Biocoagulation of Direct Red-31 and Direct Yellow-12 Azo Dyes from Synthetic Wastewater with Moringa Oleifera Seed Presscake
LU Zhi-li (鲁智礼),DENG Jian-mian (邓建绵),ZHANG Er-fei (张二飞),HAO Ya-ru (郝亚茹),HUANG Jian-ping (黄健平),LI Chong (李 翀),TIE Jing-xi (帖靖玺)*
1 School of Environmental and Municipal Engineering,North China University of Water Resources and Electric Power,Zhengzhou 450011,China
2 School of Environmental and Municipal Engineering,Xi'an University of Architecture and Technology,Xi'an 710055,China
Introduction
About 10 000 commercial dyes and pigments are used around the world,and the global annual production of dyes is more than 8×105tons[1-2].It was estimated that about 10%-15% of the dyes are released into the effluents during dyeing processes[3],and 17%-20% of industrial water pollution is attributed to textile dyeing and treatments[4].The occurrence of dyes in the aquatic system is very hazardous because of their carcinogenic,mutagenic,toxic,and allergenic nature[5].Hence,it is of great importance to remove dyes from industrial wastewaters.
Biological processes are inefficient for dye-containing wastewater treatment due to their recalcitrant and inhibitory nature resulting from their complex aromatic structures[6-9].Hence, physico-chemical treatment technologies including membrane filtration[10], ion-exchange[11], advanced oxidation[12-13],adsorption[14-15]and coagulation[16-18]are widely used for dye based effluents treatment.Among these techniques,coagulation is very attractive because of its low cost,easy operation and high efficiency[19-20].Now,synthetic coagulants are widely used for water treatment,but some coagulants have caused anxiety because of their potential impact on human health.For example,aluminum salt coagulants have the risk of causing Alzheimer's disease[17].Hence,there is an increasing demand for efficient and safe coagulant.Coagulants extracted from natural products can serve as alternatives to the synthetic coagulants because they are biodegradable and eco-friendly.
As a tropical plant,Moringa oleifera (MO)belongs to the family of Moringaceae.It has been reported that Moringa oleifera seed (MOS)was used as a natural coagulant to remove many pollutants including heavy metals[21-24], Escherichia coli[25],algae[26-27]and surfactants[28]from water.The proteins in MOS have been proved to be the main active agents for water purification,and the most likely mechanisms involved in the coagulation activity is adsorption and neutralisation of charges[29].
So far,there is little information about the use of MOS for dye removal.Hence,the purpose of the study is to explore the dye removal from its aqueous solution using MOS presscake(MOSP),the residual solids of MOS after oil extraction as natural coagulant.Two direct azo dyes,Direct Red-31 (DR-31)and Direct Yellow-12 (DY-12),were selected as model dyes because azo dyes account for 70 percent of total dye consumption,which makes them the most representative and largest class of dyes used in industries[30-31].The effect of various factors like coagulant dose,pH,temperature inorganic salts,and suspended solid were investigated.The results may provide new insight into both MOSP reuse and development of new coagulant for dye removal.
1 Experimental
1.1 Preparation of coagulants
The MOSP used in this study was supplied by Yunnan Moringa oleifera Bio-Technique Co.Ltd.The MOSP was crushed and sieved through 0.45 mm screen.The powder was used as natural coagulant.
1.2 Preparation of artificial wastewater
The solutions of the two azo dyes (Table 1)used in the experiments were prepared by adding a certain amount of the two dyes (produced by Jufaxiang chemical company,Tianjin,China)into distilled water.The pH of the solutions was adjusted by 0.1 mol·L-1KOH and 0.1 mol·L-1HCl solutions,and measured by a pH meter (pHS-3C,Leici Ltd.,China).The solution temperature was determined by a mercury thermometer.All other reagents used in the study were of analytical grade.

Table 1 Information of the two dyes
1.3 Coagulation experiments
Coagulation experiments were carried out on a Jar-Test apparatus (ZR4-6, Zhongrun water industry technology development Co.,Ltd.,Shenzhen,China).After the prepared coagulant was added into 200 mL of the solution,the solution was rapidly mixed (120 r·min-1)for 1 min and followed by slow mixing (45 r·min-1)for 20 min[20].The mixture was then allowed to settle for 30 min.Sample about 10 mL was collected from 2 cm below the water surface using a syringe.The sample was then rapidly centrifuged at 4 000 r·min-1for 15 min.The residual dye concentration in the supernatant was determined by a spectrophotometer (UVmini-1240,Shimadu,Japan)at the wavelength shown in Table 1.The dye removal rate was calculated from the following equation:

where C0and Ceare the initial and final concentrations of dye in solution (mg·L-1),respectively.
1.4 Infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR) was employed to characterize MOSP.Potassium bromide pellets were prepared by mixing 1 mg of dried MOSP with 200 mg of KBr (spectrometry grade)and then pressing at 10 000 kg/cm2for 30 min under vacuum.The FTIR spectra were recorded on a Nicolet 5 700 FTIR meter from 4 000 to 400 cm-1.
2 Results and Discussion
2.1 Infrared spectroscopy
The FTIR technique was employed to identify the main functional groups of the MOSP and Fig.1 shows the FTIR spectrum.It can be seen that the broad band centered at 3 300 cm-1is related to OH stretching.The functional group comes mainly from the protein and fatty acid structures of the MOSP.NH stretching (amide A)at band 3 300 cm-1makes another contribution in this region due to the high content of protein in the MOSP[32-33].The peaks at 2 926 and 2 855 cm-1are attributed to symmetrical and asymmetrical C—H stretching of the CH2group in fatty acids.The band at 1 657 cm-1is attributed to the CO stretching (amideⅠ).The band at 1 543 cm-1is related with CN stretching and NH bending (amide II).The bands at 1 236 cm-1is corresponded to the CN stretching (amide III),and the band at 644 cm-1is attributed to OCN bending (amide IV).The bands at 592 cm-1is related to CO out-of-plane bending (amide VI).The existence of the bands proves the protein structures of the MOSP.The proteins in MOS have been proved to be an efficient coagulant for turbidity removal from water[33].

Fig.1 FTIR spectrum of MOSP
2.2 Effect of temperature
The effect of solution temperature on the two dyes' removal is shown in Fig.2.The removal rate increased from 33.9% to 95.4% for DR-31 and from 23.2% to 93.8% for DY-12 as the temperature increased from 11 to 25℃.The results indicated that higher temperature was favorable for the two dyes'removal.The probable reason might be the following aspects:(1)the viscosity of the solution increased as the temperature decreased and the floc settlement was slowed down by the high viscosity of the solution at low temperature,resulting in poor dye removal[34];(2)the aggregation rate of the particles in solution was weakened by low temperature,leading to a poor dye removals[34];(3)furthermore,some particles of MOSP were observed to cohere together to form pastes at low temperature.The pastes decreased the total surface areas for the electrostatic adsorption of the two dyes,which in turn led to the poor performance of dye removal.

Fig.2 Influence of temperature on coagulation removal of the two dyes (pH:7,T:25℃,MOSP dosage:250 mg·L -1 for DR-31 and 350 mg·L -1 for DY-12,C0:50 mg·L -1)
2.3 Effect of pH
The effect of pH on coagulation of DR-31 and DY-12 by MOSP were studied in the pH range of 3.0-11.0 (Fig.3).It can be seen that the removal rates of the two dyes decreased with increasing pH.The removal rates decreased from 98.1%and 99.0% at pH 3.0 to 93.4% and 77.1% at pH 9.0 for DR-31 and DY-12,respectively.The respective removal rate dramatically drops to 20.8% and 1% when pH further increased to 11.0.The result indicated that the coagulation removal of the two dyes was a pH dependent process,and lower pH was favorable for their removal due to the fact that the pH of the solution could influence both the properties of MOSP and the and dissociation of the dye molecules.There are four basic mechanisms of classic coagulation theory,namely,double layer compression,adsorption and charge neutralization,adsorption and bridging, and colloid entrapment.The most likely mechanism involved in the removal of the two azo dyes by MOSP was adsorption and charge neutralization.It can be explained by the fact that the water-soluble cationic proteins in the MOSP have isoelectric points between pH value of 10.0 and 11.0[29].Hence,the MOSP surface was positively charged at pH values from 3.0 to 9.0.As a result,the electrostatic interaction between the positively charged MOSP and the negatively charged anionic dyes took place.The dye molecules were first adsorbed onto the surfaces of the MOSP,and then the flocs that formed using the MOSP particles as nuclei were settled down by gravity.The reaction was strengthened when pH decreased,leading to higher removal efficiencies of the two dyes.In contrast,the MOSP surface became negatively charged at pH 11.0,so the competition effects between the OH-ions and DY-12 or DR-31 anions and also the electrostatic repulsion between the MOSP surface sites and the dye molecules increased,resulting in decrease of removal of DY-12 or DR-31.

Fig.3 Influence of initial pH on coagulation removal of the two dyes (T:25℃,MOSP dosage:250 mg·L -1 for DR-31 and 350 mg·L -1 for DY-12,C0:50 mg·L -1)
2.4 Effect of MOSP dosage
The effect of MOSP dosage on the two dyes removal is shown in Fig.4.At the initial concentration of 30 mg·L-1,the DR-31 removal increased from 30.0% to 96.7% and the DY-12 removal increased from 19.6% to 90.1% as the MOSP dosage increased from 100 to 400 mg · L-1.The DR-31 removal increased from 6.7% to 97.0% and the DY-12 removal increased from 11.8% to 97.7% as the MOSP dosage increased from 50 to 100 mg·L-1at the initial concentration of 50 mg·L-1.The results indicated better dye removal occurred at larger MOSP dosage at a fixed dye concentration due to the fact that the increasing MOSP dosage provided larger surface area,more active sites and soluble proteins.

Fig.4 Influence of MOSP dosage on coagulation removal of the two dyes (pH:7,T:25℃)
2.5 Effect of inorganic salts
Inorganic salts are often used to improve the dyeing property in the dyeing process.Hence,the industrial dyeing effluents contain various salts.A quantity of 1 g·L-1of salt was added to the dye solution to investigate their influence on dye removal.It can be seen from Fig.5 that all the three sodium salts improved the two dyes' removal,and the improvement for DR-31 was more significant than that for DY-12.The salting-in effect increased the protein-protein dissociations,resulting in increasing release of the proteins acting as natural coagulant from the MOSP particles,which in turn led to more chance for the coagulating reaction.Okuda et al.examined the turbidity removal efficiencies by using Moringa oleifera coagulant extracted by distilled water (MOCDW)and 1.0 mol·L-1NaCl solution (MOC-SC).The results indicated that the efficiency of MOC-SC was 7.4 times better than that of MOC-DW[35].

Fig.5 Influence of inorganic salts on coagulation removal of the two dyes (T:25℃,MOSP dosage:225 mg·L -1 for DR-31 and 275 mg·L -1 for DY-12,C0:50 mg·L -1)
2.6 Effect of suspended solid
Apart from inorganic salts, the effluents from dyeproducing industries also contain various types of suspended solids.To investigate the effect of suspended solids on dye removal,kaolin was used to simulate the suspended solid in the effluent.It can be seen from Fig.6 that suspended solid simulated by kaolin at two dosages decreased removal rates of the two because the kaolin particles were also removed by the MOSP coagulation,which decreased the opportunity of reaction between the dye molecules and the MOSP.
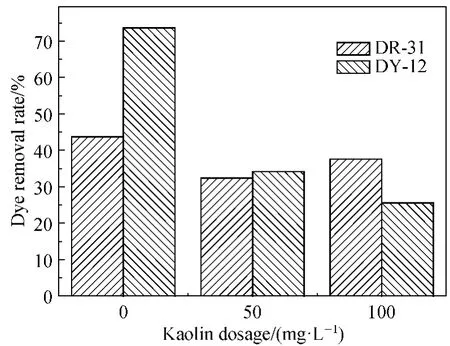
Fig.6 Influence of suspended solid on coagulation removal of the two dyes (T:25℃,MOSP dosage:225 mg·L -1 for DR-31 and 275 mg·L -1 for DY-12,C0:50 mg·L -1)
3 Conclusions
The present work investigated the removal characteristics of DR-31 and DY-12 from water by using MOSP.The FTIR result idicated the existence of cationic proteins in MOSP which were active agents acting as natural coagulant for dye removal.The combined effects of adsorption and neutralisation of charges and the nucleation of the MOSP particles were the main mechanism for the removal the two dyes.Higher temperature,lower pH,and larger MOSP dosage were favorable for the removal of the two dyes.Inorganic salts prompted the release of proteins from the MOSP,which in turn increased the dye removal.The suspended solid in the dye soutions decreased the dye removal efficiencies.The results indicated that MOSP could be a potential coagulant for dye removal.
[1]Sanja P,Natalija K,Ana L B,et al.Removal of Some Reactive Dyes from Synthetic Wastewater by Combined Al (III)Coagulation/Carbon Adsorption Process [J].Dyes and Pigments,2004,62(3):291-298.
[2]Agata S,Eric G,Montserrat R,et al.The Removal of Sulphonated Azo-Dyes by Coagulation with Chitosan [J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2008,330(2/3):219-226.
[3]Vaidya A A,Datye K V.Environmental Pollution during Chemical Processing of Synthetic Fibres[J].Colourage,1982,14:3-10.
[4]Chan S H S,Wu T Y,Juan J C,et al.Recent Developments of Metal Oxide Semiconductors as Photocatalysts in Advanced Oxidation Processes (AOPs)for Treatment of Dye Waste Water[J].Journal of Chemical Technology and Biotechnology,2011,86(9):1130-1158.
[5]Bakshi D K, Gupta K G, Sharma P, et al.Enhanced Biodecolorization of Synthetic Textile Dye Effluent by Phanerochaete Chrysosporium under Improved Culture Conditions[J].World Journal of Microbiology &Biotechnology,1999,15(4):507-509.
[6]Daneshvar N,Rabbani M,Modirshahla N,et al.Critical Effect of Hydrogen Peroxide Concentration in Photochemical Oxidative Degradation of C.I.Acid Red 27 (AR27)[J].Chemosphere,2004,56(10):895-900.
[7]Valderrama C,Cortina J L,Farran A,et al.Evaluation of Hyper-cross-linked Polymeric Sorbents (Macronet MN200 and MN300)on Dye (Acid red 14)Removal Process[J].Reactive&Functional Polymers,2008,68(3):679-691.
[8]Wang L,Wang A Q.Adsorption Behaviors of Congo Red on the N, O-carboxymethyl-Chitosan/Montmorillonite Nanocomposite[J].Chemical Engineering Journal,2008,143(1/2/3):43-50.
[9]Wang L,Wang A Q.Adsorption Properties of Congo Red from Aqueous Solution onto N,O-carboxymethyl-Chitosan [J].Bioresource Technology,2008,99(5):1403-1408.
[10]Zheng Y P,Yao G H,Cheng Q B,et al.Positively Charged Thin-Film Composite Hollow Fiber Nanofiltration Membrane for the Removal of Cationic Dyes through Submerged Filtration[J].Desalination,2013,328(1):42-50.
[11]Jordi L,Josep S,Joan L.Modeling of the Dynamic Adsorption of an Anionic Dye through Ion-Exchange Membrane Adsorber[J].Journal of Membrane Science,2009,340(1/2):234-240.
[12]Zhu H Y, Jiang R, Xiao L, Y et al.Photocatalytic Decolorization and Degradation of Congo Red on Innovative Crosslinked Chitosan/Nano-CdS Composite Catalyst under Visible Light Irradiation [J].Journal of Hazardous Materials,2009,169(1/2/3):933-940.
[13]Guo X Y,Tan Y Q,Wei Z B,et al.Ozonation of Some Dyes in Aqueous Solution and Toxicological Assessment of Their Oxidation Products[J].Fresenius Environmental Bulletin.2013,22(6):1689-1696.
[14]Yusra S,Haq N B.Kinetic and Thermodynamic Modeling for the Removal of Direct Red-31 and Direct Orange-26 Dyes from Aqueous Solutions by Rice Husk[J].Desalination,2011,272(1/2/3):313-322.
[15]Ghaedi M,Sadeghian B,Amin Pebdani A,et al.Kinetics,Thermodynamics and Equilibrium Evaluation of Direct Yellow Removal by Adsorption onto Silver Nanoparticles Loaded Activated Carbon [J].Chemical Engineering Journal,2012,187:133-141.
[16]Himanshu P,Vashi R T.Removal of Congo Red Dye from Its Aqueous Solution Using Natural Coagulants [J].Journal of Saudi Chemical Society,2012,16(2):131-136.
[17]Agata S,Eric G,María A P,et al.Removal of an Anionic Dye(Acid Blue 92)by Coagulation-Flocculation Using Chitosan[J].Journal of Environmental Management,2009,90(10):2979-2986.
[18]Shi B Y,Li G H,Wang D S,et al.Removal of Direct Dyes by Coagulation:The Performance of Preformed Polymeric Aluminum Species[J].Journal of Hazardous Materials,2007,143(1/2):567-574.
[19]Huang X,Bo X W,Zhao Y X,et al.Effects of Compound Bioflocculant on Coagulation Performance and Floc Properties for Dye Removal[J].Bioresource Technology,2014,165:116-121.
[20]Szygua A,Guibal E,Aríňo Palacín M,et al.Removal of an Anionic Dye (Acid Blue 92)by Coagulation-Flocculation Using Chitosan[J].Journal of Environmental Management,2009,90(10):2979-2986.
[21]Parul S,Pushpa K,Srivastava M M,et al.Ternary Biosorption Studies of Cd (II),Cr (III)and Ni (II)on Shelled Moringa Oleifera Seeds[J].Bioresource Technology,2007,98(2):474-477.
[22]Sharma P,Kumari P,Srivastava M M,et al.Removal of Cadmium from Aqueous System by Shelled Moringa Oleifera Lam Seed Powder[J].Bioresource Technology,2006,97(2):299-305.
[23]Kumari P,Sharma P,Srivastava S,et al.Biosorption Studies on Shelled Moringa Oleifera Lamarck Seed Powder:Removal and Recovery of Arsenic from Aqueous System [J].International Journal of Mineral Processing,2006,78(3):131-139.
[24]Obuseng V,Nareetsile F,Kwaambwa H M.A Study of the Removal of Heavy Metals from Aqueous Solutions by Moringa oleifera Seeds and a Mine-Based Ligand 1,4-bis[N,N-bis(2-picoyl)amino]Butane[J].Analytica Chimica Acta,2012,730:87-92.
[25]Pritcharda M,Craven T,Mkandawire T,et al.A Comparison between Moringa Oleifera and Chemical Coagulants in the Purification of Drinking Water - an Alternative Sustainable Solution for Developing Countries[J].Physics and Chemistry of the Earth,2010,35(13/14):798-805.
[26]Nishi L,Salcedo Vieira M,Fernandes Vieira M,et al.Hybrid Process of Coagulation/ Flocculation with Moringa Oleifera Followed by Ultrafiltration to Remove Microcystis sp.Cells from Water Supply[J].Procedia Engineering,2012,42:865-872.
[27]Teixeira C M L L,Kirsten F V,Teixeira P C N.Evaluation of Moringa Oleiferaseed Flour as a Flocculating Agent for Potential Biodiesel Producer Microalgae [J].Journal of Applied Phycology,2012,24(3):557-563.
[28]Beltrán-Heredia J,Sánchez-Martín J,Barrado-Moreno M.Long-Chain Anionic Surfactants in Aqueous Solution Removal by Moringa Oleifera Coagulant[J].Chemical Engineering Journal,2012,180:128-136.
[29]Anselme N,Subba N K,Brian G,et al.Active Agents and Mechanism of Coagulation of Turbid Waters Using Moringa Oleifera[J].Water research,1995,29(2):703-710.
[30]Sponza D T,Işik M.Decolorization and Inhibition Kinetic of Direct Black 38 Azo Dye with Granulated Anaerobic Sludge[J].Enzyme and Microbial Technology,2004,34(2):147-158.
[31]Işik M,Sponza D T.Monitoring of Toxicity and Intermediates of C.I.Direct Black 38 Azo Dye through Decolorization in an Anaerobic/Aerobic Sequential Reactor System [J].Journal of Hazardous Materials,2004,114(1/2/3):29-39.
[32]Alves V N,Mosquetta R,Coelho N M,et al.Determination of Cadmium in Alcohol Fuel Using Moringa Oleifera Seeds as a Biosorbent in an On-Line System Coupled to FAAS [J].Talanta,2010,80(3):1133-1138.
[33]Kong J,Yu S N.Flourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures[J].Acta Biochimica et Biophysica Sinica,2007,39(8):549-559.
[34]Xiao F,Huang J C H,Zhang B J,et al.Effects of Low Temperature on Coagulation Kinetics and Floc Surface Morphology Using Alum [J].Desalination,2009,237(1/2/3):201-213.
[35]Okuda T,Baes A U,Nishijima W,et al.Improvement of Extraction Method of Coagulation Active Components from Moringa Oleifera Seed [J].Water Research,1999,33(15):3373-3378.
 Journal of Donghua University(English Edition)2015年3期
Journal of Donghua University(English Edition)2015年3期
- Journal of Donghua University(English Edition)的其它文章
- Evaluation of Cadmium Bioavailability in Soils Using Diffusive Gradients in Thin Film Technique and Traditional Methods
- Structured Query Language Injection Penetration Test Case Generation Based on Formal Description
- Graph Regularized Sparse Coding Method for Highly Undersampled MRI Reconstruction
- Application of Monetary Unit Sampling Based on Extended Audit Game
- Group Performance Evaluation in Universities with Entropy Method
- On Augmented Zagreb Index of Molecular Graphs
