Comparative studies on leaf epidermal micromorphology and mesophyll structure of Elaeagnus angustifoliaL.in two different regions of desert habitat
MengMeng Li ,YuBing Liu ,MeiLing Liu ,Dan Liu
1.Key Laboratory of Stress Physiology and Ecology in Cold and Arid Regions of Gansu Province,Cold and Arid Regions Environmental and Engineering Research Institute,Chinese Academy of Sciences,Lanzhou,Gansu 730000,China
2.Shapotou Desert Research &Experiment Station,Cold and Arid Regions Environmental and Engineering Research Institute,Chinese Academy of Sciences,Lanzhou,Gansu 730000,China
3.University of Chinese Academy of Sciences,Beijing 100049,China
1 Introduction
Plant epidermis possesses a number of important diagnostic characters that offer valuable clues for identification,such as size,shape and orientation of stomata,guard cells and subsidiary cells,structural peculiarities of epidermal cell walls,and distinctive or specialized form of trichomes(Dickison,2000).Variations of epidermal characteristics amongst species may be the result of the effects of the environment(Uphof and Hummel,1962;Zoricet al.,2009).Knowledge of the plant's leaf anatomy is crucial for understanding how these plants adapt to the environment(Maet al.,2012).The responses of leaf structure to the environment are well-documented(Feiet al.,1998;Zhanget al.,2005).Plants with different taxonomies or from different functional groups may have different responses to environmental change.Thus,variations of leaf structure inevitably lead to different responses to environmental factors(Rury and Dickison,1984;P'yankovet al.,1999;Cuiet al.,2006).In most dicotyledonous plants,leaf mesophyll is divided into palisade and spongy tissues,which differ in their location,cell morphology,and functional activity(Ivanova and P'yankov,2001).The proportion of palisade to spongy mesophyll tissues vary with plant species and growth conditions.It is generally accepted that palisade tissue prevails in the mesophyll of dorsiventral leaves and provides a major contribution to their photosynthesis.The spongy tissue was assumed to be of minor importance for CO2fixation.The assumption was that the major function of the spongy tissue is to reflect light that passed through the palisade layer(Ivanova and P'yankov,2001).
Elaeagnaceae is a small family with three genera:
ElaeagnusL.,HippophaeL.andShepherdiaNutt..Temperate EurasianHippophaeas well as North AmericanShepherdiaconsists of only three species,whereasElaeagnusL.contains the largest number of species(reached 80 species)with a wide distribution in eastern Asia,extending to Southeast Asia and Queensland in northeastern Australia(Huanget al.,2005).The diversity ofElaeagnusis centered in China,mainly distributed from the Yangtze River valley to its southern region,but is also found in Northwest China.Elaeagnusgrows in diverse habitats at elevations of 50–3,100 m,such as thickets near the seashore,streamside in valleys,forests,roadside,shrub land,and rocky slopes(Huang and Maimaitijiang,2005).Elaeagnus angustifoliaL.,locally also called Guixiangliu,Xiangliu,Yinliu,Hongdou,Jinlinghua and Shilixiang in China,grows in the desert or sandy soils.It is a deciduous tree or shrub with a height of 4–15 m,mainly distributed in the arid desert areas of Northwest Xinjiang,Gansu,Ningxia and West Inner Mongolia,China.E.angustifoliais highly adaptive to drought,and is one of the pioneer trees which play an important role for soil improvement and ecological restoration along desert margins and saline-alkali land.To a certain extent,this genus still remains poorly known(Sun and Lin,2010).
In order to obtain qualitative and quantitative characteristics of leaf epidermal micromorphology and mesophyll structure to evaluate the responses ofE.angustifoliato different environmental factors,leaf materials were selected from fixed sandy land of Linze County,Gansu Province and pioneer forest on former farmland of Qitai County,Xinjiang Uygur Autonomous Region,China.This study will help us to further understand plant adaptation to their environment,and can also facilitate better management of desert vegetation.
2 Materials and methods
2.1 Materials and study sites
Leaf samples were selected from the artificial forest ofTamarix chinensisLour.andE.angustifoliain Linze County,Gansu Province(material A),and Qitai County,Xinjiang Uygur Autonomous Region(material B)(Table 1).The habitats of materials A and B are fixed sandy land in Linze Inland River Basin Research Station and naturally-regenerated forest of ten years after cessation of farming in Qitai Desert Forest Management Station,respectively(Figure 1).Linze belongs to a continental arid climate,with an annual average temperature of 7.6 °C,highest temperature of 39.1 °C and lowest temperature of 27 °C.The frost-free period is about 165 d in one year and the annual average wind speed is 5.84 m/s.Annual average precipitation is 117 mm and average evaporation is 2,390 mm,which is more than 20 times of the rainfall.The zonal soil is gray brown desert soil and zonal vegetation is desert shrubs.Qitai is located in Changji Hui Autonomous Prefecture in Xinjiang Uygur Autonomous Region.Samples were taken from the Qitai Desert Forest Management Station.Qitai belongs to a temperate continental semi-desert arid climate with an annual average temperature of 6 °C,extreme maximum temperature of 44 °C,and minimum temperature of-43 °C.Annual average wind speed is 2.9 m/s,and maximum wind force can reached 12 grades.An average of 155 d is frost-free period(from late April to early October).Annual average rainfall is 110–130 mm,and annual average evaporation is more than 2,160 mm.
2.2 Materials selected and treatment
E.angustifoliawas sampled from the aforementioned regions in June of 2012 and 2013.New growth mature leaves were harvested from the sunny slope of the trees.The leaves were cut into 0.5cm×0.5cm sections after 0.5 cm segment from the leaf tip,and then immediately fixed in a phosphate buffer(pH 7.2)containing 3% glutaraldehyde for 24 hours.
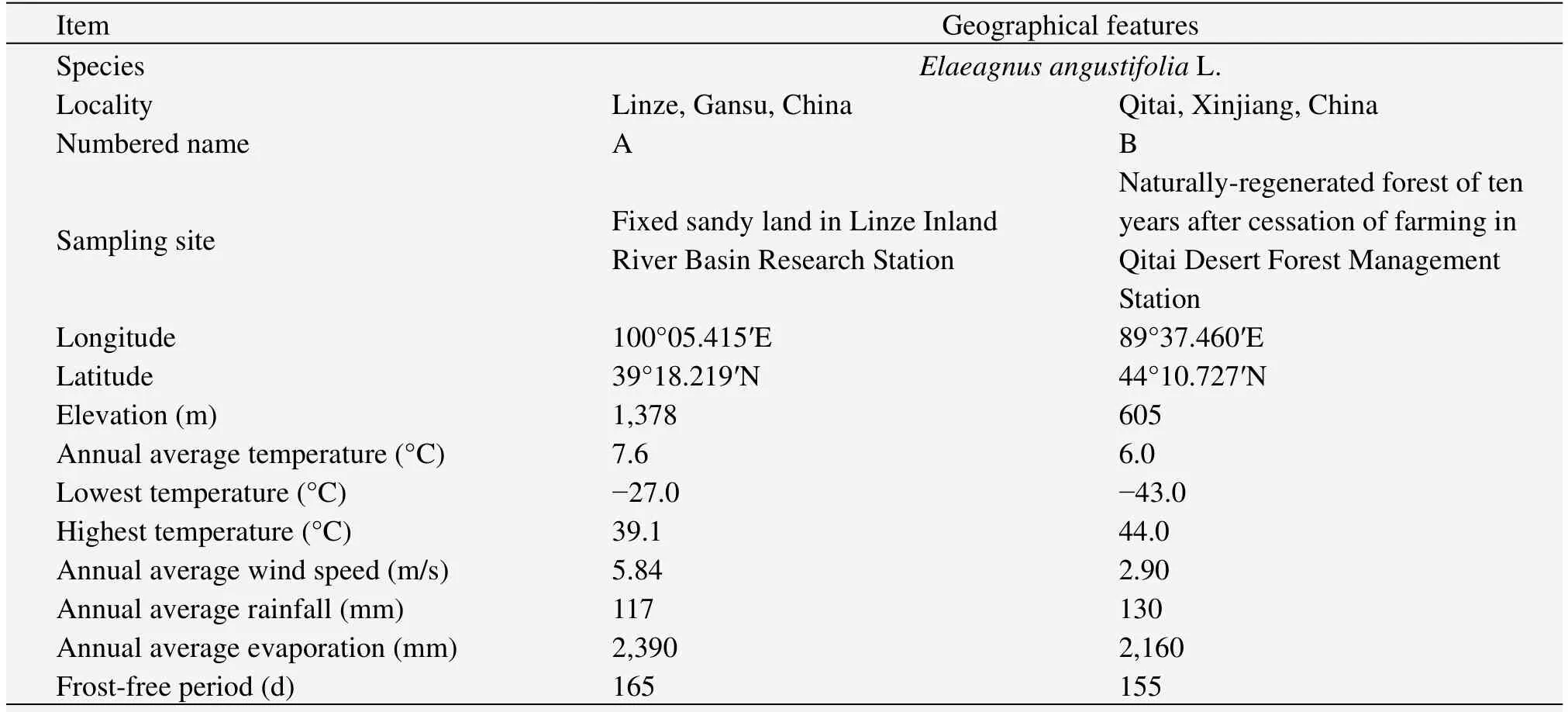
Table 1 General situation of sampling sites

Figure 1 E.angustifolia in natural habitats of fixed sandy land in Linze Inland River Basin Research Station(A)and naturally-regenerated forest of ten years after cessation of farming in Qitai Desert Forest Management Station(B)
2.3 Mesophyll structure analyzed by the light microscopy
Sample preparation is according to the method of Liuet al.(2012).Samples were washed twice for 10 min in 0.1 M phosphate buffer at pH 7.2,and then post-fixed with 1.0% OsO4in the same buffer overnight.After post-fixing,samples were washed with the same phosphate buffer and dehydrated in increasing concentrations of ethanol(from 60% to 95%).Pre-inclusion of samples in acetone:epon812(1:1)at 35 °C(24 hours)was followed by final inclusion in freshly prepared resin for 12 hours at 45 °C and 24 hours at 65 °C.
For light microscopy(LM),semi-thin sections(1.0 µm)were stained with 1% toluidine blue.Photographs were taken with a Zeiss Q500 IW light microscope,and digital images captured using a BX41-32P02+MD20 camera.Three independent experiments were performed for each staining analysis.To determine the cross-sectional size of different tissues,4–6 slices of different trees were analyzed with an image analyzer(ImageJ Launcher-1.4.3.67).
2.4 Chloroplast ultrastructure analyzed by transmission electron microscopy
For transmission electron microscopy(TEM),3–5 ultra-thin sections(70 nm)from different trees were obtained using an ultramicrotome(LKB-V,Sweden)and stained with 3% uranylacetate followed by lead citrate before observation with a TEM(JEM-1230,Japan),and images were analyzed with an image analyzer(ImageJ Launcher-1.4.3.67).
2.5 Leaf epidermal micromorphology analyzed by scanning electron microscopy
For scanning electron microscopy(SEM),4–6 different tree samples were fixed,post-fixed and dehydrated as above,dried using JFD-310 apparatus(JEOL Ltd.,Tokyo,Japan),covered with 20 nm of gold,and observed in a JSM-6380(JEOL Ltd.)microscope,and images were analyzed with an image analyzer(Smile View-6.31.100.1190).
2.6 Statistical analysis
Results are expressed as means ± standard errors.SPSS 16.1 software(SPSS,Chicago,IL,USA)is used for statistical analysis.To assess the statistical significance between the sections and the micromorphological differentiation of examined species at the level of epidermal characteristics,a one-way analysis of variance(ANOVA)by Duncan's multiple range test atP<0.05 is performed(Zoricet al.,2009;Liuet al.,2012).
3 Results
3.1 Mesophyll structural characteristics
The transverse section of the leaf lamina ofE.angustifolia(Figure 2)is a typical bifacial leaf.There are two kinds of mesophyll tissue in the leaf structure,palisade tissue and spongy tissue.The leaf lamina was thicker in material A than in B.Meanwhile,the ratio of palisade to spongy tissue was significantly different,1.44 in A and 1.15 in B(Table 2).There was one layer of epidermal cells in both adaxial and abaxial surfaces of the leaf,with thicker upper epidermal cells in A,but thicker lower epidermal cells in B.A thick layer of trichomes covered the leaf epidermis,being thicker for A in both adaxial and abaxial surfaces of 73.58 µm and 68.58 µm,and thinner for B of 55.55 µm and 65.66 µm,respectively.Obviously,the thickness of lower epidermal cells and trichomes of material A were larger than that of the upper epidermis in materials B.Owing to the thicker leaf lamina in material A,the ratio of palisade to spongy tissue,thickness of epidermal cells and trichomes measurements were larger compared to material B.
3.2 Epidermal cells characteristics
Figure 3 shows the transmission electron micrographs of epidermal cell walls and the covered epidermal wax on the leaf surface cell walls ofE.angustifolia.The largest thickness of the epidermal cell wall and cuticular wax were in the adaxial surface of material B of 6.383 µm and 1.05 µm,respectively.In contrast to the mesophyll structural data,the epidermal cell wall and epidermal cuticular wax were thicker in material B than that in A in both adaxial and abaxial surfaces,while larger in the adaxial surface of both materials A and B.

Figure 2 Leaf cross-sections of E.angustifolia in Linze(A)and Qitai(B).A1,B1,10x,scale bars = 50 µm.A2,B2,40x,scale bars = 40 µm.EC,epidermis cells;EA,epidermal appendages;PT,palisade tissue;ST,spongy tissue;VB,vascular bundle
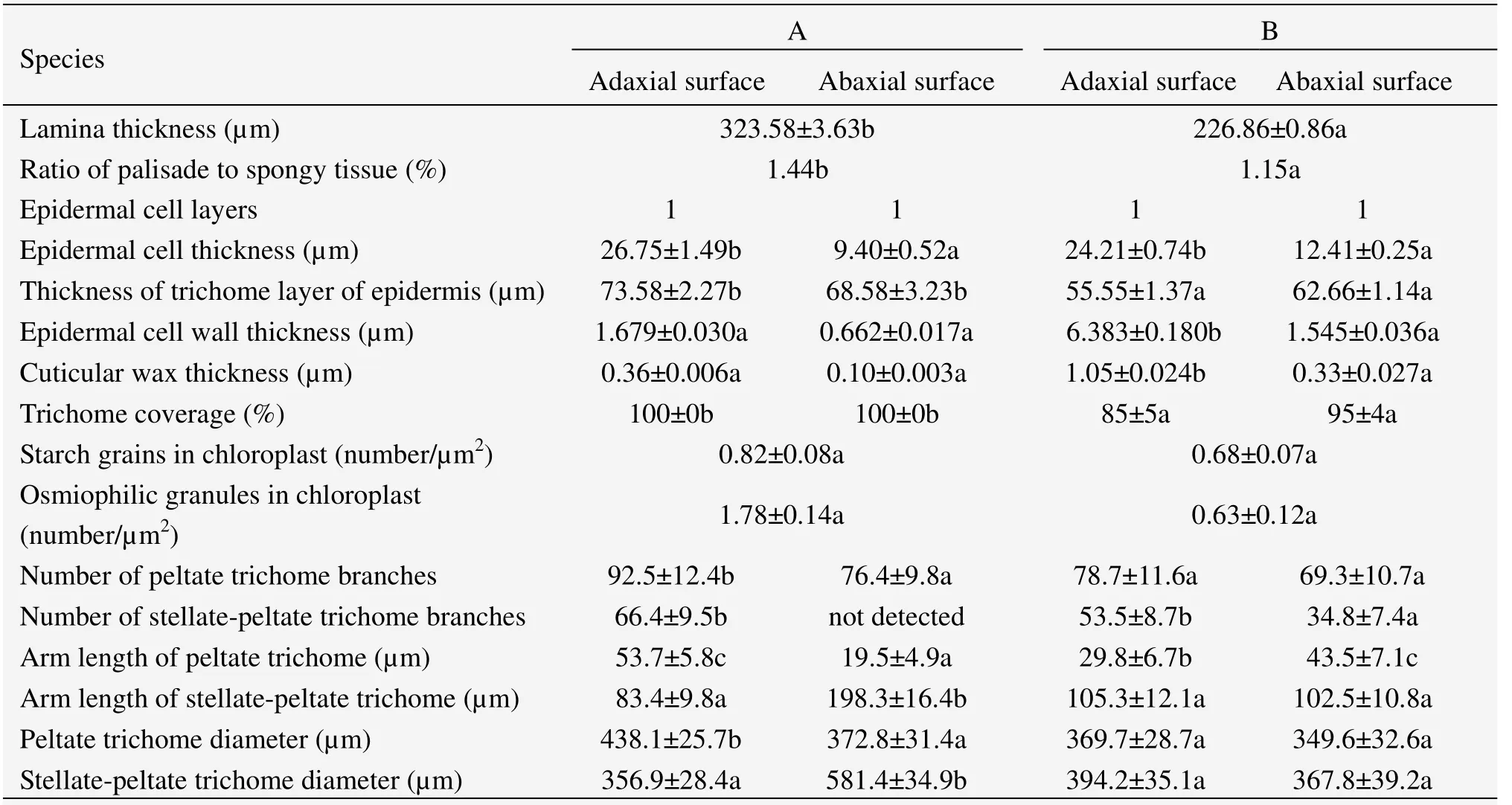
Table 2 Statistical data of epidermal micromorphology and mesophyll structure
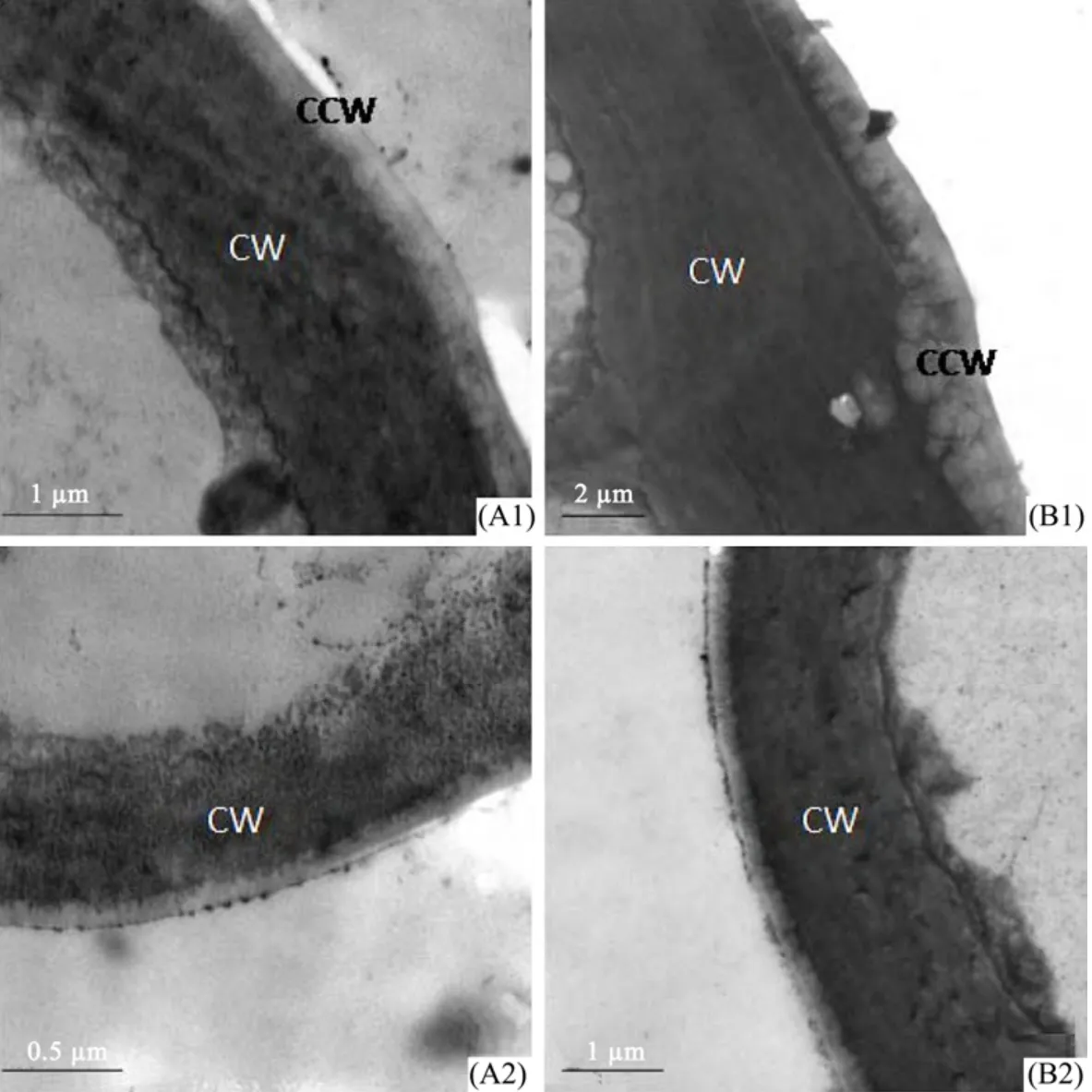
Figure 3 Transmission electron micrographs of epidermal cell walls and epidermal wax of E.angustifolia leaf surface in Linze(A)and Qitai(B).A1 and B1,cell wall and epidermal wax of epidermal cell in adaxial surface.A2 and B2,cell wall and epidermal wax of epidermal cell in abaxial surface.A1,B2,scale bars = 1 µm.A2,scale bar = 0.5 µm.B1,scale bar = 2 µm.CW,cell wall;CCW,cuticular wax
3.3 Chloroplast ultrastructural characteristics
From the transmission electron micrographs of mesophyll cells and chloroplasts ofE.angustifolialeaves(Figure 4),we find that the mesophyll cells were almost packed with chloroplasts.Chloroplast shapes are different in the two materials,spherical in material A,and spindly in material B(Figures 4 A2 and B2).There are a certain number of starch grains and osmiophilic granules in chloroplasts of material A.In addition,the starch grains are of different shapes such as cylindrical,oval or spherical.
3.4 Leaf epidermal micromorphological characteristics
Figures 5 and 6 show the epidermal micromorphology of the two materials with adaxial and abaxial surface,respectively.Numerous layers of peltate or stellate-peltate trichomes cover the two surfaces of materials A(Figures 5 A1 and 6 A1)and B(Figures 5 B1 and 6 B1),reaching up to 100% on both surfaces of A,and 85% and 95% on the adaxial and abaxial sides of B,respectively(Table 2).Peltate is the main type of trichome forE.angustifolia,which overlap each other(Figures 5 A2,A3 and Figures 6 A2,B2).In addition,trichome diameter,arm length,and number of branches(Table 2)of the two materials are significantly discrepant for both adaxial and abaxial surfaces.Because of trichome density,it was difficult to study the stomatal and epidermal cell microcharacteristics of the species.Therefore,the stomatal index and cell density were not examined in this study.
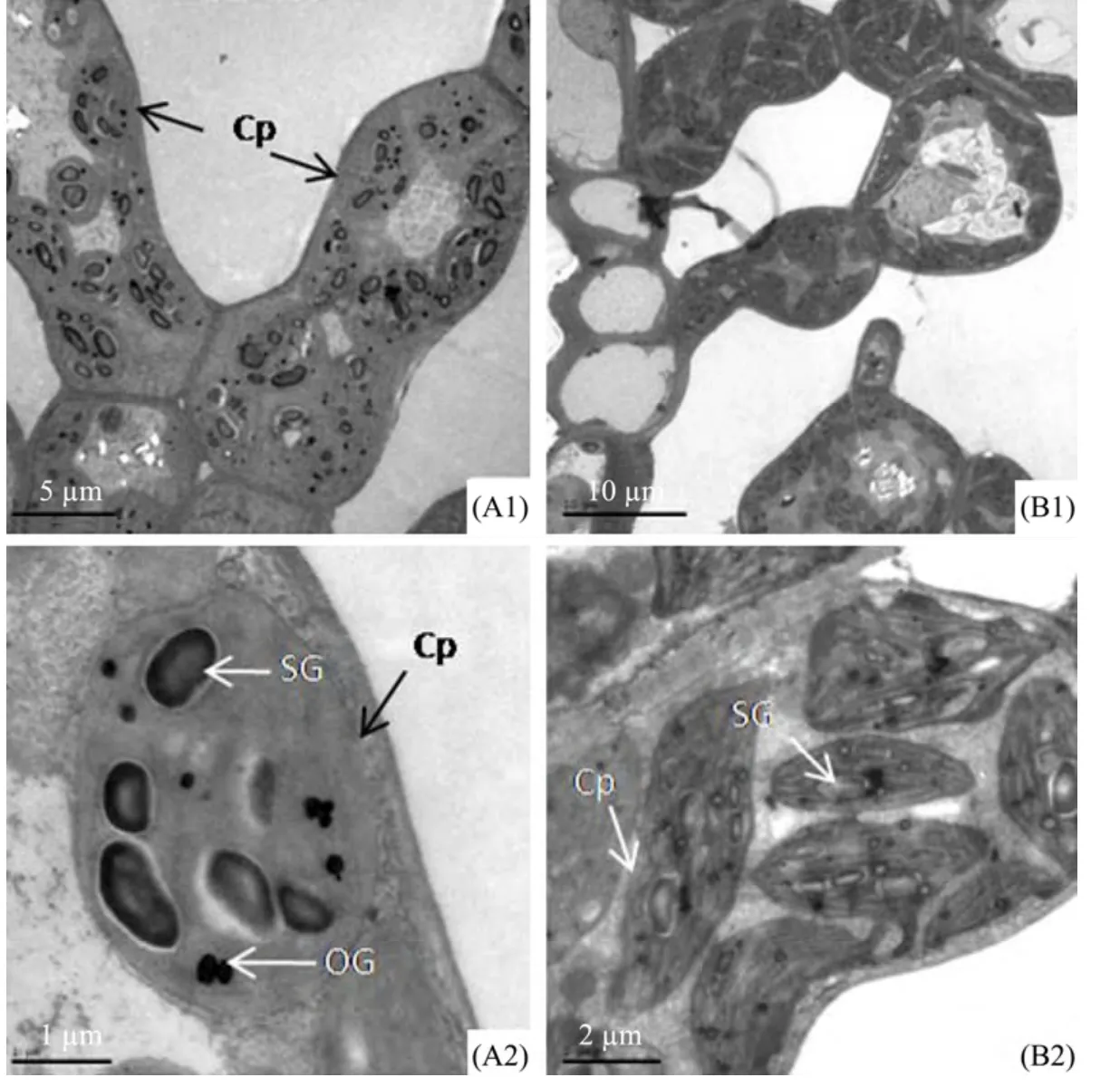
Figure 4 Transmission electron micrographs of mesophyll cells and chloroplasts of E.angustifolia leaves in Linze(A)and Qitai(B).A1 and B1,mesophyll cells.A2 and B2,chloroplasts of mesophyll cells.A1,scale bar = 5 µm.A2,scale bar = 1 µm.B1,scale bar = 10 µm.B2,scale bar = 2 µm.Cp,chloroplasts;SG,starch grains;OG,osmiophilic granules
4 Discussion
Plants have evolved to exist in conditions which are rarely ideal for maintenance of normal physiology and may be at the limit for survival.They are subject to a wide range of abiotic stresses,and their cuticular wax layer provides a protective barrier(Shepherd and Griffiths,2006).Leaves are more sensitive to environmental change and have large plasticity,thus can take on various forms(e.g.,simple to lobed leaves)which are adaptations to the local environment.The structure of the laminae can reflect the influence of environmental factors or the plant's adaptation to environmental factors.Environmental change often leads to changes of length,width and thickness of the leaf,leaf epidermal trichomes,epidermal cells,epicuticular wax crystals,mesophyll palisade tissue,spongy tissue,intercellular space and collenchyma(Li and Bao,2005).
In order to overcome a harsh environment,there are often numerous forms of epidermal trichomes on the leaf surface of desert plants.In this study,mesophyll structure and cuticular tissue were examinedinE.angustifolialeaves from two different regions.Studies show thatE.angustifoliahave specialized and diverse leaf micromorphology in the desert habitats of Qitai and Linze.Numerous layers of overlapping trichomes cover both the adaxial and abaxial surfaces ofE.angustifoliain the two regions,except for the material of Qitai where the adaxial surface contain few trichomes.The trichomes of Elaeagnaceae are multicellular structures which are an adaptation to the local environment,and have important physiological and ecological significance(Ehleringer and Mooney,1978;Kenzoet al.,2008).The thickly covered trichomes are closely related to the environmental factors of Linze,such as lower annual average rainfall,higher average wind speed and annual average evaporation.Trichome functions include reducing water evaporation or lowering lamina temperature to avoid dehydration,while densely white trichomes of the leaf epidermis can reflect sunlight and can provide effective absorption of air moisture(Fahn,1986).SEM studies have shown that Elaeagnaceae of China have five different types of leaf epidermal trichomes,namely,stellate,stellate-peltate,peltate,stalked stellate,and odd shaped stalked trichomes(Zhanget al.,1992).In this study we found three types from the materials ofE.angustifolia:stellate,stellate-peltate and peltate.Peltate trichomes help reduce transpiration,appear to be an adaptation to cold environments,and are highly evolved compared to other trichome types(Zhanget al.,1992).
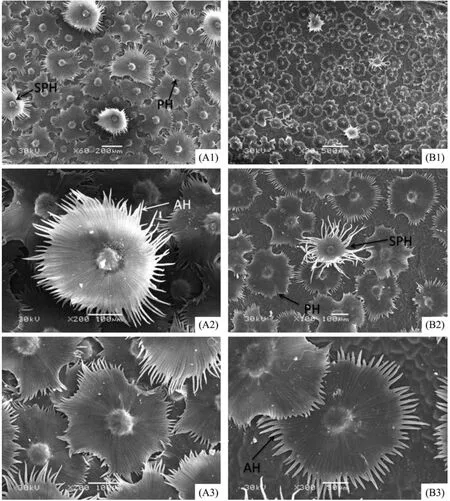
Figure 5 Scanning electron micrographs showing the epidermis of the adaxial surface of E.angustifolia leaves in in Linze(A)and Qitai(B).A1,scale bar = 200 µm.A2,B2,A3,scale bars = 100 µm.B1,scale bar = 500 µm.B3,scale bar = 50 µm.SPH,stellate-peltate trichome;PH,peltate trichome;AH,trichome arm

Figure 6 Scanning electron micrographs showing the epidermis of the abaxial surface of E.angustifolia leaves in in Linze(A)and Qitai(B).A1,scale bar = 200 µm.A2,B2,scale bars = 50 µm.B1,scale bar = 500 µm
Similarly,from sectioned material,the thicker lamina and larger ratio of palisade to spongy tissue were found in the Linze material.The mesophyll cells with developed palisade tissue increased the plant's drought tolerance,thus the increasing cell layer,the reduction of spongy tissue,and also the decreasing intercellular space are responses of plants to water shortages(Chartzoulakiset al.,2002).The spherical chloroplasts have a higher photosynthetic ability in the Linze material,and also more starch grains in the chloroplast were found.These results indicate thatE.angustifoliais equipped with epidermal and mesophyll structures adapted to a harsh environment.
The natural habitat ofE.angustifoliain the two research regions is desert,with low precipitation and high evaporation.By comparison,higher altitude and evaporation are found in Linze,and the material of Linze show larger data of lamina thickness,ratio of palisade to spongy tissue,thickness of epidermal cells and trichome layers,and trichome coverage.A thick lamina and high ratio of palisade to spongy tissue are important characters which adapted to drought and cold stress.Thicker epidermal cells and trichome layers are thought to possibly increase the thickness of the leaf boundary layer(Wooley,1970;Wuenscher,1970)and reduce leaf absorptance,resulting in a reduced heat load,and as a consequence lower leaf temperatures and transpiration rates(Ehleringeret al.,1976;Ehleringer and Mooney,1978).These characters are important to inhibit transpiration and improve the drought tolerance in plants.
This work was financially supported by the National Natural Science Foundation of China(Grant No.91125029)and the State Key Development Program for Basic Research of China(973 Program,Grant No.2013CB429904).
Chartzoulakis K,Patakas A,Kofidis G,et al.,2002.Water stress affects leaf anatomy,gas exchange,water relations and growth of two avocado cultivars.Scientia Horticulturae,95(1–2):39–50.DOI:10.1016/S0304-4238(02)00016-X.
Cui XP,Liu GH,Zhang RL,2006.Comparison of leaf anatomical structure betweenSalix gordejeviigrowing under contrasting habitats of Otingdag Sandland andSalix microtachyavar.bordensisgrowing on the lowlands of dunes.Acta Ecologica Sinica,26(6):1842–1847.
Dickison WC,2000.Integrative Plant Anatomy.San Diego:Academic Press,pp.186–195.
Ehleringer JR,Björkman O,Mooney HA,1976.Leaf pubescence:effects on absorptance and photosynthesis in a desert shrub.Science,192(4237):376–377.DOI:10.1126/science.192.4237.376.
Ehleringer JR,Mooney HA,1978.Leaf hairs:effects on physiological activity and adaptive value to a desert shrub.Oecologia,37:183–200.DOI:10.1007/BF00344990.
Fahn A,1986.Structural and functional properties of trichomes of xeromorphic leaves.Annals of Botany,57(5):631–637.
Fei SL,Fang JY,Fan YJ,et al.,1998.Anatomical characteristics of leaves and woods ofFagus lucidaand their relationship to ecological factors in Mountain Fanjingshan,Guizhou,China.Acta Botanica Sinica,41(9):1002–1009.
Huang J,Maimaitijiang,2005.Study on the classification of Elaeagnus in Xinjiang.Bulletin of Botanical Research,25:268–271.DOI:10.3969/j.issn.1673-5102.2005.03.006.
Huang J,Maimaitijiang,Yang C,et al.,2005.Present situation and prospect about the study ofElaeagnus angustifoliaL..Chinese Wild Plant Resources,24(3):26–33.
Ivanova LA,P'yankov VI,2001.Structural adaptation of the leaf mesophyll to shading.Russian Journal of Plant Physiology,49(3):419–431.DOI:10.1023/A:1015513607202.
Kenzo T,Yoneda R,Azani MA,et al.,2008.Changes in leaf water use after removal of leaf lower surface hairs onMallotus macrostachyus(Euphorbiaceae)in a tropical secondary forest in Malaysia.Journal of Forest Research,13(2):137–142.DOI:10.1007/s10310-008-0062-z.
Li FL,Bao WK,2005.Responses of the morphological and anatomical structure of the plant leaf to environmental change.Chinese Bulletin of Botany,22:118–127.
Liu YB,Li XR,Liu ML,et al.,2012.Responses of three different ecotypes of reed(Phragmites communisTrin.)to their natural habitats:leaf surface micro-morphology,anatomy,chloroplast ultrastructure and physio-chemical characteristics.Plant Physiology and Biochemistry,51:159–167.DOI:10.1016/j.plaphy.2011.11.002.
Ma JJ,Ji CJ,Han M,et al.,2012.Comparative analyses of leaf anatomy of dicotyledonous species in Tibetan and Inner Mongolian grasslands.Science China Life Sciences,55(1):68–79.DOI:10.1007/s11427-012-4268-0.
P'yankov VI,Kondratchuk AV,Shipley B,1999.Leaf structure and specific leaf mass:the alpine desert plants of the Eastern Pamirs,Tadjikistan.New Phytologist,143(1):131–142.
Rury P,Dickison W,1984.Structural correlations among wood,leaves and plant habit.In:White RA,Dickison WC(eds.).Contemporary Problems in Plant Anatomy.Orlando:Academic Press,pp.495–540.
Shepherd T,Griffiths DW,2006.The effects of stress on plant cuticular waxes.New Phytologist,171(3):469–499.DOI:10.1111/j.1469-8137.2006.01826.x.
Sun M,Lin Q,2010.A revision ofElaeagnusL.(Elaeagnaceae)in mainland China.Journal of Systematics and Evolution,48(5):356–390.DOI:10.1111/j.1759-6831.2010.00085.x.
Uphof JCT,Hummel K,1962.Plant hairs.Encyclopedia of Plant Anatomy,4(5):1–292.
Wooley JT,1970.Water relations of soybean leaf hairs.Agronomy Journal,56(6):569–571.DOI:10.2134/agronj1964.0002196 2005600060014x.
Wuenscher JE,1970.The effect of leaf hairs ofVerbascum thapsuson leaf energy exchange.New Phytologist,69(1):65–73.DOI:10.1111/j.1469-8137.1970.tb04050.x.
Zhang HX,Liu GH,Cui XP,2005.Affection of aridity to anatomical structure of leave ofUlmus pumilaL.var.sabulosa.Bulletin of Botanical Research,25(1):39–44.
Zhang ZX,Gao ZQ,Zhang Y,1992.A SEM study on the morphology of foliar surface of seabuckthorn andElaeagnusand their implication on taxonomy.1.The morphology of foliar surface and their attachment.Bulletin of Botanical Research,12(2):169–176.
Zoric L,Merkulov L,Lukovic J,et al.,2009.Leaf epidermal characteristics ofTrifoliumL.species from Serbia and Montenegro.Flora-Morphology,Distribution,Functional Ecology of Plants,204(3):198–209.DOI:10.1016/j.flora.2008.02.002.
 Sciences in Cold and Arid Regions2015年3期
Sciences in Cold and Arid Regions2015年3期
- Sciences in Cold and Arid Regions的其它文章
- The characteristics of oasis urban expansion and drive mechanism analysis:a case study on Ganzhou District in Hexi Corridor,China
- Characterizing changes in ecosystem service values in China's eastern Loess Plateau
- Elemental composition and its environmental significance for the varicolored hills in the northern foothills of the Qilian Mountains of Sunan Yugur Autonomous County,China
- Aesthetic evaluation of yardang landforms landscape:the Dunhuang Yardang National Geo-park example
- Characterizing stand structure in a spruce forests:effects of sampling protocols
- Biomass and water partitioning in two age-related Caragana korshinskii plantations in desert steppe,northern China
