昆虫转座子在转基因技术中的应用
许 军, 张宏波, 韩民锦, 谭安江, 黄勇平, 张 泽*
1中国科学院上海生命科学研究院植物生理生态研究所,昆虫发育与进化生物学重点实验室,
上海 200032; 2中国科学院大学,北京 100049; 3重庆大学生命科学学院,重庆 400044
昆虫转座子在转基因技术中的应用
许军1,2, 张宏波3, 韩民锦3, 谭安江1, 黄勇平1, 张泽3*
1中国科学院上海生命科学研究院植物生理生态研究所,昆虫发育与进化生物学重点实验室,
上海 200032;2中国科学院大学,北京 100049;3重庆大学生命科学学院,重庆 400044
摘要:转座子是基因组中一段可移动的DNA重复片段。越来越多的研究表明,转座子是真核生物基因组的主要组成成分,是基因组和表型进化的主要动力之一,并且对基因表达调控网络的进化具有重要的贡献。由于转座子在基因组内具有可移动性,使其在生物技术和分子生物学领域备受重视,尤其在转基因技术上得到了广泛应用。本文综述了转座子在昆虫中的分布、类型及功能,重点阐述不同昆虫转座子在转基因技术中的应用,并对转基因安全性和转座子稳定性进行了讨论。
关键词:转座子; 昆虫; 转基因; 安全性
1956年McClintock在玉米中发现了控制元件,这种控制元件是基因组中一段可移动的DNA序列,可以通过不同转座机制从基因组的一个位置“跳跃”到另一个位置,后来这种控制元件被命名为转座元件或转座子(Transposon)(McClintock,1956)。根据其转座机制的不同主要分为DNA转座子(DNA transposon,Class 2)和反转录转座子(Retrotransposon,Class 1)(Wickeretal.,2007)。DNA转座子以“剪切和粘贴”(Cut and paste)机制进行转座,在其过程中转座酶将供体位点的DNA转座子以双链DNA的形式切割并整合到靶位点完成转座。反转录转座子以“拷贝和粘贴”(Copy and paste)机制转座,其先以双链DNA为模板转录成RNA,再逆转录合成cDNA,最后整合到基因组中完成转座(图1)。反转录转座子包括长末端重复元件(Long terminal repeats,LTRs)和非长末端重复元件(Non-long terminal repeats,Non-LTRs);非长末端重复元件又可分为自主的长散布元件(Long interspersed elements,LINEs)和非自主的短散布元件(Short interspersed elements,SINEs)。
转座子是真核生物基因组的主要组成成分,如转座子约占人类基因组序列的45%、玉米基因组序列的75%、果蝇Drosophilamelanogaster基因组序列的15%~22% 和飞蝗Locustamigratoria基因组的41%等(Ashburner & Bergman,2005; Kazazian,2004; Quesnevilleetal.,2005; Wangetal.,2014)。转座子具有转座能力,并且可以通过转座增加其在基因组中的拷贝,因此,转座子是基因组进化的主要动力之一。另外,无论是在转录水平还是转录后水平,转座子均可以通过多种机制改变邻近基因的结构和表达(图2)。某些转座子内部可能包含一些顺式调节元件,当其发生转座插入到功能基因附近时就有可能改变邻近基因的表达(Feschotte,2008)。例如,人类基因组中大约有22%的启动子序列来自转座子 (Jordanetal.,2003; van de Lagemaatetal.,2003)。当转座子插入到一些基因上游的顺式调控元件并且破坏了原有的顺式调节元件时,有可能导致其邻近基因表达的关闭,还有一些转座子可能会作为表观沉默的靶位点影响邻近基因的表达(Girard & Freeling,1999; Grewal & Jia,2007; Slotkin & Martienssen,2007; Wallaceetal.,1991)。此外,转座子可以通过外显子化改变邻近基因的结构和表达(Nietal.,2007; Piriyapongsaetal.,2007)。

图1 3类主要转座子的转座机制图Fig.1 Translocation mechanisms of three types of transposable elements
转座子可以通过自身或其他自主转座子编码的转座酶完成其在基因组中的转座,近年利用转座子开发的转基因载体已成为功能基因组学研究的重要工具,并且极大地推动了基础理论和应用生物学的发展。Nelson & Klein (1984)用转座子标签法克隆了玉米bronze基因。Khillanetal.(1985)将目的基因置于P因子中,在转座酶的帮助下,将携带目的基因的P因子从质粒转到老鼠的染色体上,从而达到转基因的目的。Couplandetal.(1988)在烟草中证明了负责玉米Ac转座子转座的核心序列。目前,研究人员已经开发出了适用于多种生物体的转基因载体,如应用piggyBac转座子在包括小鼠在内的许多物种中成功获得了转基因个体。同时,由于转座子在物种内或不同物种基因组间具有可移动性或不稳定性,给转基因过程中的生物安全带来了潜在风险。
1 昆虫中转座子的分布
随着大规模基因组测序技术的发展和5000个昆虫基因组计划(i5k)的开展,目前已有近123个昆虫基因组被测序(http:∥www.ncbi.nlm.nih.gov/genome/browse/),对已测序昆虫基因组进行注释后发现,转座子广泛分布于昆虫基因组中,并且不同昆虫基因组中转座子含量存在很大的差别。如家蚕Bombyxmori基因组中已鉴定了1308个转座子家族,约占整个基因组序列的45%,仅次于埃及伊蚊Aedesaegypti基因组中的转座子含量(47%) (Neneetal.,2007; Osanai-Futahashietal.,2008)。家蚕转座子多数集中于LTR、LINE和TIR等3类,其中,LINE类转座子含量最多,TIR 和LTR类转座子次之(图3)(Xuetal.,2013)。在双翅目模式昆虫果蝇属中转座子含量相对较低,约占整个果蝇属基因组序列的15%~22%,其中,LTR类反转录转座子最多,LINE类反转录转座子和TIR类转座子次之。另外,在已测序果蝇属的12 个种中存在一种特异的转座子家族DINE-1,该转座子家族仅分布于双翅目昆虫中(Clarketal.,2007)。亲缘关系较近的物种间转座子种类也存在巨大差异。如埃及伊蚊基因组中只含有一种高度退化的Mariner 元件,而这种元件在冈比亚按蚊Anophelesgambiae中达到了20种以上;在埃及伊蚊中发现冈比亚按蚊中没有的3种特异元件,即Non-LTR的LOA 元件、LTR的Osvaldo元件和Penelope家族(Holtetal.,2002; Neneetal.,2007)。这一结果证实转座子在不同系统中进化方式不同。

图2 转座子对基因结构和表达的影响Fig.2 The influence of transposable elements on structures and expression of genes
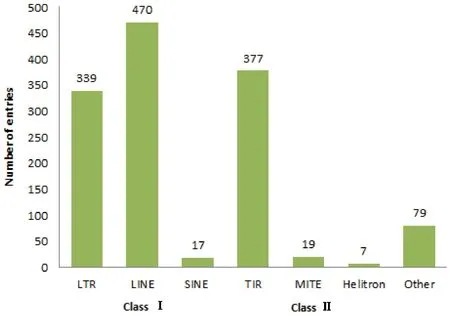
图3 家蚕基因组中不同转座子的种类Fig.3 The classification of transposable elements in the silkworm, Bombyx mori
2 昆虫转座子的应用
转座元件的发现为遗传学研究提供了一种分子操作手段。如转座子插入位点的多样性可以作为遗传标记用于连锁和进化分析,还可以开发出转基因载体。转基因昆虫是被认为继转基因微生物和转基因植物之后又一项可以带动工业生产的分子生物学技术。通过该技术对经济昆虫进行改良,可以阻断媒介昆虫对疾病的传播和农林业害虫的危害。昆虫转基因系统中的转座子主要有5种(图4;Handler,2001)。
2.1 Minos转座子
Minos转座子是从海德尔果蝇Drosophilahydei中分离得到,其长度为1.4 kb,具有100 bp的末端反向重复序列(Franz & Savakis,1991)。Minos转座子在地中海实蝇Ceratitiscapitata中的转座效率为1%~3%(Loukerisetal.,1995)。Minos转座子还可以在双翅目和鳞翅目的细胞系中发生转座,并且已应用该转座子成功获得斯氏按蚊Anophelesstephensi和黑果蝇Drosophilavirilis转基因个体(Catterucciaetal.,2000a、2000b; Klinakisetal.,2000)。
2.2 Mos1 (Mariner)转座子
Mos1转座子是从马里塔尼亚果蝇Drosophilamauritiana中发现,并且与体细胞不稳定性等位基因whitepeach相关联(Haymer & Marsh,1986; Jacobsonetal.,1986)。其长度为1286 bp,具有28 bp的末端反向重复序列,其中有4个碱基错配。与其他TC1转座子相似,其在插入位点两端通常会形成2 bp(TA)的靶位点正向重复序列。利用Mariner转座子作为载体已经成功获得果蝇和埃及伊蚊转基因个体(Coatesetal.,1998; Garzaetal.,1991; Lidholmetal.,1993; Lohe & Hartl,1996)。
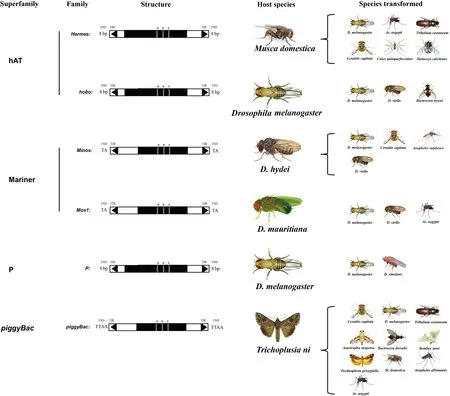
图4 昆虫转基因系统中的转座子Fig.4 Transposable elements in insect transgenic vector systems
2.3 P因子
由于P因子能引起黑腹果蝇Drosophilamelanogaster生殖障碍而被鉴定出来,目前,P因子已经广泛应用于果蝇遗传学研究,是目前研究最详细的一类昆虫转座子(Kidwell,1977)。P转座子长度为2.9 kb,具有31 bp的末端反向重复序列。中间含有可编码转座酶的序列,已经建立了成熟的P转座子和转座酶辅助系统(Rubin & Spradling,1982)。但是,该转座子在除果蝇以外的昆虫中没有转座活性,因此,其在应用上受到了极大限制。
2.4 hobo转座子
系统发生分析表明,hobo和植物中的Ac和Tam3转座子具有同源性(Calvietal.,1991)。这暗示hobo转座子作为转基因载体可能具有更为广泛的应用前景。昆虫中的hobo转座子包括家蝇Muscadomestica中的Hermer、果实蝇中的Homer、橘小实蝇Bactroceradorsalis中的hopper等(Handler & Gomez,1997; Pinkertonetal.,1999; Warrenetal.,1994)。目前,已应用这些转座子成功获得埃及伊蚊、赤拟谷盗Triboliumcastaneum、厩螫蝇Stomoxyscalcitrans、致倦库蚊Culexquinquefasciatus和地中海实蝇转基因个体(Berghammeretal.,1999; Jasinskieneetal.,1998; O′Brochtaetal.,2000)。
2.5 piggyBac转座子
piggyBac是来源于鳞翅目昆虫的DNA转座子,最初从杆状病毒侵染粉纹夜蛾Trichoplusiani的TN-368细胞中分离得到(Fraseretal.,1983)。其全长2472 bp, 并且具有一对13 bp末端反向重复序列和一对19 bp副末端重复序列,在副末端重复序列之间是2.1 kb转录单元,包含一个高频率切除和转座必需的1.8 kb编码转座酶的开放阅读框。piggyBac转座子常在TTAA目标位点插入,因此,也被归纳为TTAA特殊的可移动因子家族(Caryetal.,1989)。目前,该转座子系统已经在地中海实蝇、果蝇、赤拟谷盗、加勒比按实蝇Anastrephasuspensa、橘小实蝇、家蚕、棉红铃虫Pectinophoragossypiella、家蝇、淡色按蚊Anophelesalbimanus、埃及伊蚊中成功实现了转基因(Berghammeretal.,1999; Handleretal.,1998; Handler & Harrell,1999; Handler & McCombs,2000; Peloquinetal.,2000; Tamuraetal.,2000; Thibaultetal.,1999)。
3 转座子稳定性与转基因生物安全
转基因安全涉及2个方面:(1)转入的基因是否会对其他生物造成危害,包括对环境造成不利影响或影响取食者的发育及繁殖;(2)转基因是否会发生水平转移,即转座元件是否会发生二次转座。在转座酶的作用下转座子只发生一次移动,固定于转入生物的基因组内,而通常这种转座酶不存在于转入生物体内,所以不会因发生跳动而侵入其他生物体。但是,在与环境互作的过程中是否会发生再次转座或跳动尚不清楚。
例如,棉红铃虫是世界上最具破坏性的棉花害虫。据美国棉花协会统计,其每年造成将近2400万美元的经济损失。美国农业部在加州圣华金河谷选择了25000 hm2田块开展了携带绿色荧光蛋白基因筛选标记的转基因棉红铃虫的小规模试验,遗传转化式是由piggyBac转座子介导的转基因。野外释放试验充分考虑了多重物理和生物学因素,包括地理隔离、笼子隔离、生殖不育、雄性信息素陷进、移除可能含有野生型棉红铃虫的棉花、释放大量不育的棉红铃虫、必要时杀虫剂处理等。检测发现,与野生型相比,在实验室饲养20代左右的能稳定遗传绿色荧光蛋白的棉红铃虫的产卵量下降了19.8%,而piggyBac转座子的基因组整合位点没有发生任何变化,即转座子可以稳定地固定在基因组中而不消失或发生二次转座(Milleretal.,2001)。但是,由于试验的短期性和局限性,并不能够证实转座子可以一直稳定地遗传下去。
4 结语
在转基因昆虫中,载体基因是随目的基因一同导入的非必需元素。用于昆虫转基因的载体主要是转座子。目前,piggyBac转座子在昆虫遗传转化中应用最广泛,稳定性相对较高,应用该转座子已成功转化了多种昆虫。但是,还需要进一步评估这些转基因昆虫的遗传稳定性、各个世代基因表达的一致性,以及是否会发生基因水平转移等。因此,开发高效稳定的转座子系统对于昆虫遗传转化体系的建立和生物安全具有十分重要的意义。
参考文献
Ashburner M and Bergman C M. 2005.Drosophilamelanogaster: a case study of a model genomic sequence and its consequences.GenomeResearch, 15: 1661-1667.
Berghammer A J, Klingler M and Wimmer E A. 1999. A universal marker for transgenic insects.Nature, 402: 370-371.
Calvi B R, Hong T J, Findley S D and Gelbart W M. 1991. Evidence for a common evolutionary origin of inverted repeat transposons inDrosophilaand plants: hobo, Activator, and Tam3.Cell, 66: 465-471.
Cary L C, Goebel M, Corsaro B G, Wang H G, Rosen E and Fraser M J. 1989. Transposon mutagenesis of baculoviruses: analysis ofTrichoplusianitransposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses.Virology, 172: 156-169.
Catteruccia F, Nolan T, Blass C, Muller H M, Crisanti A, Kafatos F C and Loukeris T G. 2000a. TowardAnophelestransformation: Minos element activity in anopheline cells and embryos.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 97: 2157-2162.
Catteruccia F, Nolan T, Loukeris T G, Blass C, Savakis C, Kafatos F C and Crisanti A. 2000b. Stable germline transformation of the malaria mosquitoAnophelesstephensi.Nature, 405: 959-962.
Clark A G, Eisen M B, Smith D R, Bergman C M, Oliver B, Markow T A, Kaufman T C, Kellis M,etal. 2007. Evolution of genes and genomes on theDrosophilaphylogeny.Nature, 450: 203-218.
Coates C J, Jasinskiene N, Miyashiro L and James A A. 1998. Mariner transposition and transformation of the yellow fever mosquito,Aedesaegypti.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 95: 3748-3751.
Coupland G, Baker B, Schell J and Starlinger P. 1988. Characterization of the maize transposable element Ac by internal deletions.EMBOJournal, 7: 3653-3659.
Feschotte C. 2008. Transposable elements and the evolution of regulatory networks.NatureReviewsGenetics, 9: 397-405.
Franz G and Savakis C. 1991. Minos, a new transposable element fromDrosophilahydei, is a member of the Tc1-like family of transposons.NucleicAcidsResearch, 19: 6646.
Fraser M J, Smith G E and Summers M D. 1983. Acquisition of host cell DNA sequences by Baculoviruses: relationship between host DNA insertions and FP mutants ofAutographacalifornicaandGalleriamellonellanuclear polyhedrosis viruses.JournalofVirology, 47: 287-300.
Garza D, Medhora M, Koga A and Hartl D L. 1991. Introduction of the transposable element mariner into the germline ofDrosophilamelanogaster.Genetics, 128: 303-310.
Girard L and Freeling M. 1999. Regulatory changes as a consequence of transposon insertion.DevelopmentalGenetics, 25: 291-296.
Grewal S I and Jia S. 2007. Heterochromatin revisited.NatureReviewsGenetics, 8: 35-46.
Handler A M. 2001. A current perspective on insect gene transformation.InsectBiochemistryandMolecularBiology, 31: 111-128.
Handler A M and Harrell R A. 1999. Germline transformation ofDrosophilamelanogasterwith the piggyBac transposon vector.InsectMolecularBiology, 8: 449-457.
Handler A M and Gomez S P. 1997. A newhobo,Ac,Tam3 transposable element,hopper, fromBactroceradorsalisis distantly related tohoboandAc.Gene, 185: 133-135.
Handler A M and McCombs S D. 2000. The piggyBac transposon mediates germ-line transformation in the Oriental fruit fly and closely related elements exist in its genome.InsectMolecularBiology, 9: 605-612.
Handler A M, McCombs S D, Fraser M J and Saul S H. 1998. The lepidopteran transposon vector,piggyBac, mediates germ-line transformation in the Mediterranean fruit fly.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 95: 7520-7525.
Haymer D S and Marsh J L. 1986. Germ line and somatic instability of a white mutation inDrosophilamauritianadue to a transposable genetic element.DevelopmentalGenetics, 6: 281-291.
Holt R A, Subramanian G M, Halpern A, Sutton G G, Charlab R, Nusskern D R, Wincker P, Clark A G,etal. 2002. The genome sequence of the malaria mosquitoAnophelesgambiae.Science, 298: 129-149.
Jacobson J W, Medhora M M and Hartl D L. 1986. Molecular structure of a somatically unstable transposable element inDrosophila.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 83: 8684-8688.
Jasinskiene N, Coates C J, Benedict M Q, Cornel A J, Rafferty C S, James A A and Collins F H. 1998. Stable transformation of the yellow fever mosquito,Aedesaegypti, with the Hermes element from the housefly.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 95: 3743-3747.
Jordan I K, Rogozin I B, Glazko G V and Koonin E V. 2003. Origin of a substantial fraction of human regulatory sequences from transposable elements.TrendsinGenetics, 19: 68-72.
Kazazian H H Jr. 2004. Mobile elements: drivers of genome evolution.Science, 303: 1626-1632.
Khillan J S, Overbeek P A and Westphal H. 1985.DrosophilaP element integration in the mouse.DevelopmentalBiology, 109: 247-250.
Kidwell M G. 1977. Reciprocal differences in female recombination associated with hybrid dysgenesis inDrosophilamelanogaster.GeneticsResearch, 30: 77-88.
Klinakis A G, Loukeris T G, Pavlopoulos A and Savakis C. 2000. Mobility assays confirm the broad host-range activity of the Minos transposable element and validate new transformation tools.InsectMolecularBiology, 9: 269-275.
Lidholm D A, Lohe A R and Hartl D L. 1993. The transposable element mariner mediates germline transformation inDrosophilamelanogaster.Genetics, 134: 859-868.
Lohe A R and Hartl D L. 1996. Germline transformation ofDrosophilaviriliswith the transposable element mariner.Genetics, 143: 365-374.
Loukeris T G, Livadaras I, Arcà B, Zabalou S and Savakis C. 1995. Gene transfer into the medfly,Ceratitiscapitata, with aDrosophilahydeitransposable element.Science, 270: 2002-2005.
McClintock B. 1956. Controlling elements and the gene.ColdSpringHarborSymposiaonQuantitativeBiology, 21: 197-216.
Miller E R, Staten T, Claus J, Sledge M, Peloquin J and Miller T. 2001. A multiple generation life history study on rearing a genetically altered (EGFP) strain of pink bollworm (Lepidoptera: Gelechiidae)∥Proceedings of Belt Wide Cotton Conf. Anaheim, CA.
Nelson O E and Klein A S. 1984. Characterization of an spm-controlled bronze-mutable allele in maize.Genetics, 106: 769-779.
Nene V, Wortman J R, Lawson D, Haas B, Kodira C, Tu Z J, Loftus B, Xi Z,etal. 2007. Genome sequence ofAedesaegypti, a major arbovirus vector.Science, 316: 1718-1723.
Ni J Z, Grate L, Donohue J P, Preston C, Nobida N, O′Brien G, Shiue L, Clark T A, Blume J E and Ares M Jr. 2007. Ultraconserved elements are associated with homeostatic control of splicing regulators by alternative splicing and nonsense-mediated decay.Genes&Development, 1: 708-718.
O′Brochta D A, Atkinson P W and Lehane M J. 2000. Transformation ofStomoxyscalcitranswith a Hermes gene vector.InsectMolecularBiology, 9: 531-538.
Osanai-Futahashi M, Suetsugu Y, Mita K and Fujiwara H. 2008. Genome-wide screening and characterization of transposable elements and their distribution analysis in the silkworm,Bombyxmori.InsectBiochemistryandMolecularBiology, 38: 1046-1057.
Peloquin J J, Thibault S T, Staten R and Miller T A. 2000. Germ-line transformation of pink bollworm (Lepidoptera: gelechiidae) mediated by thepiggyBactransposable element.InsectMolecularBiology, 9: 323-333.
Pinkerton A C, Whyard S, Mende H A, Coates C J, O′Brochta D A and Atkinson P W. 1999. The Queensland fruit fly,Bactroceratryoni, contains multiple members of the hAT family of transposable elements.InsectMolecularBiology, 8: 423-434.
Piriyapongsa J, Rutledge M T, Patel S, Borodovsky M and Jordan I K. 2007. Evaluating the protein coding potential of exonized transposable element sequences.BiologyDirect, 2: 31.
Quesneville H, Bergman C M, Andrieu O, Autard D, Nouaud D, Ashburner M and Anxolabehere D. 2005. Combined evidence annotation of transposable elements in genome sequences.PLoSComputerBiology, 1: 165-166.
Rubin G M and Spradling A C. 1982. Genetic transformation ofDrosophilawith transposable element vectors.Science, 218: 348-353.
Slotkin R K and Martienssen R. 2007. Transposable elements and the epigenetic regulation of the genome.NatureReviewsGenetics, 8: 272-285.
Tamura T, Thibert C, Royer C, Kanda T, Abraham E, Kamba M, Komoto N, Thomas J L, Mauchamp B, Chavancy G, Shirk P, Fraser M, Prudhomme J C and Couble P. 2000. Germline transformation of the silkwormBombyxmoriL. using apiggyBactransposon-derived vector.NatureBiotechnology, 18: 81-84.
Thibault S T, Luu H T, Vann N and Miller T A. 1999. Precise excision and transposition of piggyBac in pink bollworm embryos.InsectMolecularBiology, 8: 119-123.
van de Lagemaat L N, Landry J R, Mager D L and Medstrand P. 2003. Transposable elements in mammals promote regulatory variation and diversification of genes with specialized functions.TrendsinGenetics, 19: 530-536.
Wallace M R, Andersen L B, Saulino A M, Gregory P E, Glover T W and Collins F S. 1991. A de novo Alu insertion results in neurofibromatosis type 1.Nature, 353: 864-866.
Wang X, Fang X, Yang P, Jiang X, Jiang F, Zhao D, Li B, Cui F,etal. 2014. The locust genome provides insight into swarm formation and long-distance flight.NatureCommunications, 5: 2957.
Warren W D, Atkinson P W and O′Brochta D A. 1994. The Hermes transposable element from the house fly,Muscadomestica, is a short inverted repeat-type element of the hobo, Ac, and Tam3 (hAT) element family.GeneticalResearch, 64: 87-97.
Wicker T, Sabot F, Hua-Van A, Bennetzen J L, Capy P, Chalhoub B, Flavell A, Leroy P, Morgante M, Panaud O, Paux E, SanMiguel P and Schulman A H. 2007. A unified classification system for eukaryotic transposable elements.NatureReviewGenetics, 8: 973-982.
Xu H E, Zhang H H, Xia T, Han M J, Shen Y H and Zhang Z. 2013. BmTEdb: a collective database of transposable elements in the silkworm genome.Database(Oxford), doi: 10.1093/database/bat055.
(责任编辑:杨郁霞)
The application of insect transposons in transgenic technology
Jun XU1,2, Hong-bo ZHANG3, Min-jin HAN3, An-jiang TAN1, Yong-ping HUANG1, Ze ZHANG3*
1Key Laboratory of Insect Developmental and Evolutionary Biology, Institute of Plant Physiology and Ecology, Shanghai Institutes
forBiologicalSciences,ChineseAcademyofSciences,Shanghai200032,China;2University of Chinese Academy of Sciences,
Beijing100049,China;3School of Life Sciences, Chongqing University, Chongqing 400044, China
Abstract:Transposon is a class of mobile repetitive DNA segments in the genome. Many studies found that transposons constitute a significant component of eukaryotic genomes. They are the main driving force of genomic and phenotypic evolution, and have an important contribution to the evolution of gene regulatory networks. Transposon mobility in the genome makes them an attractive tool in the field of biotechnology and molecular biology, especially in transgenic technology. In this review, we introduced the distribution, types and functions of transposons in the insects, reviewed the application and examples of insect transposons in transgenic techniques, and discussed the transgenic security and stability of transposon.
Key words:transposon; insect; transgene; security
通讯作者*(Author for correspondence), E-mail: wanfanghao@caas.cn
作者简介:刘桂清, 女, 助理研究员。 研究方向: 昆虫分子生物学与外来生物入侵。 E-mail: pepsiliu81@163.com
基金项目:环保公益性行业科研专项(201409061); 农业部2014年农作物病虫鼠害疫情监测与防治(外来入侵生物防治)项目; 人力资源社会保障部2014年度留学人员科技活动择优资助项目
收稿日期(Received): 2015-01-12接受日期(Accepted): 2015-02-09
DOI:10. 3969/j.issn.2095-1787.2015.02.004

