叠氮基修饰硅胶固定相在亲水模式下的色谱评价及应用
赵艳艳, 李秀玲 , 郭志谋, 梁鑫淼
(1. 大连医科大学药学院,辽宁 大连116044;2. 中国科学院分离分析化学重点实验室,中国科学院大连化学物理研究所,辽宁 大连116023)
Hydrophilic interaction liquid chromatography(HILIC)has attracted more and more interests in recent years. Samples with strong polarity could be well separated with HILIC. However,stationary phases for HILIC were scarce,which limit the application. Several novel stationary phases were synthesized by our group,which showed good performance under HILIC mode,such as Click βcyclodextrin (CD)[1],Click olio(ethylene glycol)(OEG)linked β-cyclodextrin (OEG-CD)[2-6],Click Maltose [7],Click Carboxylic acid[8],Click Aspartic acid [9]. These stationary phases have a common synthesis method—click chemistry (1,2,3-triazole forming reactions),which has been used as a facile,robust and highly efficient conjugation strategy in bonded stationary phase synthesis. Silica based azide-modified stationary phase was used as the medium product to synthesize these different types of stationary phases,which have the potential to be applied under HILIC mode,too. Azide group could provide cation ion-exchange and dipolar interactions,moreover,the existing Si-OH on the surface of silica gel could provide hydrogen bonding interactions. However,the chromatography properties of azide-modified silica gel were not studied,and the chromatographic performance of the stationary phase during application was not clear.
Protein glycosylation can modify protein properties and participate in cellular communication,thus protein glycosylation is one of the most important co-/post-translational modifications. Glycosylation analysis is necessary to understand the relationship between the biofunctions and structures of glycopeptides. However,difficulty often encounters with glycopeptide analysis due to the low abundance and ion-suppress effect brought by their high-abundance counterparts during MS analysis. HILIC shows high performance in glycopeptide enrichment,which can selectively enrich glycopeptides based on their polar difference from the counterparts. However,the commercial matrices for glycopeptide enrichment with HILIC are scarce. Novel stationary phases synthesized with Click Chemistry were prepared by our group and were applied in glycopeptide enrichment,such as Click OEG-CD [3],Click Maltose [7],Click Carboxylic acid [8],Click Aspartic acid [9].High glycopeptide enrichment selectivity was obtained with these novel stationary phases. However,their common medium product,the silica based azide-modified stationary phase’s performance in glycopeptide enrichment has not been studied before.
In the investigation,the chromatographic properties of silica based azide-modified stationary phase were evaluated. Moreover,azide-modified silica gel was applied in glycopeptide enrichment and the enrichment conditions were optimized according to the properties. The azide-modified silica gel is expected to show good performance in glycopeptide enrichment under suitable conditions.
1 Experimental
1.1 Chemicals
Silica gel (particle size,5 μm;pore size,10 nm;surface area,270 m2/g)was purchased from Fuji Silysia Chemical (Japan). The reagents used for chemical modification of the silica gel were all with identified structures. Water was purified on a Milli-Q system (USA). Acetonitrile (ACN,HPLC grade)was purchased from Fisher (USA).All the evaluation compounds were analytical grade chemicals of various origins.
Horseradish peroxidase (HRP),dithiothreitol(DTT),and iodoacetic acid (IAA)were obtained from Sigma (USA). Ammonium bicarbonate(NH4HCO3)and ammonium formate (NH4FA)were purchased from Fluka (USA). Acetonitrile(MS grade)and formic acid (HPLC grade)for analysis were from Merck (Darmstadt,Germany)and Acros (New Jersey,USA),respectively. GELoader tips were obtained from Eppendorf (Hamburg,Germany).
1.2 Instrument,columns and conditions
Elemental analysis was performed on a Vario EL III elemental analysis system (Germany). The HPLC system (Agilent 1200,USA)consisted of a quaternary pump,an autosampler,a degasser,an automatic thermostatic column compartment and a diode array detector. The FT-IR spectrum was measured on a Bruker FTIS (Tensor 27)analysis system (Germany).
LC-MS analysis was performed on an Acquity nano liquid chromatography system (Waters,Milford,MA,USA)coupled to a nano electrospray ionization-quadrupole time-of-flight (QTOF)mass spectrometer (Waters MS Technologies,Manchester,UK)in positive ion electrospray ionization (ESI)mode. MS analysis method was set according to our previous report[3].
1. 3 Preparation of the silica based azidemodified stationary phase
The azide modified silica gel was prepared according to the literature[10],and the structure is shown in Fig.1. The resulting azide-modified silica gel was evaluated with the elemental analysis and FT-IR instrument. The resulting stationary phase was packed into a steel column (150 mm×4.6 mm)for chromatographic evaluation.
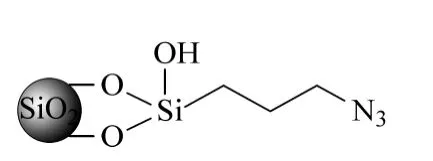
Fig.1 Structure of the silica based azide-modified stationary phase
1.4 Chromatographic evaluation of the silica based azide-modified stationary phase
Chromatographic property of the silica based azide-modified stationary phase was evaluated with typical nucleosides as the test samples. Four nucleosides (cytidine,guanosine,inosine,and uridine)were dissolved in methanol/water (1 ∶1,v/v)to 0.5 mg/mL,separately. The wavelength was 280 nm and the column temperature was 30℃. The injection volume was 1 μL,and the flow rate of mobile phase was 1.0 mL/min. The mobile phase for isolation of the four nucleosides was ACN/water (95 ∶5,v/v).
1. 5 Glycopeptide enrichment by the silica based azide-modified stationary phase
HRP was digested according to the methods in our previous report[3].
Enrichment was carried out in GELoader tips.The procedure was performed according to literature[11]with a minor modification. ACN slurry containing 1.5 mg silica based azide-modified matrix was pushed into the GELoader tip. The column was firstly equilibrated with 40 μL 50 mmol/L NH4HCO3with 90% ACN aqueous solution,50 mmol/L NH4FA with 90% ACN aqueous solution,0.1% FA with 90% ACN aqueous solution under starting condition. Then,the HRP digest (5 μL,1 mg/mL)was loaded onto the column. The column was rinsed with 60 μL of 90%,80%,70%,60%,50% (v/v)ACN aqueous solutions added with 50 mmol/L NH4HCO3,50 mmol/L NH4FA,0.1% FA. The resulting fractions were dried and dissolved in 20 μL 50% (v/v)ACN-0.1% (v/v)FA aqueous solution and analyzed with MS.
1.6 Data analysis
The instrument was controlled by Agilent ChemStation for HPLC system and Masslynkx for MS system. Data treatment was performed by Microsoft Excel and Origin 8.0 in a personal computer.
2 Results and discussion
2.1 Preparation of azide-modified silica gel
The silica based azide-modified stationary phase was characterized with the elemental analysis results. The carbon content of azide-modified silica gel was 4.0%. Moreover,the surface structure of azide-modified silica gel was evaluated with FT-IR instrument (Fig.2). The wave number of 2 112 cm-1indicated that the azide group was successfully bonded onto the surface of silica gel.

Fig.2 FT-IR spectrum of the azide-modified silica gel
2.2 Chromatography property of azide-modified stationary phase under HILIC mode
The azide-modified stationary phase might be applied under HILIC mode. To evaluate the retention properties of azide-modified stationary phase under HILIC mode,nucleosides with high polarity were selected as test compounds. It can be seen from Fig.3 that the retention factors (log k)of the four nucleosides decreased when the acetonitrile volume percentage decreased from 95% to 60%. The results indicated that the azide-modified stationary phase could be applied under HILIC mode. It can be seen from Fig.4 that the nucleosides could be well retained and isolated. The elution order of the test compounds is in accordance to that in the HILIC mode. Noticeably,the hydrophilic interaction of azide-modified stationary phase was relatively weaker compared with those of the other novel HILIC stationary phases such as Click TE-Cys[12,13]. The different surface structures contributed to the results.

Fig.3 Retention properties of the test compounds with different volume percentages of acetonitrile by using azide-modified stationary phase

Fig.4 Chromatogram of nucleosides on azide-modified silica gel under HILIC mode
The repeatability and stability of azide-modified stationary phase was also evaluated. After ten successive injections,the chromatograms were almost overlapping and the RSD of retention times was less than 2.4%. The results showed that the column has good repeatability. After washing with pure water for 72 h (1.0 mL/min),the test samples were reanalyzed with 8 h intervals and the retention times of nucleosides were almost remain stable,which indicated the good stability of the column.
2.3 Application of azide-modified stationary phase in glycopeptide enrichment
Since the silica based azide-modified stationary phase could provide several interactions,such as cation ion-exchange, dipolar, and hydrogen bonding interactions. The stationary phase was expected to be applied in glycopeptide enrichment under HILIC mode with suitable conditions. The influence of salt species was studied. Moreover,the glycopeptide enrichment selectivity under acid conditions was also studied.
In this investigation,the digest of HRP,which has nine potential glycosylation sites with hybridtype glycans,was selected as a model sample to study the glycopeptide enrichment selectivity of the azide-modified stationary phase. The glycopeptides in HRP digest have different peptide backbones,which are attached with similar glycans. Since the polarities of glycopeptides are higher than those of their counterparts,glycopeptides could be enriched under HILIC mode.
The performance of azide-modified silica gel in glycopeptide enrichment under HILIC mode was investigated. Ammonium bicarbonate and ammonium formate were added in the mobile phase for comparison. The difference between the two salts was the pH value in water solution. Water solution added with ammonium bicarbonate was prone to basic condition,while that with ammonium formate was prone to acid condition. The same concentration of salts (50 mmol/L)was added in the mobile phase,and the enrichment results were compared between the two different conditions. It can be seen in Fig.5 that the majority of glycopeptides were eluted in the ACN/50 mmol/L NH4HCO3(90 ∶10,v/v)fraction,such as glycopeptides of m/z 921.85 (2 +),1 202.49 (2+),1 224.53 (3 +),1 237.16 (3 +),1 386.09 (2+). Moreover,the signal of ACN/50 mmol/L NH4HCO3(90 ∶10,v/v)fraction was 2-10 times stronger than those of the other fractions,which indicated that most glycopeptides were not well retained on the azide-modified silica gel under the condition. Since the glycopeptides contain carboxyl groups,the glycopeptides exist with negative charges under basic condition. The glycopeptides in HRP digest will not be retained on the azide-modified silica gel under basic condition due to the cation-ion exchange interactions provided by the stationary phase.

Fig.5 Mass spectra of HRP digest after enrichment by azide-modified silica gel under HILIC mode

Fig.6 Mass spectra of HRP digest after enrichment by azide-modified silica gel under HILIC mode

Table 1 Reported glycopeptides found in HRP digest
It can be seen from Fig.6 that several glycopeptides were eluted in the ACN/50 mmol/L NH4FA (90 ∶10,v/v)fraction,such as the glycopeptides of m/z 921.89 (2 +),1 661.64 (3 +),1 836.14 (2+). The signals of the peptides in the ACN/50 mmol/L NH4FA (90 ∶10,v/v)fraction were stronger than those in the other fractions,which indicated that most peptides were eluted in the fraction. Noticeably,glycopeptides of m/z 1 224.55 (3+)and 1 237.20 (3+)were eluted in the ACN/50 mmol/L NH4FA (60 ∶10,v/v)fraction (Fig.6d),which were eluted in the ACN/50 mmol/L NH4HCO3(90 ∶10,v/v)fraction (Fig.5a). The different enrichment selectivity was attributed to the different salt species added in the mobile phase. Since the mobile phase with ammonium formate is prone to be more acidic than that with ammonium bicarbonate,the repulsive force between the glycopeptides and the matrix is weaker. Therefore,the acid condition is suitable for the glycopeptide enrichment on the azidemodified silica gel. Although some glycopeptides(m/z 1 224.55 (3 +),1 237.20 (3 +))were strongly retained on the stationary phase than their counterparts,glycopeptides of m/z 921.89(2+),1 661.64 (3+),1 836.14 (2+)were not retained when NH4FA added in the mobile phase.Therefore,it can be concluded that the salt added in the mobile phase did not benefit for the glycopeptide enrichment on azide-modified silica gel.The possible reason is that the ion-exchange interaction provided by the stationary phase is greatly reduced with the existence of the salt in the mobile phase.
Based on the above results,the enrichment process was operated under acidic condition(0.1% (v/v)formic acid)and no salt was added in the mobile phase. It can be seen from Fig.7 that the glycopeptides were not eluted in the ACN/0.1% (v/v)FA (90 ∶10,v/v)fraction,and high abundance non-glycopeptides could be removed from the glycopeptide fraction,such as peptides of m/z 958.99 (2 +),1 161.64 (1 +),1 185.57 (1+). Most glycopeptides were eluted in the ACN/0.1% (v/v)FA (70 ∶10,v/v)fraction,and nine glycopeptides were detected. Although glycopeptides of 1 301.63 (2 +),1 255.99 (4 +)were detected in other fractions,better glycopeptide selectivity and retention were obvious under the current separation conditions.
Accordingly,glycopeptides could be enriched on the azide-modified silica gel under HILIC mode with acid added in the mobile phase. Since the salt in the mobile phase could reduce the ion-exchange interaction between the glycopeptides and stationary phase,the glycopeptides tend to be coeluted with other peptides in the first fraction with salt added in the mobile phase. Moreover,acid in the mobile phase could enhance the ion-exchange interaction between glycopeptides and stationary phase.

Fig.7 Mass spectra of HRP digest after enrichment by azide-modified silica gel under HILIC mode
3 Conclusions
In the investigation,the chromatographic properties and application of silica based azide-modified stationary phase in glycopeptide enrichment under HILIC mode were studied. The stationary phase can provide ion-exchange,dipolar,and hydrogen bonding interactions due to the existing azide group on the surface of the silica gel. The retention properties of nucleosides on silica based azide-modified stationary phase were in accord with HILIC characterization. The glycopeptide enrichment selectivity was found to be high under HILIC mode under acidic condition without salt.
[1] Guo Z M,Lei A W,Zhang Y P,et al. Chem Commun,2007:2491
[2] Zhao Y Y,Guo Z M,Zhang Y P,et al. Talanta,2009,78:916
[3] Zhao Y Y,Yu L,Guo Z M,et al. Anal Bioanal Chem,2011,399:3359
[4] Zhao Y Y,Guo Z M,Li X L,et al,Chinese Journal of Chromatography,2013,31(8):763
[5] Zhao Y Y,Wang L H,Guo Z M,et al. Chem Res Chin Univ,2015,31(1):44
[6] Zhao Y Y,Li X L,Yan J Y,et al. Anal Methods,2012,4:1244
[7] Yu L,Li X L,Guo Z M,et al. Chem Eur J,2009,15:12618
[8] Yu L,Li X L,Dong J,et al. Anal Methods,2010,2:1667
[9] Li X L,Shen G B,Zhang F F,et al. J Chromatogr B,2013,941:45
[10] Guo Z M,Lei A W,Liang X M,et al. Chem Commun,2006:4512
[11] Larsen M R,Hojrup P,Roepstorff P. Mol Cell Proteomics,2005,4:107
[12] Shen A J,Guo Z M,Yu L,et al. Chem Commun,2011:4550
[13] Shen A J,Guo Z M,Cai X M,et al. J Chromatogr A,2012,1228:175

