Clinical Characteristics and Outcome of Gleason Score 10 Prostate Cancer on Core Biopsy Treated by External Radiotherapy and Hormone Therapy
Zhi-peng Mai, Wei-gang Yan*, Han-zhong Li, Zhi-gang Ji, Fu-quan Zhang, Ke Hu, and Yu Xiao
1Department of Urology,2Department of Radiation Oncology,
3Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
ORIGINAL ARTICLE
Clinical Characteristics and Outcome of Gleason Score 10 Prostate Cancer on Core Biopsy Treated by External Radiotherapy and Hormone Therapy
Zhi-peng Mai1, Wei-gang Yan1*, Han-zhong Li1, Zhi-gang Ji1, Fu-quan Zhang2, Ke Hu2, and Yu Xiao3
1Department of Urology,2Department of Radiation Oncology,
3Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
prostatic neoplasms; biopsy; neoplasm grading; outcomes
Objective To evaluate the clinical characteristics and outcomes of patients with Gleason score 10 prostate cancer treated by external radiotherapy and hormone therapy.
Methods From January 2003 to March 2014, 1832 patients with prostate cancer were treated, among which 9 patients (represented 0.49%) were identified as Gleason score 10 disease on prostate core biopsy without distant metastases when first diagnosed. All 9 patients were treated by whole pelvic external radiotherapy (The whole pelvic dose was 50.0 Gy and the boost dose ranged from 76.2 to 78.0 Gy) and long-term hormone therapy. We assessed the clinical characteristics, treatment outcomes and treatment toxicities. Survival curves were calculated using the Kaplan-Meier method.
Results The median follow-up was 4.8 years. Six patients’ pre-treatment prostate-specific antigen (PSA) levels were lower than 20.0 μg/L and three patients’ pre-treatment PSA levels were higher than 70.0 μg/L. The median percentage of positive biopsy cores was 91%. Three, four and two cases were classified as T2c, T3a and T3b stage, respectively. Three cases were assessed as N1 stage. The 5-year biochemical failure-free survival, distant metastasis-free survival, cancer specific survival and overall survival rates were 28.6%, 57.1%, 66.7% and 57.1%, respectively. Five patients experienced grade 1-2 acute gastrointestinal toxicities and six patients complained of grade 1-2 acute genitourinary toxicities. No bone fracture or cardiovascular disease was detected.
Conclusions Gleason score 10 prostate cancer on core biopsy is usually combined with other high risk factors. The pre-treatment PSA levels lie in two extremes. Timely and active treatments are urgent needed because unfavourable oncological outcomes are often presented.
Chin Med Sci J 2015; 30(2):90-94
BASED on the sum of major and minor patterns and ranged from 1 to 5, the Gleason grade serves as one of the main components in classifying prostate cancer (Pca) patients for therapy.1 Higher Gleason scores usually indicate poor oncological outcomes because of the aggressive biologica potential of the disease. In most cases, Gleason score 8-10 were classified as a single prognostic group.2-4 However recent studies have revealed that Gleason score 8, 9, or 10 may be separated from each other in outcome prediction.5, 6 Gleason score 10, as pure pattern 5 to yield a Gleason score 5+5, is the rarest and most poorly differentiated type of Gleason grade.7 To our knowledge, the results o external radiotherapy combined with hormone therapy have not been independently reported for this group o patients.
In this study, we aimed to evaluate the clinica characteristics and outcomes of patients with Gleason score 10 disease on prostate core biopsy without distant metastases when first diagnosed treated by whole pelvic external radiotherapy and long-term hormone therapy The treatment outcomes included biochemical failure-free survival (BFFS), distant metastasis-free survival (DMFS) cancer specific survival (CSS) and overall survival (OS) The combined treatment toxicities were also observed.
PATIENTS AND METHODS
Patient’s selection
From January 2003 to March 2014, 1832 patients with Pca were treated at Peking Union Medical College Hospital. Finally, 13 patients were identified as Gleason score 10 disease on prostate core biopsy. Distant metastases were assessed based on the serum levels of skeletal alkaline phosphatase, Magnetic Resonance Imaging (MRI) and bone scan. And 4 patients were excluded because bone metastases were confirmed when first diagnosed. The remaining 9 patients represented 0.49% of the total amount of Pca. The International Society of Urological Pathology 2005 Modified Gleason score was used for neoplasm grading. The Gleason score is the sum of the two most common patterns (grades 1-5) of tumor growth found. The Gleason score ranges between 2 and 10, with 2 being the least aggressive and 10 the most aggressive.
Treatment
Hormone therapy was used once one patient was first diagnosed as Pca on biopsy. The treatment protocol was maximum androgen blockade, which consisted of a luteinizing hormone-releasing hormone agonist in conjunction with an antiandrogen (bicalutamide 50 mg daily).
Follow-up
Patients were monitored using the digital rectal examination, serum prostate-specific antigen (PSA) and testosterone measurement monthly for 3 months, every 3 months over the rest of the first two years, then every 6 months thereafter. If tumor progression was detected, the interval of follow-up was shortened.
The endpoints of this analysis included BFFS, DMFS, CSS and OS. The treatment toxicities were observed. According to the American Society for Therapeutic Radiology and Oncology criteria,8the definition of biochemical failure was: an increase of 2 μg/L after the nadir had been reached. Distant metastasis was defined based on the serum levels of skeletal alkaline phosphatase, MRI and bone scan. Patients dying in the setting of metastatic disease were classified as Pca-specific death.
Statistical analysis
Statistical analysis was performed with the Statistical Package for Social Sciences, version 19.0 (SPSS, Chicago, IL, USA) software. The median and the range were used to demonstrate the central tendency and the variability. Survival curves were calculated using the Kaplan-Meier method. Factor analysis could not be expressed for the small sample size.
RESULTS
Clinical characteristics at baseline
The median age of patients was 72 (range 64-85) years and the median prostate volume was 43.2 (range25.6-60.1) ml. Nodules could be detected upon digital rectal examination, ultrasound or MRI for all patients. Four (44%) cases were found to have extracapsular extension when diagnosed and were classified as T3a stage. Two (22%) cases were assessed as T3b with tumor invading seminal vesicles. The remaining three (33%) cases were identified as T2c stage. Three (33%) cases were estimated as N1 because regional nodal invasions were found on CT or MRI. Nine (67%) cases were classified as M0 without positive evidence of distant metastasis. The pre-treatment PSA levels lied in two extremes. Those lower than 20.0 μg/L were 6.6, 7.0, 7.3, 9.1, 11.2 and 15.6 μg/L, respectively. The others higher than 70.0 μg/L were 77.4, 100.3 and 112.8 μg/L, respectively. High volume involved by Pca in this cohort with median 91% (range 45%-100%) of positive biopsy cores.
Outcome
With a median follow-up of 4.8 years, the 5-year BFFS, DMFS, CSS and OS rates for the entire cohort were 28.6%, 57.1%, 66.7% and 57.1%, respectively. Six patients experienced biochemical failure and the median biochemical failure time was 36 months. Five patients further developed bone metastatic Pca. Of all the patients, 3 died of advanced Pca and 1 passed away because of cerebral hemorrhage during the follow-up period. Since initial diagnosis, the death times were 26, 41, 57 and 72 months, respectively. The survival curve of BFFS, DMFS and CSS are depicted in Figs. 1-3.
Treatment toxicity
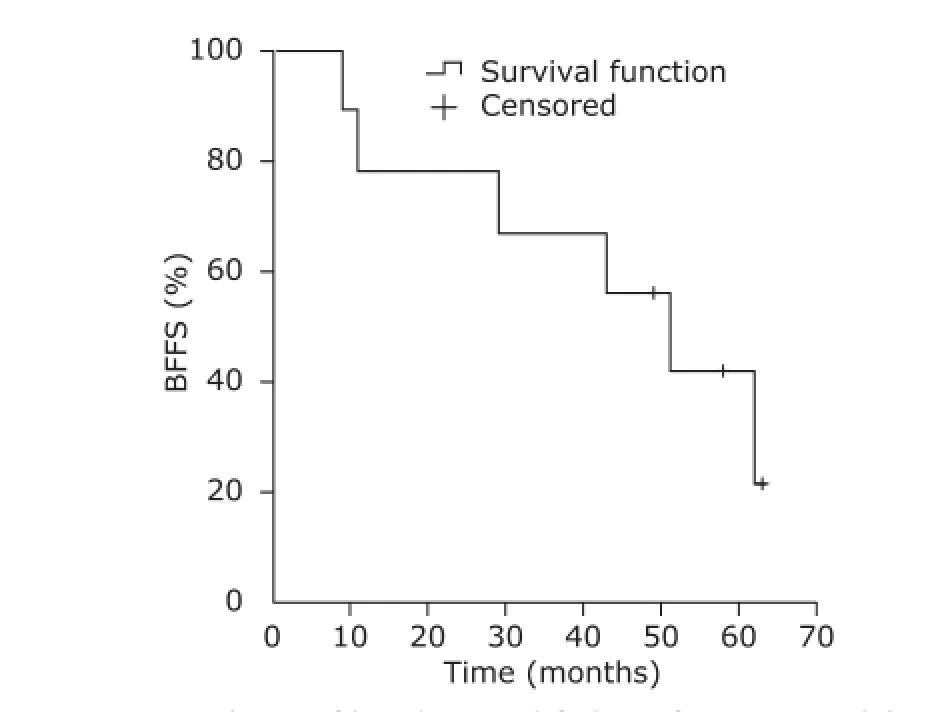
Figure 1. Analysis of biochemical failure-free survival (BFFS).

Figure 2. Analysis of distant metastasis-free survival (DMFS).

Figure 3. Analysis of cancer specific survival (CSS).
Five (56%) patients experienced grade 1-2 acute gastrointestinal (GI) toxicities. The symptoms were rectal discomfort (n=4), diarrhea (n=2) and hemafecia (n=1). Six (67%) patients complained of grade 1-2 acute genitourinary (GU) toxicities. The symptoms included increased urinary frequency (n=5), urgency (n=2), odynuria (n=4) and hematuria (n=1). None of the patients experienced grade 3 or higher acute GI and GU toxicities. No late GI and GU toxicities of any grade were found among all the patients during the follow-up period. Four (44%) patients complained of loss of libido, mammary development or breast pain. Five (56%) patients experienced hot flashes. No bone fracture or cardiovascular disease was detected.
DISCUSSION
The prostate needle biopsy tissue of Gleason pattern 5 shows little to no glandular differentiation and is composed of single cells, cords, small nests and solid sheets.7Although the biologic mechanism remains unknown, high-risk Pca with any component of Glesaon pattern 5 disease predicts an increased risk of disease progression. Sabolch et al9demonstrated that prostate adenocarcinoma with Gleason pattern 5 behaved worse. In their study, rates of 8-year freedom from metastasis and CSS with and without Gleason pattern 5 were 61% and 55%, 89% and 98%, respectively. Nanda et al1also found patients withGleason pattern 5 indicated shorter time to PSA failure. They reported the median time to PSA failure for patients with biopsy Gleason score 8 or Gleason score 9-10 were 5.4 years or 4.5 years.
Gleason score 10, as pure pattern 5 to yield a Gleason score 5+5, is the rarest type of Gleason grade.7Traditionally, Gleason score 8-10 were classified as a single prognostic group. However, recent studies have revealed that Gleason score 8, 9, or 10 may be separated from each other in outcome prediction. Stock et al5assigned a combination of brachytherapy, external radiotherapy and hormonal therapy for patients and reported the 8-year freedom from biochemical failure rates were 84%, 55% and 30% for scores of 8, 9 and 10, respectively. The corresponding freedom from distant metastases and prostate-cancer specific survival rates were 86%, 76%, 30% and 92%, 80%, 62.5%, respectively. Ellis et al6pointed out that Gleason score 9-10 on needle core biopsy could serve as the highly select subset for significant predictors of radical prostatectomy outcome.
Gleason score is a powerful parameter for predicting outcomes in patients with adenocarcinoma of the prostate.10Patients with high Gleason score are considered to be at high risk for poor oncological results.11Men with high grade Pca, especially those with Gleason 9-10 on biopsy usually have occult micrometastatic or advanced clinical disease at presentation. The optimum treatment strategy remains controversial. Generally, this series of patients are recommended to undergo radiation therapy combined with hormone therapy.12Radical prostatectomy is suitable for only a few highly selected patients. For example, Ellis et al6reported a group of patients with favourable clinical characteristics treated by radical prostatectomy. Their selection bias was evidenced by Gleason score 9-10 in only a mean of 2 cores with a mean of 56% involvement of the most involved core. The mean serum pre-PSA level was 8.9 μg/L.
Traditionally, standard dose external radiotherapy combined with hormone therapy had been accepted for high risk Pca.13,14Given a high risk for biochemical failure and cancer specific death, escalated doses of external radiotherapy can be considered. Zelefsky et al15analysed the radiation dose on treatment outcome in localized Pca patients. They found 10-year PSA relapse-free survival rate was 55% and 41% for high risk patients treated with escalated doses and with lower doses, respectively. Higher radiation dose levels were consistently associated with improved biochemical control outcomes and reduction in distant metastases. The role of hormone therapy in high risk Pca treated with escalated doses of external radiotherapy is not clearly established. Bolla et al16showed that radiotherapy combined with 6 months hormone therapy provides inferior survival as compared with radiotherapy combined with long-term hormone therapy. Zelefsky et al15also recommended long-term hormone therapy in conjunction with escalated doses of external radiotherapy for high risk patients.
Our study showed several clinical features in patients with Gleason score 10 on core biopsy without distant metastases when first diagnosed. Firstly, the pre-treatment PSA levels lied in two extremes. In our cohort, 6 patients’PSA levels were lower than 20 μg/L and the median were 8.2 μg/L. Contrarily, the remaining 3 patients’ PSA levels were higher than 70 μg/L and the median were 100 μg/L. It may be explained that a proportion of these prostate tumor was so poorly differentiated that the epithelial cells lose expression of a PSA encoding gene.17Secondly, high volume involved by carcinoma in this group of patients with median 91% of positive biopsy cores. The finding was consistent with the pathological results of Gleason pattern 5 reported by Humphrey.7The study revealed that high grade Gleason patterns were characterized by typically extensive involvement of needle core tissue and multiple cores involved by carcinoma.
In our cohort, all the patients were treated by whole pelvic radiotherapy and long-term hormone therapy. The median prescription dose was 81.0 Gy. The 5-year BFFS, DMFS, CSS and OS rates were 28.6%, 57.1%, 66.7% and 57.1%, respectively. To our knowledge, one report by Inman et al18was found to evaluate the outcomes of Gleason score 10. In their study, all patients underwent radical prostatectomy and had postoperative pathologic Gleason score 10. The 5-year BFFS and CSS rates were 53.8% and 76.9%, respectively. Because of small sample sizes, it is not surprising to find the differences in treatment outcomes between the two studies. Nevertheless, both studies demonstrated the treatment outcomes were not so optimistic which mainly accounted for the aggressive potential of the disease.
In conclusion, Gleason score 10 Pca on core biopsy is often combined with other high risk factors. The pretreatment PSA levels lie in two extremes. Because of the aggressive potential of the disease, timely and active treatments are urgent needed.
REFERENCES
1. Nanda A, Chen M, Renshaw A, et al. Gleason pattern 5 prostate cancer: Further stratification of patients with high-risk disease and implications for future random-ized trials. Int J Radiat Oncol Biol Phys 2009; 74: 1419-23.
2. McGuire BB, Helfand BT, Loeb S, et al. Outcomes in patients with Gleason score 8-10 prostate cancer: Relation to preoperative PSA level. BJU Int 2012; 109: 1764-9.
3. Krauss DJ, Hayek S, Amin M, et al. Prognostic significance of neuroendocrine differentiation in patients with Gleason score 8-10 prostate cancer treated with primary radiotherapy. Int J Radiat Oncol Biol Phys 2011; 81:119-25.
4. Serni S, Masieri L, Minervini A, et al. Cancer progression after anterograde radical prostatectomy for pathologic Gleason score 8 to 10 and influence of concomitant variables. Urology 2006; 67:373-8.
5. Stock R, Cesaretti J, Hall S, et al. Outcomes for patients with high-grade prostate cancer treated with a combination of brachytherapy, external beam radiotherapy and hormonal therapy. BJU Int 2009; 104:1631-6.
6. Ellis CL, Partin AW, Han M, et al. Adenocarcinoma of the prostate with Gleason score 9-10 on core biopsy: Correlation with findings at radical prostatectomy and prognosis. J Urol 2013; 190:2068-73.
7. Humphrey PA. Gleason pattern 5 adenocarcinoma in prostate needle biopsy. J Urol 2012; 188:1341-2.
8. Consensus Consensus statement: Guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys 1997; 37:1035-41.
9. Sabolch A, Feng F, Daignault-Newton S, et al. Gleason pattern 5 is the greatest risk factor for clinical failure and death from prostate cancer after dose-escalated radiation therapy and hormonal ablation. Int J Radiat Oncol Biol Phys 2011; 81:351-60.
10. D'Ambrosio DJ, Hanlon AL, Al-Saleem T, et al. The proportion of prostate biopsy tissue with Gleason pattern 4 or 5 predicts for biochemical and clinical outcome after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2007; 67:1082-7.
11. Lughezzani G, Gallina A, Larcher A, et al. Radical prostatectomy represents an effective treatment in patients with specimen-confined high pathological Gleason score prostate cancer. BJU Int 2013; 111: 723-30.
12. Pahlajani N, Ruth KJ, Buyyounouski MK, et al. Radiotherapy doses of 80 Gy and higher are associated with lower mortality in men with Gleason score 8 to 10 prostate cancer. Int J Radiat Int J Radiat Oncol Biol Phys 2012; 82:1949-56.
13. D'Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs. radiation alone for prostate cancer: A randomized trial. JAMA 2008; 299:289-95.
14. Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase ⅢRTOG 85-31. Int J Radiat Oncol Biol Phys 2005; 61:1285-90.
15. Zelefsky MJ, Pei X, Chou JF, et al. Dose escalation for prostate cancer radiotherapy: Predictors of longterm biochemical tumor control and distant metastases-free survival outcomes. Eur Urol 2011; 60: 1133-9.
16. Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 2009; 11:2516-27.
17. Weir EG, Partin AW, Epstein JI. Correlation of serum prostate specific antigen and quantitative immunohistochemistry. J Urol 2000; 163:1739-42.
18. Inman BA, DiMarco DS, Slezak JM, et al. Outcomes of Gleason score 10 prostate carcinoma treated by radical prostatectomy. Urology 2006; 68:604-8.
for publication January 3, 2015.
Tel: 86-10-69152520, E-mail: yanwg111@126.com
All the selected patients whole pelvic intensitymodulated radiotherapy. Computed tomography (CT) simulation was performed with the patients in the prone position. The CT scan was from the second lumbar vertebrae to 10 cm below the pubic symphysis at 5 mm intervals. Patients were required to void the bowel and bladder before simulation and each treatment session. The primary clinical target volume included the whole prostate, seminal vesicles, internal iliac, external iliac, and obturator nodal regions and the planning target volume was a margin of 1.0 cm to the block edge. The treatments utilized 6MV 4-field or 5-field intensity-modulated radiotherapy technique. The whole pelvic dose was 50.0 Gy. The boost treatment included the whole prostate, seminal vesicles and metastatic regional lymph nodes. The boost dose ranged from 76.2 to 78.0 Gy. The treatment was administered in 1.8-2.0 Gy fractions.
 Chinese Medical Sciences Journal2015年2期
Chinese Medical Sciences Journal2015年2期
- Chinese Medical Sciences Journal的其它文章
- Relationship between Ulcerative Colitis and Lung Injuries△
- Evaluation of Tubal Patency with Transvaginal Threedimensional Hysterosalpingo-contrast Sonography△
- Perinipple Broken Line Incision: a Novel Approach for Breast Augmentation
- Impact of Laparoscopic Versus Open Hepatectomy on Perioperative Clinical Outcomes of Patients with Primary Hepatic Carcinoma
- Effect of Neoadjuvant Chemotherapy Treatment on Prognosis of Patients with Advanced Gastric Cancer: a Retrospective Study△
- Assessment of Stroke Volume Variation Perioperatively by Using Arterial Pressure with Cardiac Output
