Comparative study of adsorptive role of carbonaceous materials in removal of UV-active impurities of paclitaxel extracts☆
Jaber Nasiri,Elaheh Motamedi,Mohammad Reza Naghavi
Department of Agronomy and Plant Breeding,Division of Molecular Plant Genetics,College of Agricultural&Natural Resources,University of Tehran,Karaj,Iran
Short Communication
Comparative study of adsorptive role of carbonaceous materials in removal of UV-active impurities of paclitaxel extracts☆
Jaber Nasiri,Elaheh Motamedi*,Mohammad Reza Naghavi**
Department of Agronomy and Plant Breeding,Division of Molecular Plant Genetics,College of Agricultural&Natural Resources,University of Tehran,Karaj,Iran
A R T I c L E I N F o
Article history:
25 March 2015
Accepted 15 April2015
Available online 24 April 2015
Paclitaxel Reduced graphene oxide Graphite oxide Puri fi cation Carbon nanotube
Graphite oxide(GO)and reduced graphene oxide(rGO)nanosheets were synthesized with a low-cost manufacturing method.The morphology and structures of the synthesized samples were studied using X-ray diffraction(XRD),atomic force microscopy(AFM),Fourier-transform infrared(FTIR)and Raman spectroscopy.The ef fi ciencies of GO and rGO as novel candidate adsorbents in the pre-puri fi cation of paclitaxel were compared and contrasted with those of commercial graphite(Gt),graphene(G)and multi-wall carbon nanotube(MWCNT).According to UV–vis and HPLC analyses,rGO was evaluated as the best absorbent for the removal of impurities in pre-puri fi cation of paclitaxel from plant cell cultures. In contrast,the GO had the poorest pro fi ciency for paclitaxel pre-puri fi cation in comparison with the other carbonaceous adsorbents.This is attributed to the existence of many localized defects in theπstructure of GO that is related to weakness ofπ–πstacking interactions between crude extract impurities and GO.
©2015 Xi'an Jiaotong University.Production and hosting by Elsevier B.V.All rights reserved.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Paclitaxel,as a contemporary exciting plant-derived anticancer drug,was fi rst isolated from the inner bark of the Taxus brevifolia [1].Using high performance liquid Chromatography(HPLC)is essential to obtain high level of purity and yield of this drug.However,puri fi cation by HPLC has no effective resolving power so long as the feed materials are not previously enriched for paclitaxel[2]. Pre-puri fi cation procedures before fi nal HPLC runs often requires use of some adsorbents that improve purity of crude extracts.This increases the purity and yield and decreases the cost of the fi nal paclitaxel products.
On the other hand,graphite(Gt)is one of the naturally occurring crystalline forms of carbon used as industrial adsorbents. Carbon nanotubes(CNTs)are relatively new ef fi cient carbonaceous sorbents because of their large speci fi c surface area and hollow structure[3].Graphene(G)is a new member of the carbon family with a one-atom-thick sheet of honeycomb carbon lattice structure[4].Graphite oxide(GO)is a layered compound that can be synthesized by oxidizing graphite.The oxygenated functional groups on GO along with its large surface area indicate it can be used as an ef fi cient adsorbent[5].The chemical reduction of GO is a typical method for the production of reduced graphene oxide (rGO).However,few studies have been made for exploring the possibility of using this cost effective rGO as an adsorbent.
In this investigation,pro fi ciencies of fi ve different carbonbased adsorbents(i.e.rGO,GO,Gt,G and CNT)were evaluated in pre-puri fi cation of paclitaxel.We focused on comparing the effectiveness and removal ability of color and impurities from crude paclitaxel extract obtained from plant cell culture,for the above mentioned adsorbents.To the best of our knowledge,this is the fi rst report to compare the adsorptive role of carbonaceous materials in pre-puri fi cation of paclitaxel.
2.Experimental
Natural fl ake graphite was provided by Qingdao Dingding Graphite Products Factory(Shandong,China),Graphene and multi-wall carbon nanotube were purchased from US Research Nanomaterials,Inc.(Houston,USA).GO was prepared from natural graphite using modi fi ed Hummer’s method[6].For preparation of rGO,100 mg of GO in 100 mL of water was ultrasonicated for 30 min,to which ammonia solution was added to increase the pH up to 10 and then 1 mL of hydrazine hydrate was added and the solution was heated at 100°C for 24 h.After cooling the solution, the resulting black precipitates were centrifuged and washed three times with deionized(DI)water and fi nally dried at 60°C.
Fresh stems of Taxus baccata were collected from Botanical Garden of University of Tehran,Karaj,Iran.After sterilization,theexplants were cultured on a B5 medium and fi nally kept in a growth chamber at 24°C under dark condition[7].The pH of the medium was carefully adjusted to 5.8 prior to autoclaving.Calli cultures were transferred to a fresh B5 medium every 2 weeks[8]. Concisely,20 g of dry weight freeze-dried biomass was fi rst subjected to 800 mL of hexane at room temperature for 12 h.Then the mixture was centrifuged(5000 rpm)for 20 min and the supernatant was discarded.The pellet,in the following,was extracted with 400 mL of MeOH:CH2Cl2(1:1)by sonication for 1 h at room temperature,centrifuged at 5000 rpm for 20 min and the supernatant was transferred to the next glass tubes.The extracts were dried at 25°C under vacuum and redissolved in 200 mL of dichloromethane plus 200 mL of distilled water.Subsequent to vortexing for 10 s and centrifuging at 5000 rpm for 20 min,the organic fraction was isolated and dried under vacuum.This dark brown color dried crude extract was utilized as starting material for adsorbent treatments.
A stock solution of 1500 mg/L extract was prepared by dissolving of dried crude in dichloromethane.Since then,certain amounts of each adsorbent(i.e.rGO,GO,Gt,G and CNT)were added separately to this extract solution in 5 mL tubes.The mixture was shaken for 45 min at 40°C and then centrifuged.During adsorption treatment, the solution turned colorless.The decolorization ef fi ciency of adsorbents was investigated using a UV–vis spectrophotometer (Shimadzu UV-2100,Kyoto,Japan).Decolorization(%)was calculated from the total absorbance of solution in the visible range(400–800 nm)of UV–vis spectrum as Eq.(1):

where A0and Aeare the total absorbance of crude extract(from 400 to 800 nm)before and after treatment with adsorbent, respectively.
3.Results and discussion
3.1.Characterization of synthesized GO and rGO
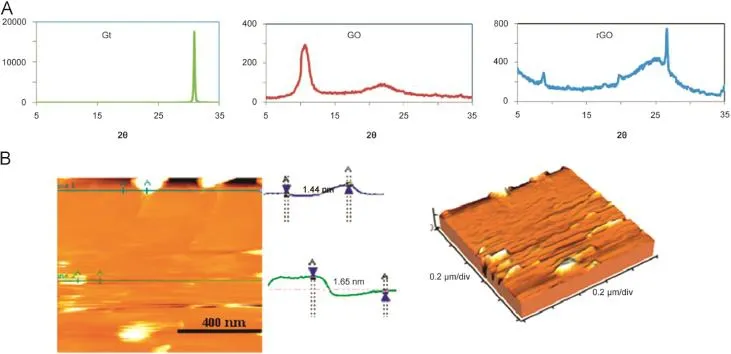
Fig.1.(A)XRD patterns of Gt along with synthesized GO and rGO.(B)AFM image of GO.
XRD(Philips Xpert MPD,Eindhoven,Netherlands),and AFM (VEECO CP-Research,New York,USA)analyses were used to confi rm the micro-structures and morphology of GO and rGO.XRD spectra obviously showed the oxidation of graphite and preparation of GO(Fig.1A).The diffraction peak of GO appeared at 10.58° which originated from the diffraction on its(0 0 2)layer planes with the basal spacing of 0.959 nm.This was along with a broad diffraction peak at 22.29 nm which was interpreted in terms of short-range order in stacked graphene sheets[9].The XRD pattern of rGO showed the broad peak at about 25°with interlayer spacing of approximately 0.378 nm(Fig.1A).This was much smaller than the 0.959 nm for GO,and was closer to the(0 0 2)graphite peak of 0.334 nm.The sharp peak at 2θ=26.6°indicated a highly organized crystal structure with an interlayer spacing of 0.339 nm, which was consistent with the layer spacing of normal graphite. Moreover,a weak peak at 2θ=8.7°,(d-spacing of 0.768 nm)appeared in the XRD pattern of rGO which was similar to the typical diffraction peak of GO.
AFM was used for quantifying the degree of exfoliation,thickness,and surface roughness of GO(Fig.1B).The samples were prepared by drop casting onto a silicon oxide wafer(0.05 mg/mL). AFM image revealed that isolated GO nanosheets were well exfoliated and dispersed with a thickness of about 1.5 nm for each.
In the FTIR(Thermo spectrometer,Madison,USA)spectrum of GO(Fig.2A),the peak at 1722 cm-1corresponded to the stretching band of C=O in carboxylic acid or carbonyl moieties.The intense band at 3426 cm-1was attributed to O–H stretching.The peak at 1638 cm-1(aromatic C=C)could be assigned to the skeletal vibrations of un-oxidized graphitic domains of GO and the deformation of the C–O was observed at 1191 cm-1[10].FTIR spectrum of rGO also con fi rmed the reduction of GO,as the carboxyl peak at 1722 cm-1disappeared entirely.In addition,the O-H(1432 cm-1)and the C-O stretching peaks decreased after the reduction treatment,indicating that most oxygenated functional groups in the GO were removed.The presence of phenol C=C ring stretching(1531 cm-1)was additional proof on the reduction of GO(Fig.2B)[11].Raman spectrum(Almega Thermo Nicolet,Madison,USA)of GO displayed two characteristic peaks, the D band around 1340 cm-1and the G band at 1584 cm-1(Fig.2C).The intensity ratio of D over G band(the R-value=ID/IG) was usually used as a measure of the degree of disorder and the average size of the sp2domain which was calculated to be 0.91from the spectrum[12].The Raman spectrum of rGO also contained both G and D bands(at 1565 and 1330 cm-1,respectively), but the intensity ratio of D over G band(1.80)increased compared to that in GO(Fig.2D).Maybe this was due to a decrease in the average size of the sp2domains upon GO reduction and production of new graphitic structures[12].
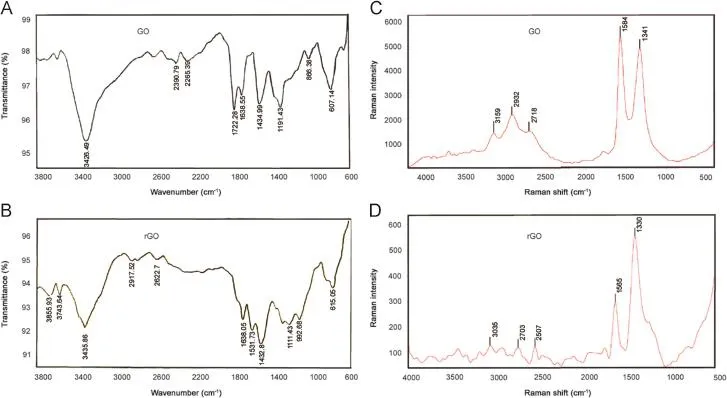
Fig.2.FTIR spectra of synthesized(A)GO and(B)rGO,Raman spectra of synthesized(C)GO and(D)rGO.
3.2.Selection of the best adsorbent
The removal performances of waxy compounds and plant pigments from crude paclitaxel extract,for fi ve candidate adsorbents(i.e.rGO,GO,Gt,G and CNT)were compared and contrasted,by batch sorption treatments.Table 1 summarizes the decolorization results for these adsorbents with various amounts (20 and 30 g/L)in dichloromethane as a solvent.
The UV–vis spectra of crude extract with light brown color showed the presence of carotenoids as plant pigments which are produced from eight isoprene molecules with large delocalizedπ electron system.Considering the structural features of these pigments as adsorbates and chemical structure and functionalities of carbonaceous hydrophobic adsorbents(i.e.rGO,CNT,G and Gt), the main plausible mechanism for decolorization of the crude extract wasπ–πelectron interactions taking place betweendelocalized and conjugated electrons of adsorbent andπelectron system of carotenoids[13].However,in the case of hydrophilic GO adsorbent,oxygen functionalities in GO surfaces made many localized defects in itsπ-structure;So,the poorer ef fi ciency of GO in comparison with the other adsorbents(i.e.G,rGO and CNT)may result from more weakness ofπ–πstacking interactions between carotenoids and GO.In contrast,chemical reduction of graphene oxide can restore the graphitic network in the basal plane of rGO. Consequently,on the basis of chemistry involving the adsorption treatment,π–πstacking interactions were occurred more effectively between rGO and adsorbate which was probably the reason for superiority of rGO over GO in decolorization of taxol crude extracts,particularly in dichloremethane.
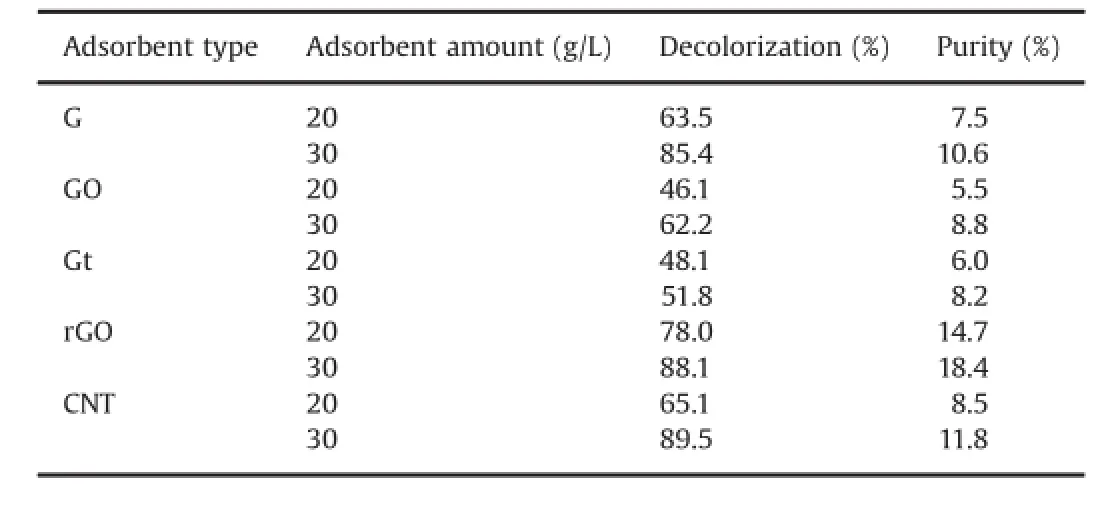
Table 1 The effect of adsorbent treatment on decolorization and purity of crude extract from plant cell culture in two different adsorbent amounts.
On the other hand,comparison between decolorization(%) results of rGO and commercial G and CNT displayed that in higher amounts(30 g/L)of adsorbent the decolorization ef fi ciency was almost the same for all three adsorbents,but in the lower dosages (20 g/L)the superiority of rGO over G and CNT was con fi rmed.This difference can be attributed to the availability of smaller surface area and fewer sorption sites in lower amounts of adsorbents. While,with the increasing adsorbent content,the number of available adsorption sites was expected to increase removal ef ficiency for all adsorbents.Furthermore,the purity of paclitaxel was evaluated by HPLC(Shimadzu 20AD,Kyoto,Japan),to determine the effectiveness of each adsorbent.Separation conditions involved C18column with mobile phase composition of water/acetonitrile(70:30,v/v)at fl ow rate of 1 mL/min,with an injection volume of 20μL.UV detector at wavelength of 227 nm was used for detection.Authentic paclitaxel(purity:97%)was purchased from Sigma-Aldrich and used as standard.HPLC analyses confi rmed the decolorization results and purity of paclitaxel decreased in the order of rGO(18.8%)>CNT(11.8%)>G(10.6%)>GO (8.7%)>Gt(8.8%)(Table 1).These results proved that adsorption treatments using these carbonaceous materials could be a suitable way to improve paclitaxel purity.Accordingly,treatment of crude with rGO resulted in more than four times of increase in the purity of paclitaxel from 3.9%to 18.8%.Therefore,from an economical perspective,rGO was eventually selected as the optimumadsorbent.Our fi ndings displayed promising applications of rGO, as a cost-effective nano-adsorbent,to provide a suitable vehicle toward improvement of paclitaxel pre-puri fi cation.
Acknowledgments
The project was fi nancially supported by Iran National Science Foundation(INSF,No.91058040).
References
[1]M.C.Wani,H.L.Taylor,M.E.Wall,et al.,Plant antitumor agents.VI.Isolation and structure of taxol,a novel antileukemic and antitumor agent from Taxus brevifolia,J.Am.Chem.Soc.93(1971)2325–2327.
[2]H.R.Jang,H.J.Oh,J.H.Kim,et al.,Synthesis of mesoporous spherical silica via spray pyrolysis:pore size control and evaluation of performance in paclitaxel pre-puri fi cation,Microporous Mesoporous Mater.165(2013)219–225.
[3]X.M.Ren,C.L.Chen,M.Nagatsu,et al.,Carbon nanotubes as adsorbents in environmental pollution management:a review,Chem.Eng.J.170(2011) 395–410.
[4]K.S.Novoselov,A.K.Geim,S.Morozov,et al.,Electric fi eld effect in atomically thin carbon fi lms,Science 306(2004)666–669.
[5]S.Stankovich,R.D.Piner,X.Chen,etal.,Stable aqueous dispersions ofgraphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate),J.Mater.Chem.16(2006)155–158.
[6]W.S.Hummers,R.E.Offeman,Preparation of graphitic oxide,J.Am.Chem.Soc. 80(1958)1339.
[7]O.L.Gamborg,R.A.Miller,K.Ojima,Nutrient requirements of suspension cultures of soybean root cells,Exp.Cell Res.50(1968)151–158.
[8]N.Tapia,A.Zamilpa,M.Bon fi ll,et al.,Effect of the culture medium and biotic stimulation on taxane production in Taxus globosa Schltdl in vitro cultures, Acta Physiol.Plant.35(2013)3447–3455.
[9]C.Nethravathi,M.Rajamathi,Chemically modi fi ed graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide,Carbon 46(2008)1994–1998.
[10]S.Stankovich,R.D.Piner,S.T.Nguyen,et al.,Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets,Carbon 44(2006)3342–3347.
[11]M.El Achaby,F.Z.Arrakhiz,S.Vaudreuil,et al.,Piezoelectricβ-polymorph formation and properties enhancement in graphene oxide–PVDF nanocomposite fi lms,Appl.Surf.Sci.258(2012)7668–7677.
[12]A.Ferrari,J.Robertson,Interpretation of Raman spectra of disordered and amorphous carbon,Phys.Rev.B 61(2000)14095–14098.
[13]M.R.Naghavi,E.Motamedi,J.Nasiri,et al.,Evaluation of magnetic-and carbon-based nano-adsorbents application in pre-puri fi cation of paclitaxel from needles of Taxus baccata,J.Nanopart.Res.17(2015)17.
24 January 2015
in revised form
☆Peer review under responsibility of Xi'an Jiaotong University.
.Tel.:+98 2612824809;fax:+98 2612224809.**
.Tel.:+98 2612227609;fax:+98 2612227608.
E-mail addresses:motamedi.elaheh@gmail.com(E.Motamedi),
mnaghavi@ut.ac.ir(M.Reza Naghavi).
 Journal of Pharmaceutical Analysis2015年6期
Journal of Pharmaceutical Analysis2015年6期
- Journal of Pharmaceutical Analysis的其它文章
- Application of analytical instruments in pharmaceutical analysis
- In vitro–in vivo studies of the quantitative effect of calcium, multivitamins and milk on single dose cipro fl oxacin bioavailability☆
- Optimization,validation and application of an assay for the activity of HMG-CoA reductase in vitro by LC–MS/MS☆
- Antimicrobial and antiproliferative prospective of kosinostatin–a secondary metabolite isolated from Streptomyces sp.☆
- Quanti fi cation of tolvaptan in rabbit plasma by LC–MS/MS:Application to a pharmacokinetic study☆
- Identi fi cation,synthesis and characterization of process related des fl uoro impurity of ezetimibe and HPLC method validations☆
