particle size of 20-22 μm. In the one case of an attempt using magnesium flake for fireworks, the particle size ranged from 100 to 500 μm.
1.2 Preparation of mixture
A weighed amount of urea (U) and/or UHP was mixed mechanically with ammonium nitrate (AN), ammonium perchlorate (AP) and sodium nitrate (SN). The actual mass fraction are shown in Table 1. These mixtures were formed so as to have an oxygen balance (OB) close to zero thus generating gaseous products with a high level of power and low toxicity. Four mixtures based on triacetone triperoxide (3,3,6,6,9,9-hexamethyl-1,2,4,5,7,8-hexoxonane, TATP) with AN and different mass fractions of water (4.2%, 9.9%, 15.5% and 24.5%, respectively) have previously been studied[8,11-12]and compared with the mixtures studied here. Data for urea nitrate (UN) and a mixture of fuel oil with ammonium nitrate (ANFO) were also taken from recent papers[8].
1.3 Calculations of detonation characteristics
The theoretical detonation characteristics (i.e. detonation velocity, detonation energy and volume of gaseous products) of the mixtures tested were calculated using the EXPLO5 thermochemical code, version V6.3. The calculation of detonation parameters is based on the chemical equilibrium steady-state ideal detonation model. The state of gaseous detonation products is described by the EXP-6 equation of state[15-16], based on intermolecular potentials and fundamental statistical mechanical theories for the calculation of the thermodynamic properties of a classical fluid of molecules interacting with a central pair potential are available today. In our calculations we used the WCA/Ree perturbation theory to generate excess thermodynamic data of a pure fluid with an EXP-6 potential, and by interpolating them with a Chebyshev polynomial. The method, i.e. EOS, is described by Byers Brown[17]. The detonation velocity, detonation energy and amount of gaseous detonation products calculated by EXPLO5 V6.3 are summarized in Table 2.
It is very likely that some of the explosive mixtures tested have non-ideal detonation behavior, particularly those containing aluminum and ammonium nitrate. However, in this study we calculated detonation properties assuming an ideal detonation model for all the mixtures (such calculation gives the theoretically maximum detonation properties, i.e. properties at infinite explosive charge diameter), except for aluminum-containing mixtures for which we carried out calculations in two different ways-one assuming Al completely reacts at the CJ state (this gives higher D, P, Q, T values) and the second assuming Al does not react, i.e. remains as solid Al.
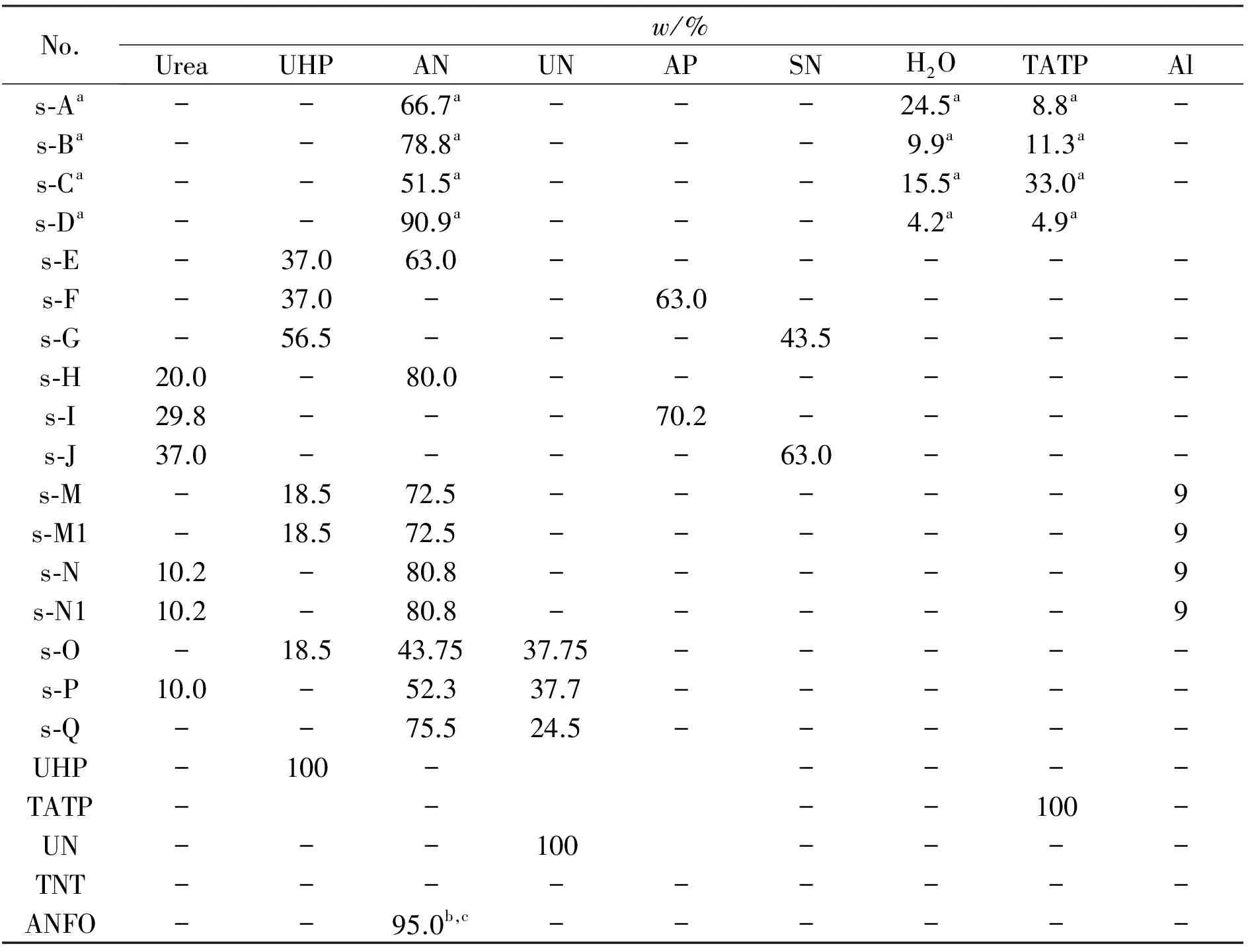
Table 1 Formulations of energetic mixtures
Notes:(a) taken from Ref.[1];(b) taken from Ref.[8];(c) this mixture contains 5% by wt.fuel oil;Data for mistures N and M were calculated taking aluminum as an inert admixture,for mixtures N1 and M1 this aluminum has reacted fully in the CJ point.
1.4 Relative explosive strength measurement
A ballistic mortar test was used for the determination of the relative explosive strength of the samples studied, using TNT as the reference[18-19]. This substitutes for the Trauzl test in the lead block, which was used in the past but which had certain disadvantages such as high cost, the use of toxic lead and the rupture of a lead block[20]. A fixed amount of an explosive (10g) was wrapped in polypropylene foil and inserted into the mortar enclosed by a steel projectile and initiated using a non-electric detonator (No.8). For each measurement, a part of the non-electric detonator was inserted in the sample and was fired by a match. Three measurements were made for each sample and the mean values are reported in Table 2. The determination is based on measuring the swing angle of the pendulum and by comparing the measurement with a calibration curve for the standard TNT explosive at different masses. The explosive strength of the explosive tested was thus expressed relative to TNT (relative explosive strength, RS as % TNT) and compared with previously studied TATP samples from Refs.[8,11]. UHP and the mixture of urea with sodium nitrate (J) gave practically zero swing angles; therefore, their RS values, in the conditions used, were taken as to be equal to zero. However, both these mixtures are energetic materials which, in specific conditions, can succumb to explosive decomposition (for UHP see Ref. 10, and the J mixture after adding hydrogen peroxide (HP)-see mixture G).
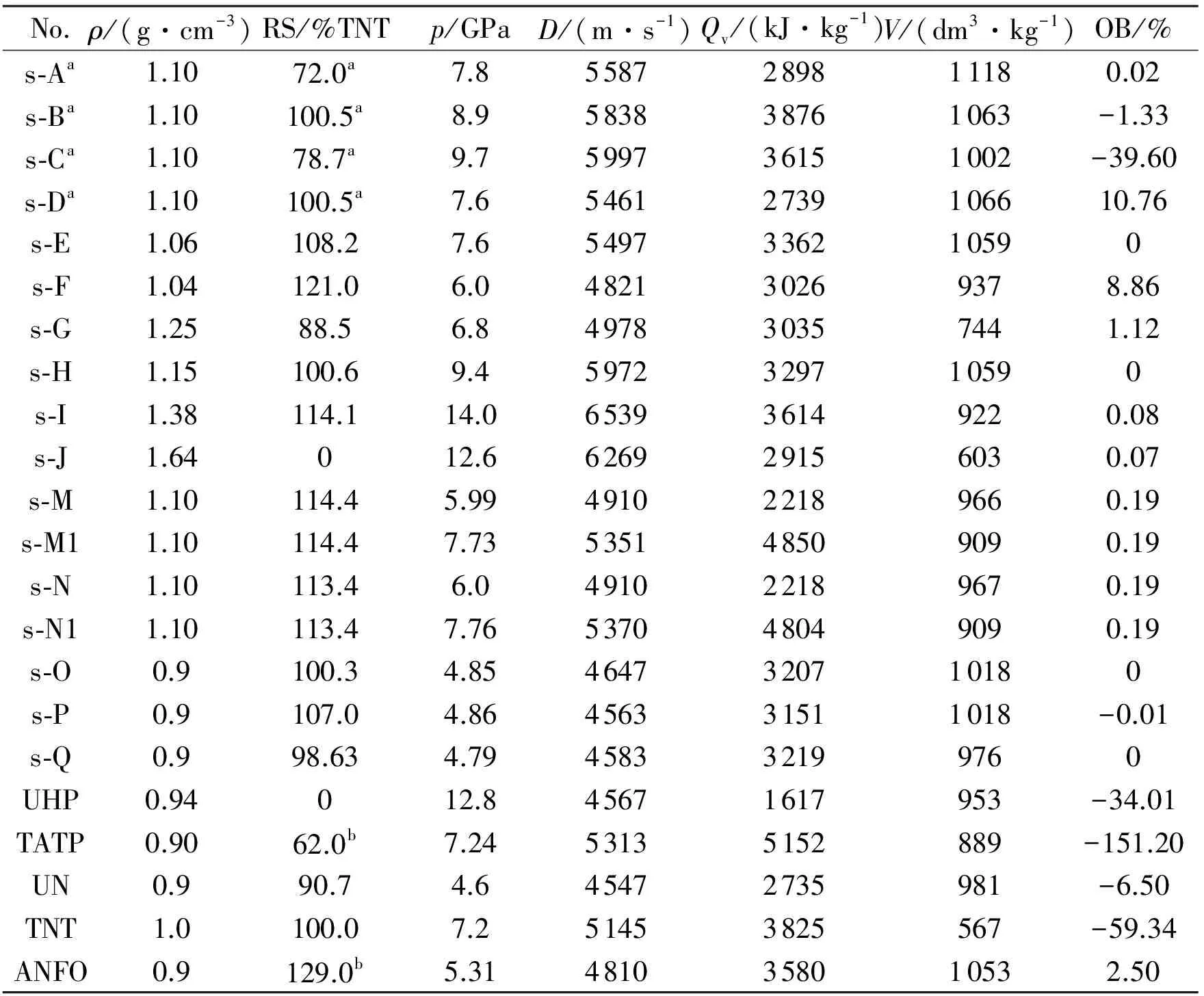
Table 2 Characteristics of energetic mixtures
Notes:(a) taken from Ref[11];(b) taken from Ref[8];Datas for mixtures N and M were calculated taking aluminum as an inert admixture, for mixtures N1 and M1 this aluminum has reacted fully in the CJ point.
1.5 Differential thermal analysis (DTA)
Due to the heterogeneity of the mixtures studied, the DSC and TGA techniques, which use samples of only a few mg, were unsuitable for a study of their thermal reactivity. Therefore, a DTA 550 Ex apparatus was used for differential thermal analysis[21]of the explosives under study. The measurements were carried out at atmospheric pressure, with the tested sample in direct contact with the air. The sample (0.05 g) was placed in a test tube made of Simax glass, 5 mm in diameter and 50 mm long. The reference standard was 0.05 g aluminum oxide. Different linear heating rates of 5, 10 and 15℃/min were used. The output of these measurements was evaluated by the Kissinger relationship (1)[22]
(1)
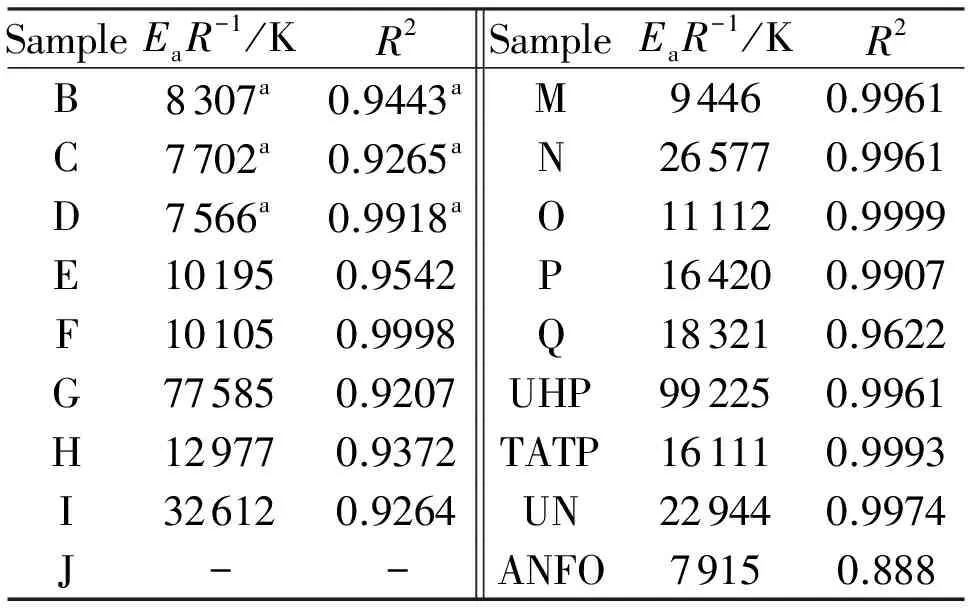
whereφis the linear heating rate andTis the peak temperature of the exothermic decomposition. The thermal reactivity, expressed as theEaR-1slopes, and its regression value from this relationship (in a similar sense to that in references[23-25]), are summarized in Table 3. Because the mixture J did not decompose with liberation of heat (see Fig.1), its exothermic thermal reactivity was taken as being zero.
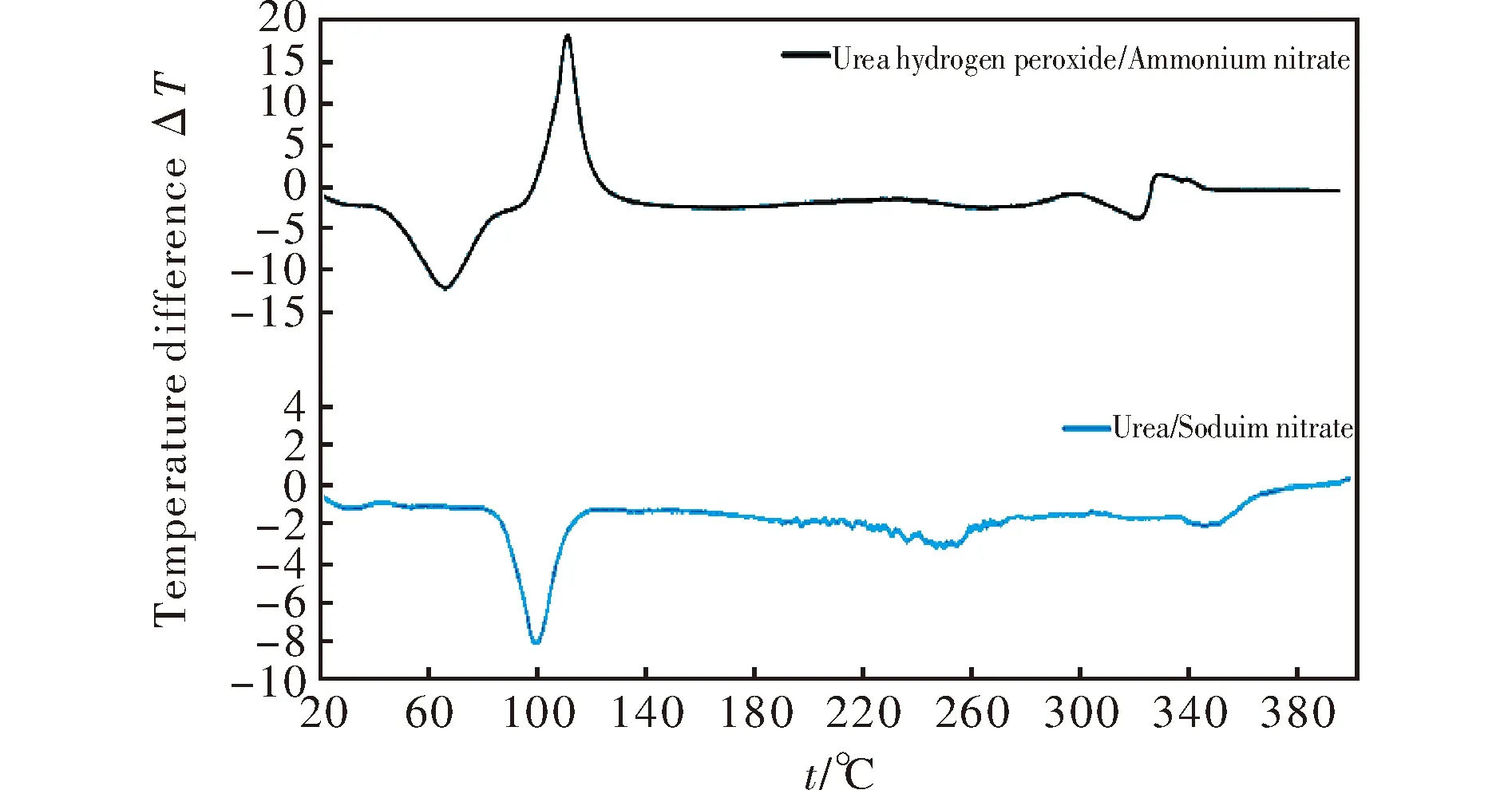
Fig.1 DTA records of mixture of UHP with ammonium nitrate (upper curve) and mixture of urea with sodium nitrate (lower curve)
Note: (a) Taken from Ref[12]
2 Results and Discussion
2.1 Explosive strength versus detonation pressure
In PBX explosives, the relative explosive strength (RS) is directly proportional to the productρD2[16], whereρD2is representative of detonation pressure. The RS values were also determined by ballistic mortar measurements[18](more about influence of the sample quality on outputs of measurements see in Ref.[18]). However, in the mixtures studied, only lines I, II and III in Fig.2 correspond to this relationship. The data for TNT (ρ= 1.0 g/cm3) are situated on line III. Hydrogen peroxide (HP) is included in appropriate mixtures through the presence of UHP (UHP contains 36.1% by wt. of HP). Thermal decomposition of hydrogen peroxide, i.e. homolysis of the peroxide HO-OH bond, has been identified as the dominant chain-branching reaction[23]that controls any given charge ignition. This fact has a positive effect on the performance of the corresponding mixtures (see RS of the mixture s-E versus s-H), whereas comparison of theirpvalues shows the reverse effect.
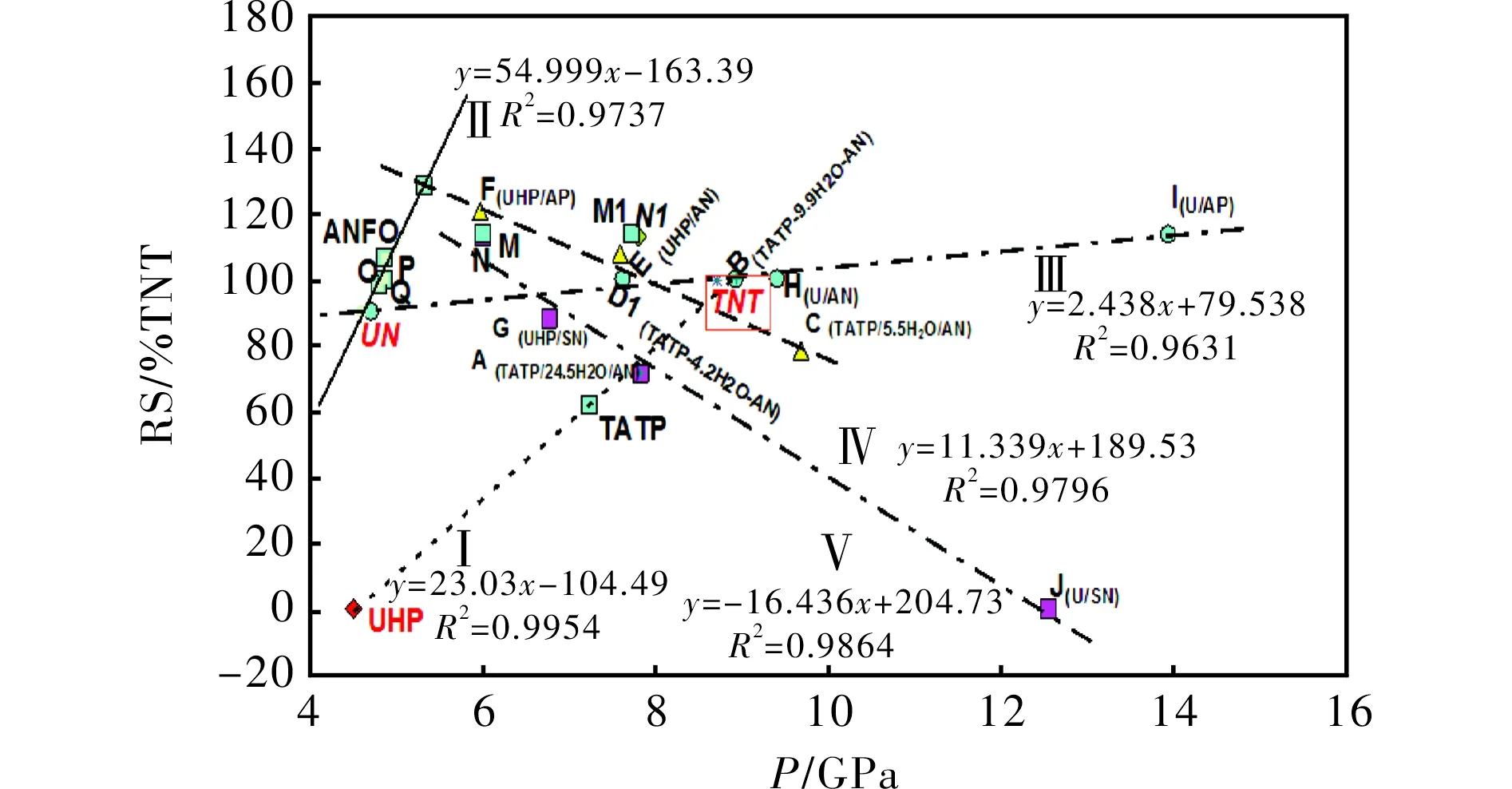
Fig.2 Relationship between experimental relative explosive strength and calculated detonation pressure of the mixtures studied
The reactive radicals (OH-radicals and also radicals derived from oxo-chlorine intermediates of decomposition in the mixture F) might have a strong influence on the reverse slope of line IV in the case of absence of cooling water admixture in samples s-F and s-E, i.e. their decomposition velocity in a ballistics mortar chamber could have been higher than in the case of other mixtures. Mixture s-C is already "cooled" by the water content and the negative oxygen balance (-39.6%). The theory about the influence of very reactive radicals (in this case·OH) may be evidenced by the comparison of the positions in Fig.2 of the mixture pair s-F and s-I, but mainly by the s-G and s-J pair. The difference in densities of the pair s-E and s-H, and thus also in their calculated detonation parameters, may well be removed due to the use of the ballistic mortar for testing their performance (for a discussion about influences in these measurements, see Ref.[16]). The influence of cooling admixtures mentioned above is very well demonstrated by the W/O emulsion explosives fortified by admixtures of high explosives[21]; such admixtures cause a relatively strong increase in the detonation velocity (D) of the fortified W/O explosives and thus the values of the productρD2, while their relative explosive strength is roughly the same[23]due to the presence of water.
Line V is influenced by solid particles in the decomposition products and, in the case of mixture A, by a relatively high water content. As for the aluminized mixtures s-N and s-M, the points N1 and M1 in Fig.2 correspond with the assumption that aluminum completely reacts in the CJ point, and the points N and M correspond with unreacted solid Al (aluminum, depending on the type of explosive used, either behaves as an inert substance or participates in the chemical reactions proceeding inside the detonation wave[27]). It seems that the data for the s-N and s-M mixtures correlate logically with line V.
2.2 Thermal reactivity versus detonation pressure
From our study of the relationship between thermal reactivity and performance of energetic materials[23-25,29,30]it was found that there exists a simple relationship between a slope of the Kissinger relation (1),EaR-1, and the productρD2(from experimental data)[25]or detonation pressure,p. Its version for the mixtures studied in this paper is represented by Fig.3. Here the trends, corresponding to linesCandD, are in agreement with analogous trends in PBXs[25]. The different positions of these lines in a grid system of Fig.3 is caused by the fact that urea can substantially increase the thermal stability of AN (lineCin Fig.3 corresponds to this)[30,31]. Also, with a content of 50% by wt. of urea, its mixture with AN can still produce a steady detonation in the UN gap test[31]. Urea cannot reduce the ability to propagate a detonation[31].
LineAin Fig. 3 mainly groups mixtures based on UHP; here again mixture M with aluminum has two possible positions in a grid system-if we took Al as being inert, the corresponding point belongs in the group around lineA. However, taking Al as a reactant in the CJ plane, the corresponding point M1 is close to lineD; it might be related to the participation of this metal powder in redox-reactions in this plane. A registered auto-ignition of the mixture with Mg could provide evidence for this theory; in this light, the analogous mixture M, in which aluminum was substituted by magnesium, self-ignited 5 hours after its preparation.
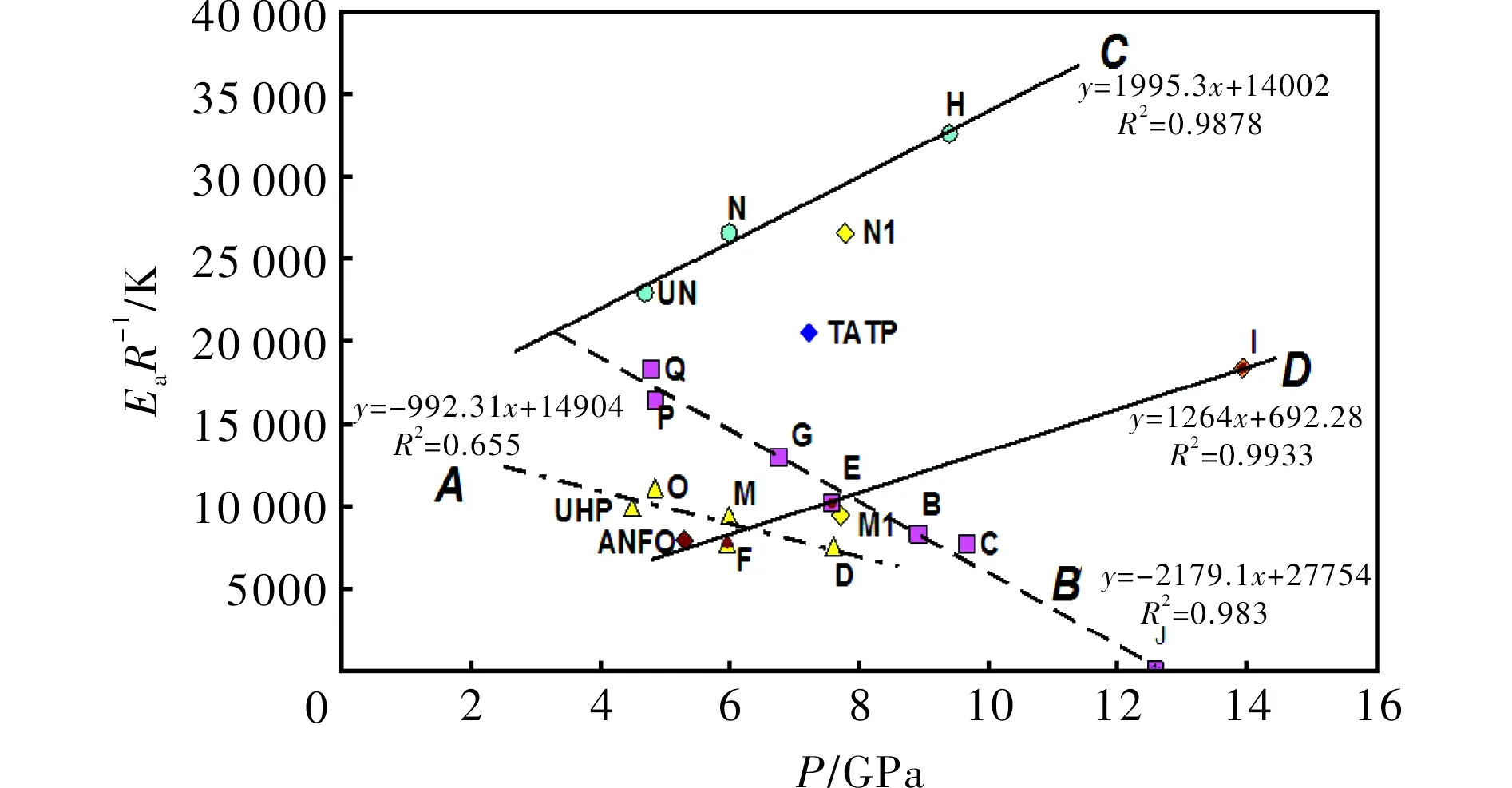
Fig.3 Relationship between thermal reactivity, expressed as the slopes of the Kissinger relationship (1), and calculated detonation pressure (see in Table 1) of the energetic materials studied
In both theAandBlines it is logical that decreasing activation energies correlates with an increase in performance of the corresponding mixtures which correspond to a general relationship between sensitivity and performance[29]. Data for TATP should belong to a group of lineBbut it has a higherEaR-1value in comparison with its mixtures with the "acidic" AN. Almost all mixtures, inherent to these lines, contain peroxides which are their most reactive component. As has already been mentioned, the starting reaction for their initiation should be homolysis of the peroxide O-O bond[26,32]. On the other hand, the most reactive component of the mixtures belonging to linesCandDis ammonium nitrate (eventually urea nitrate) which in its solid state is dissociated according to the following equilibrium[33]
(2)
With subsequent decomposition through nitration of ammonia according to the following equation[33]
H2O+H3O++N2O
(3)
The primary step in the decomposition of UN is its dissociation into urea and nitric acid with subsequent formation of isocyanuric acid from urea[34]. Dissociation into ammonia and perchloric acid is the first step in the decomposition of AP, with subsequent decomposition of the perchloric acid[33]. The difference in the primary fission steps in initiation (ionic versus radical) of the mixtures studied should be the main reason for their division into groups belonging to linesAandB, on the one hand, and to linesCandD, on the other. This difference should be also a reason for the different slopes of the first pair of these lines in comparison with the second one.
3 Conclusions
From ballistic mortar measurements and the theoretical calculation of explosive mixtures based on urea derivatives with some inorganic oxidants. It can be concluded that it is possible to prepare explosive mixtures based on urea with an explosive strength better than TNT. Their performance is seen to be positively influenced by the presence of hydrogen peroxide in their composition, with a distinct advantage being shown by its complex with urea (UHP). This influence is notable namely in the use of sodium nitrate as an oxidant in these mixtures. The chemical nature of this effect lies in the reactive hydroxy-radical formation as the first intermediate in the given mixture′s initiation. On the basis of the relationship between thermal reactivity and performance, it is possible to split the mixture into those with the ionic primary fission and those with the radical one in their initiation.
Theoretical calculation is done assuming an ideal detonation model, so the calculated detonation properties of the mixtures correspond to the theoretical maximum that can be obtained at infinite diameter of explosive charge. In accordance with the current knowledge on burning of metal powders in the reaction zone, it can be assumed that the calculation carried out assuming aluminum does not react at the CJ point gives more realistic results for those mixtures without peroxide content that were studied. However, the OH-radicals, generated during the initiation of mixtures with peroxide content, might initiate (at least partially) powdered aluminum into oxidation in CJ plane of the detonation wave. Mixtures containing both UHP and magnesium are dangerous because of potential auto-ignition.
Acknowledgement
This study was supported by means of the financial resources of Students Grant Projects No. SGSFCHT_2016002 of the Faculty of Chemical Technology at the University of Pardubice.
[1] Raczkowski Ch W, Kissel D E, Vigil M F,et al. Fertilizers placement to maximize nitrogen use by fescue [J]. Journal of Plant Nutrition, 2016, 39(4):581-587.
[2] Dubnov L V, Bakharevich N S, Romanov V I. Promyshlennye vzryvchatye veschestva (Industrial Explosives) [M].Moscow:Izdat. Nedra,1988.
[3] Litovka O B, Kozak G D, Chugreeva E Z,et al.Cast porius charges on a base of ammonium nitrate/Urea eutectic[J]. Central European Journal of Energetic Materials, 2008, 5(2):57-66.
[4] Yin H, Wang G, Du F, et al. Study on non-TNT rock explosive based on nitrates of urea and ammonia [J]. Chinese Journal of Energetic Materials, 1994, 2(4):12-19.
[5] Zhou R, Cao D, Wang J, et al. Relation between formulation and performance of explosives without TNT [J]. Applied chemical Industry, 2006, 35(8):615-617.
[6] Phillips S A, Lowe A, Marshall M,et al. Physical and chemical evidence remaining after the explosion of large improvised bombs. Part 1: firings of ammonium nitrate/sugar and urea nitrate [J]. Journal of Forensic Sciences A, 2000, 45(2):324-332.
[7] Tsippy T, Rinat R, Nitay L,et al. Urea nitrate, an exeptionally easy-to-make improvised explosive: studies towards trace characterization [J]. Analytical and Bioanalytical Chemistry, 2009, 396 (2):421-428.
[8] Matyas R, Selesovsky J. Power of TATP based explosives [J]. Journal of Hazardous Materials, 2009, 165:95-99.
[9] Chemical Trading Guide "Guiechem", http://www.
guidechem.com/cas-124/124-43-6.html, Aug.2016.
[10] Matyas R, Selesovsky J, Pelikan V, et al. Hazardous aspects of urea peroxide adduct [J]. Propellants, Explosives, Pyrotechnics,2016-DOI:10.1002/prep.201600101.
[11] Zeman S, Trzciński W A, Matyáš R. Some properties of explosive mixtures containing peroxides. Part I.Relative performance and ddtonation of mixtures with triacetone triperoxide [J]. Journal of Hazardous Materials, 2008, 154:192-198.
[12] Zeman S, Bartei C. Some properties of explosive mixtures containing peroxides. Part II. relationships between detonation parameters and thermal reactivity of the mixtures with triacetone triperoxide [J]. Journal of Hazardous Materials, 2008, 154:199-203.
[13] Matyáš R, Zeman S, Trzciński W A,et al. Detonation performance of TATP/AN based explosives [J]. Propellants, Explosives, Pyrotechnics, 2008, 33:296-300.
[14] Ball M, Steven M, The thermal decomposition of solid urea hydrogen peroxide [J]. Thermochimica Acta, 1995, 261:95-106.
[15] Fried L E , Howard W H, Souers P C. Exp-6: a new equation of state library for high pressure thermochemistry [C]∥12th International Detonation Symposium. San Diego:Naval Surface Weapons Center,2002.
[16] Victorov S.B. The effect of Al2O3phase transitions on detonation properties of aluminized explosives [C]∥12th International Detonation Symposium. San Diego:Naval Surface Weapons Center,2002.
[17] Byers Brown W. Analytical representation of the excess thermodynamic equation of state for classical fluid mixtures of molecules interacting with a-exponential-six pair potentials up to high densities [J]. Journal of Chemical Physics,1987, 87 (1):566-577.
[18] Elbeih A, Jungova M, Zeman S, et al. Explosive strength and impact sensitivity of several PBXs based on attractive cyclic nitramines [J]. Propellants, Explosives, Pyrotechnics, 2012, 37:329-334.
[19] Establishing More Detailed Conditions for Allowing Explosives, Explosive Objects and Aids into Use, and their Testing,No. 246/1996[R]. Czech Mining Authority,1966:3200-3208.
[20] Suceska M. Test Methods for Explosives [M].New York:Springer-Verlag,1995.
[21] Krupka M, Devices and equipment for testing of energetic materials [C]∥New Trends Res. Energ. Mater., Proc. Semin. Pardubice:[s.n.],2001:222-227.
[22] Kissinger H E. Reaction kinetics in differential thermal analysis [J]. Analytical Chemistry, 1957, 29(11):1702-1706.
[23] Zeman S, Kohlíěek P, Maranda M. A study of chemical micromechanism governing detonation initiation of condensed explosive mixtures by means of differential thermal analysis [J].Thermochimica Acta, 2002, 398:185-194.
[24] Nemec O, Jungova M, Zeman S. Modification of W/O emulsions by demilitarized composition B [J]. Propellants, Explosives, Pyrotechnics, 2013, 38(1):142-146
[25] Zeman S, Yan Q L, Elbeih A. Recent advances in the study of the initiation of energetic materials using the characteristics of their thermal decomposition Part II. Using simple differential thermal analysis [J]. Central European Journal of Energetic Materials, 2014, 11 (3): 395-404.
[26] Hong Z, Farooq A, Barbour E.A, et al. Hydrogen peroxide decomposition Rate: A Shock tube study using tunable laser absorption of H2O near 2.5 μm [J]. Journal of Physical Chemistry A, 2009, 113(46):12919-12925.
[27] Maranda A, Trzcinski W A, Cudzilo S,et al. Detonation problems of non-ideal explosives containing powdered aluminum [C]∥International Pyrotechnics Seminar.Tsukuba:[s.n.], 1997:509-517.
[28] Zeman S. Sensitivities of High Energy Compounds [M]. Heidelberg:Springer,2007:195-271.
[29] Zeman S, Jungova M. Sensitivity and Performance of Energetic Materials [J]. Propellants, Explosives, Pyrotechnics, 2016, 41(3):426-451.
[30] Oxley J C, Smith J L, Rogers E,et al. Ammonium nitrate: thermal stability and explosibility modifiers [J]. Thermochimica Acta, 2002, 384:23-45.[31] Tan L, Xia L H, Wu Q J,et al. Effect of urea on detonation characteristics and thermal stability of ammonium nitrate [J]. Journal of Loss Prevention in the Process Industries,2015, 38:169-175.
[32] Talouba I B, Balland L, Mouhab N,et al. Kinetic parameter estimation for decomposition of organic peroxides by means of DSC measurements [J]. Journal of Loss Prevention in the Process Industries, 2011, 24:391-396.
[33] Manelis G B, Nazin G M, Rubtsov Yu I,et al. Thermal decomposition and combustion of explosives and propellants [M].New York: Taylor & Francis, 2003.
[34] Désiletsa S, Brousseaua P, Chamberlanda D,et al. Degradation mechanism and thermal stability of urea nitrate below the melting point [J].Thermochimica Acta, 2011, 521:76-183.
10.14077/j.issn.1007-7812.2016.05.003
TJ55;TQ560 Document Code:A Article ID:1007-7812(2016)05-0022-06
Received date:2016-06-01; Revised date:2016-06-17
Biography:Ahmed K.HUSSEIN(1984-),male,MSC.,research field:Energetic materials.E-mail:ahmed92eqypt@gmail.com

