两种磺胺喹噁啉锌配合物的合成、晶体结构和荧光性质
康晶燕 黄世杰 赵秀华 赵亚云 李 星*,
(1宁波大学理学院材料科学与化学工程学院,宁波315211)
(2中华人民共和国北仑出入境检验检疫局,宁波315800)
康晶燕1黄世杰2赵秀华1赵亚云1李 星*,1
(1宁波大学理学院材料科学与化学工程学院,宁波315211)
(2中华人民共和国北仑出入境检验检疫局,宁波315800)
以磺胺喹噁啉为配体合成了2种锌的配合物[Zn(L)2(phen)]·H2O(1)和{[Zn(L)2(bpy)]·2EtOH}n(2)(L=磺胺喹噁啉,phen=菲咯啉,bpy=4,4′-联吡啶)并对其进行了单晶X-射线衍射,粉末X-射线衍射,热重,元素分析,红外光谱,紫外光谱和荧光光谱测定。单晶X-射线分析表明化合物1是单核结构,属单斜晶系,P21/c空间群。化合物2是一维结构,属单斜晶系,C2/c空间群。热分析表明化合物1和2都有很高的热稳定性。
磺胺喹噁啉;Zn配合物;晶体结构;荧光性质
0 Introduction
In the field of supramolecular chemistry,great interest has recently been focused on the crystal engineering of coordination frameworks due to their new topologies,intriguing architectures,intertwining phenomena,and potential applications in microeletronics,nonlinear optics,molecular selection, ion exchange,and catalysis[1-4].It has been documented that the geometries and properties of organic ligands exert great effect on structural frameworks of supramolecules[5-6].As we all know that sulfaquinoxalineis not only an excellently versatile ligand but also widely used in medicine due to their pharmaceutical properties such as antibacterial[7]and antitumor[8-13]activity.Sulfonamides belong to one class of synthetic antibiotics[14].They have been widely used for treatment of many human and animal diseases,such as infectious diseases of gastrointestinal and respiratory[15-17].The use of metal complexes as pharmaceutical has shown great promise in recent years as anticancer agents[18]. For example,Agcoordination compound[19],Cecoordination compound[20],Nicoordination compound[21]and Cucoordination compound[22-24]have shown higher antimicrobial activity than free ligands.These findings have aroused considerable interests and much more effort has been devoted to design and synthesize new multifunctional coordination compounds.Currently, the complexes which are formed by sulfaquinoxaline and transition metals have not been storied.In this paper,we report the syntheses and crystal structures of two Zncomplexes,namely,[Zn(L)2(phen)]·H2O(1)and{[Zn(L)2(bpy)]·2EtOH}n(2),which characterized by single crystal X-ray diffraction,powder X-ray diffraction, thermal analysis,elemental analysis,UV-Vis and fluorescence.
1 Experimental
1.1 M aterials and m easurem ents
All commercially available chemicals are of reagent grade and were used as received without further purification.Elemental analyses(C,H,and N) were performed using an EA1110CHNS-0 CE elemental analyzer.The FTIR spectra were recorded from KBr pellets in the range of 4 000~400 cm-1on a Shimadzu FTIR-8900 spectrometer.UV-visible spectra were recorded(190~400 nm region)on a TU-1901 UV-Vis spectrophotometer,Beijing pgeneral.Thermal analyses were carried out in air atmosphere with a heating rate of 10℃·min-1on a SⅡTG/DTA 7300 integration thermal analyzer.The solid state fluorescence spectra were measured on F-4600 Fluorescence spectrophotometer.Powder X-ray diffraction(PXRD)data were collected on a Bruker D8 Focus X-ray diffractometer using Cu Kα1(λ=0.154 06 nm)radiation.The calculated PXRD patterns were produced using the SHELXTL-XPOW program.Single crystal X-ray diffraction was performed on a Bruker SMART APEX-II CCD area detector.
1.2 Synthesis of[Zn(L)2(phen)]·H2O(1)
The sample of Zn(NO3)2·6H2O(1 mg,4 μmol), sulfaquinoxaline sodium(26 μg,4 μmol)and 1,10-phen(8 μg,4 μmol)were placed in a small vial including ethanol(0.06 mL)and H2O(0.5 m L).The vial was sealed and heated to 80℃for two days and thereafter cooled slowly to room temperature,and yellow bulk crystal was gained(Yield:27%).Anal. Calcd.(%)for 1(C40H32N10O5S2Zn):C 55.72,H 3.74,N 16.24;Found(%):C 54.21,H 3.86,N 17.16.IR(KBr, cm-1):3 442(m),1 623(m),1 596(m),1 542(m),1 518 (w),1 502(w),1 490(w),1 465(w),1 418(s),1 347(s), 1 328(w),1 298(m),1 277(m),1 247(w),1 183(w),1 137 (vs),1 086(vs),1 037(w),1 014(w),949(m),764(m), 724(m),685(vs),609(s),543(vs).
1.3 Synthesis of{[Zn(L)2(bpy)]·2EtOH}n(2)
A aqueous solution(5.0 mL)of Zn(OAc)2·2H2O (0.022 g,0.10 mmol)was placed at the bottom of a glass tube.Ethanol(6.0 mL)were added slowly in middle part of glass tube,finally upon which a solution of sulfaquinoxaline 0.032 g(0.10 mmol)and 0.020 g(0.10 mmol)4,4-bpy in ethanol(5.0 mL)was carefully layered.The glass tube at room temperature was allowed to stand for 15 days,and the yellow crystal precipitated in the middle of tube(Yield: 59%).Anal.Calcd.(%)for 2(C42H42N10O6S2Zn):C 55.29; H 4.64;N 15.35;Found(%):C 56.02,H 4.77;N 15.47.IR(KBr,cm-1):3 525(m),3 315(m),3 195(m), 2 967(w),1 642(m),1 597(m),1 543(m),1 493(m), 1 426(m),1 352(vs),1 299(s),1 287(s),1 250(m), 1 217(s),1 180(m),1 134(m),1 086(s),951(m),899 (s),853(s),832(s),817(s),775(s),761(s),733(s),719 (s),671(s),635(w),613(s),556(m),537(m),510(w).
1.4 X-ray diffraction data collection and refinement
Crystallographic data for 1(size:0.48 mm×0.41 mm×0.34 mm)and 2(size:0.48 mm×0.45 mm×0.38 mm)were selected under a Bruker-AXS diffractometer with graphite monochromated Mo Kα radiation(λ=0.710 73nm)for cell determination and subsequent data collection at room temperature.All absorption corrections were applied using the ABSCOR(Higashi, 1995)program[25].The Lp factor and empirical absorption were performed to correct and store the data and correct the unit cell parameters by using SAINT and SMART software[26-27].The structure was solved by using direct method and followed by successive Fourier and difference Fourier syntheses.The metal atoms were located from the E-maps,and other nonhydrogen atoms were derived from the successive difference Fourier synthesis,All non-hydrogen atoms were refined with anisotropic displacement parameters by full-matrix least-squares technique.All hydrogen atoms were placed geometrically and were refined in a riding-model approximation.The structure were refined on F2by full-matrix least-square using SHELX-97 programs package[28].The crystallographic data as well as details of the data collection and refinement for complexes 1 and 2 were listed in Table 1,the selected bond lengths and bond angles in Table 2 and hydrogen bond lengths and bond angles in Table 3.
CCDC:991934,1;991935,2.
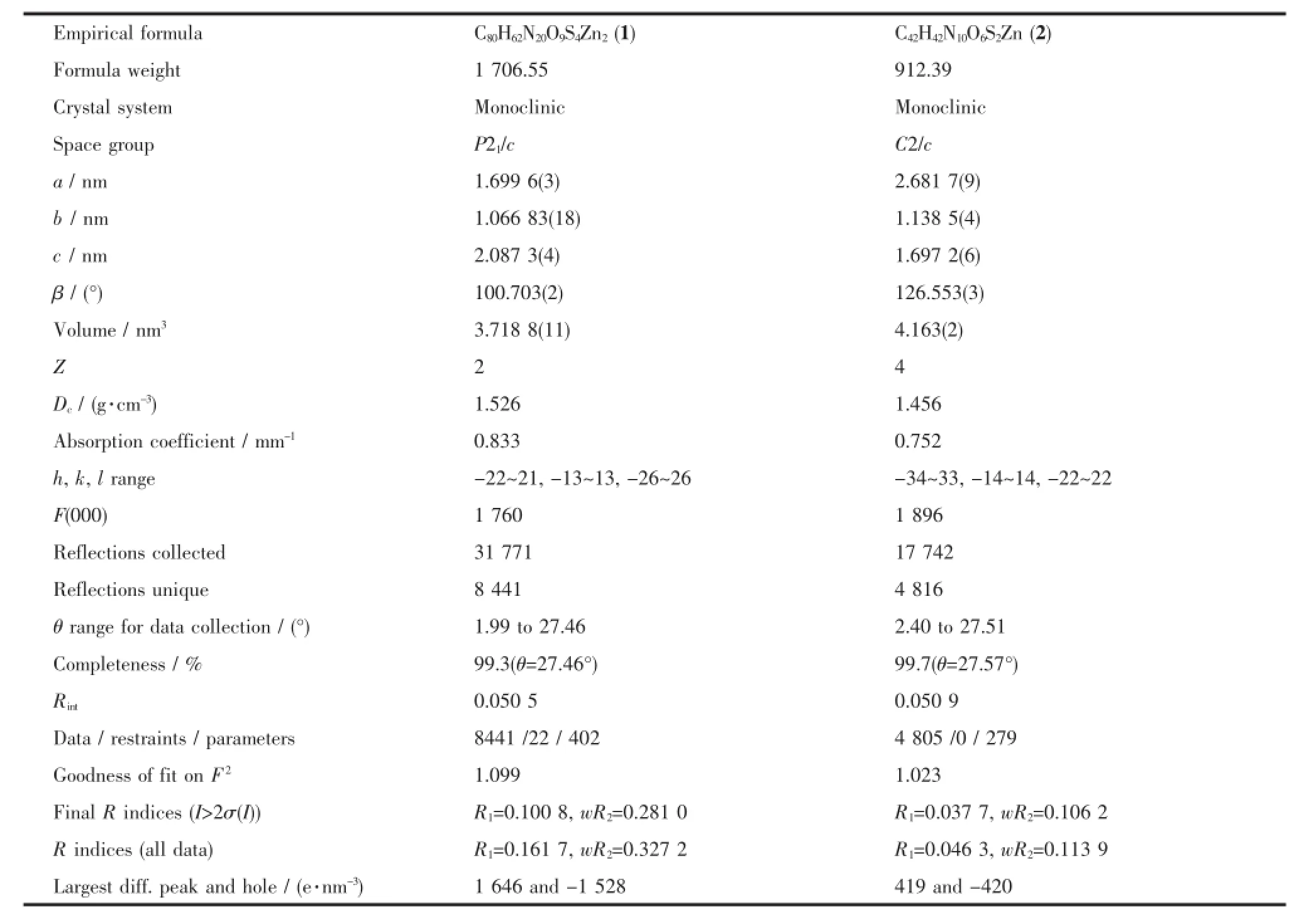
Tab le 1 Crystal data and structu re refinem ent for com p lexes 1 and 2

Table2 Selected bond lengths(nm)and bond angles()for complexes 1 and 2
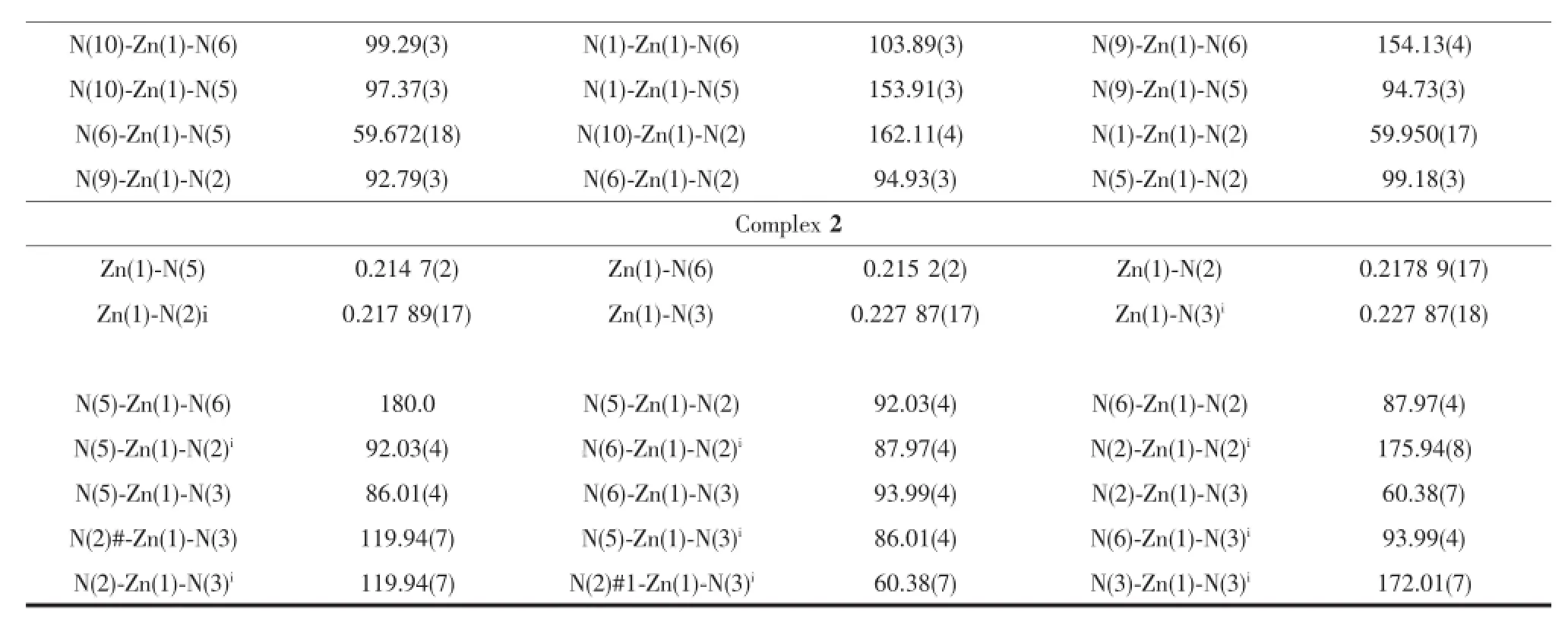
Continued Table 2
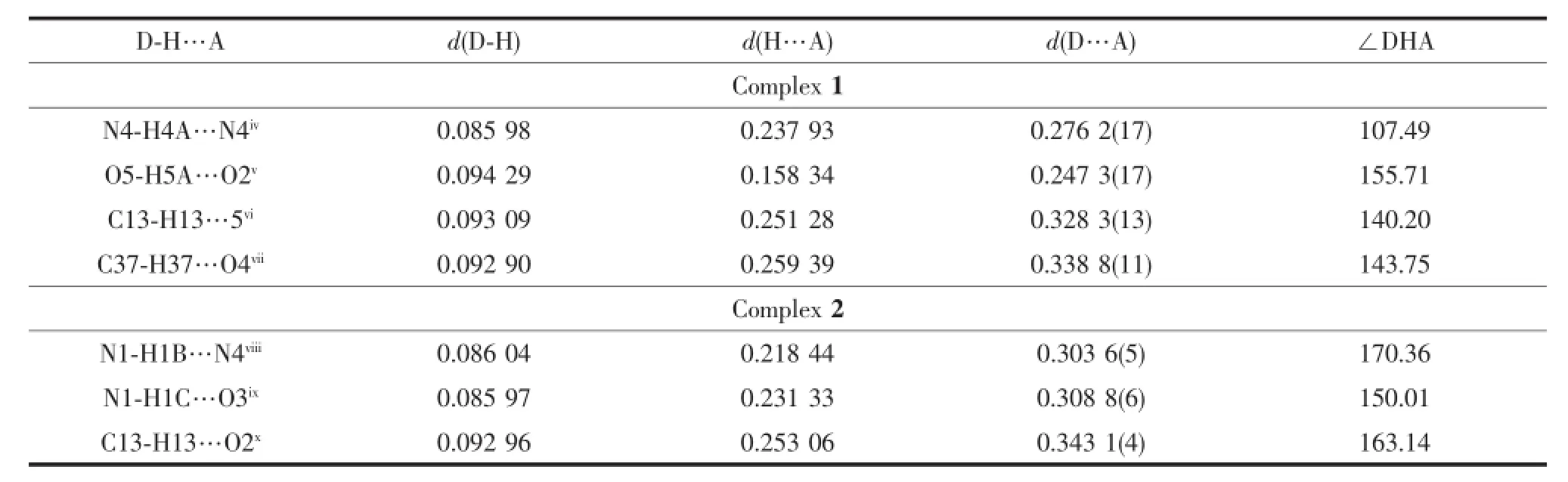
Tab le 3 Hyd rogen bond lengths(nm)and bond angles(°)for com p lexes 1 and 2
2 Results and discussion
2.1 Crystal and molecular structures
2.1.1 Structure description for complex 1

Fig.1 Coordination environment of metal ion in 1 with thermal ellipsoids at 30%probability
Single-crystal X-ray diffraction analysis reveals that complex 1 is mononuclear structure crystallizes in the monoclinic with space group P21/c and Z=4,the asymmetric unit of complex 1 contains one zinc ion, one 1,10-phen,two sulfaquinoxalinum ligands and one free water molecules.The local coordination environment of around Znion is shown in Fig.1,in which Znexhibits six-coordinated octahedral distortion coordination geometry,of which four nitrogen atoms from two sulfaquinoxalinum ligands(N1,N2,N5,N6)and two nitrogen atoms from 1,10-phen ligand(N9,N10)with Zn-N distance from 0.210 44(7)to 0.234 67(7)nm[29].As showed in Fig.2, the N4 from sulfaquinoxalinum acts as hydrogen-bond donor,contributing an H atom to amine atom N4ivof the other sulfaquinoxalinum forming N4-H4A…N4iv; the water molecule serves as hydrogen-bond donor to atom O2v,forming O5-H5A…O2v;the benzene rings acts as the donors,contributing the H atoms to the O atomsof water molecules forming C13-H13…O5vi,and C37-H37…O4vii.Through π…π stacking(d=0.398 4 nm)and hydrogen bond interactions(d(N4…N4iv)= 0.277 06 nm,d(O5…O2v)=0.250 70 nm,Symmetry code:iv1-x,1-y,-z,vx,-1+y,z,vi1-x,-y,-z,vii1-x, 1/2+y,1/2-z)[30],the complex 1 extends into a network structure(Fig.3).
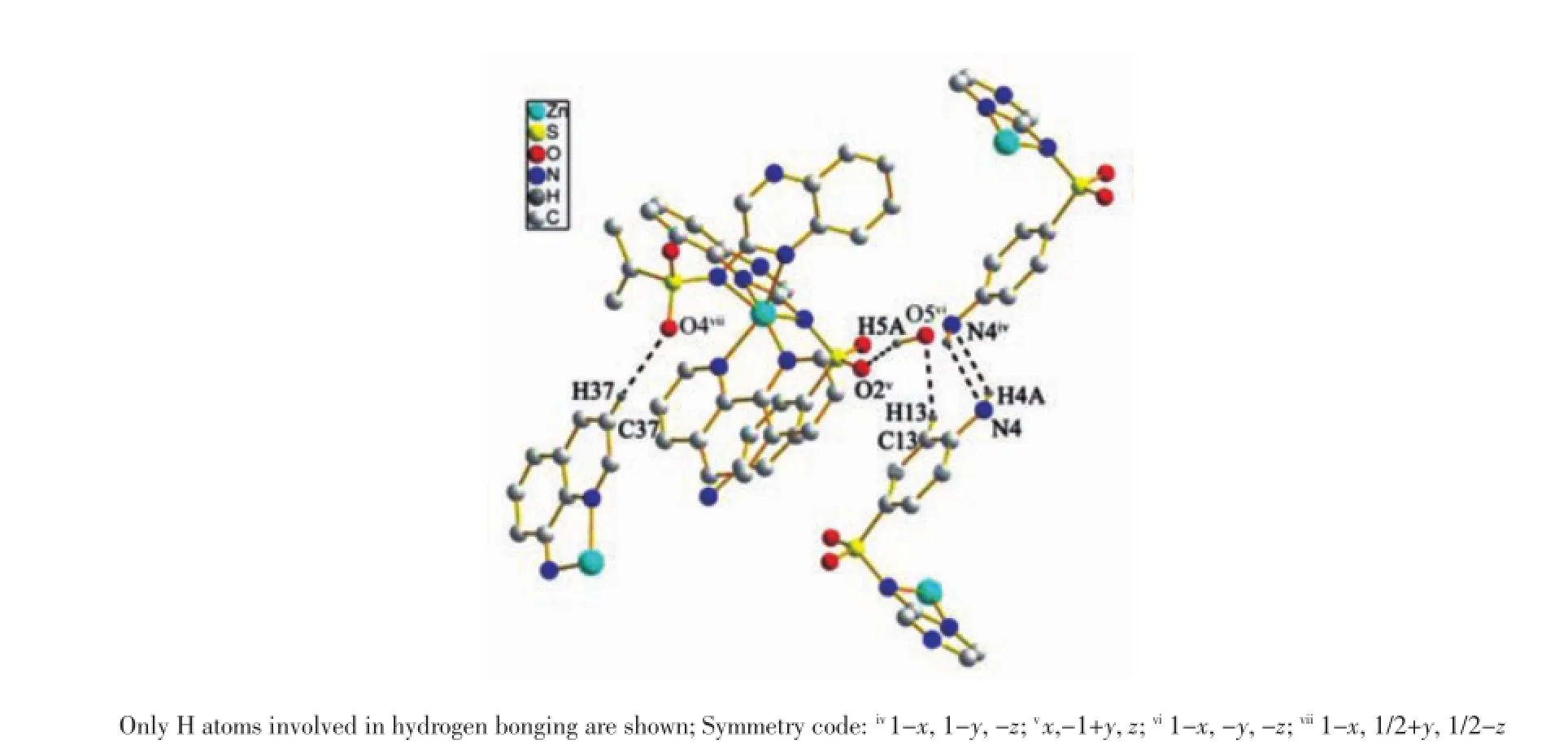
Fig.2A view of the N-H…N and C-H…O interactions(dashed lines)in 1
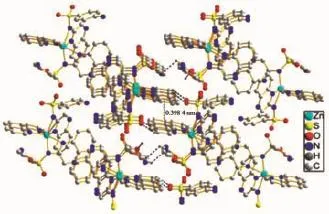
Fig.3A view of packing framework in 1
2.1.2 Structure description for complex 2
Single crystal X-ray diffraction analysis reveals that complex 2 has a one-dimensional chain structure. It crystallizes in the monoclinic system with space group C2/c.The asymmetric unit consists of one Zn, one 4,4′-bpy,two sulfaquinoxalinum ligands and two freeethanolmolecules.Thelocalcoordination environment of around zinc ion is shown in Fig.3,in whichZnexhibitssix-coordinatedoctahedral distortion coordination geometry with four nitrogen atoms from two sulfaquinoxalinum ligands(N2,N3, N2i,N3i)and two nitrogen atoms from two different 4,4′-bpy ligand(N5,N6).The Zn-N distance ranges from 0.214 7(2)to 0.227 87(18)nm.The structure extends into one-dimensional chain by bridging of 4,4′-bpy ligand with the adjacent Zndistance of1.138 5 nm(Fig.4).Different kinds of hydrogen bonds can be observed in the structure of 2(Fig.5).The benzene ring of sulfaquinoxalinum acts as a hydrogenbond donor,contributing an H atom to O2 of the other sulfaquinoxalinum forming C13-H13…O2xhydrogenbonding interaction.The N1-H1 from sulfaquinoxalinum and N4 from the neighboring sulfaquinoxalinum ligand form the N1-H1…N4viiihydrogen-bonding interaction. The ethanol molecule serves as the hydrogen-bond receptor,accepting H atoms from amine atom N1 forming N1-H1C…O3ixhydrogen-bonding interaction (Table 3).The structural units further extend into a two-dimensional hydrogen-bonding network(Fig.6).
2.2 Pow der X-ray diffraction analysis
In an attempt to confirm the homogeneity of the synthesized material,we have analyzed the PXRD

Fig.4 Coordination environment of metal ion in 2 with thermal ellipsoids at 30%probability

Fig.5 A view of the N-H…N,N-H…O and C-H…O interactions(dashed lines)in 2
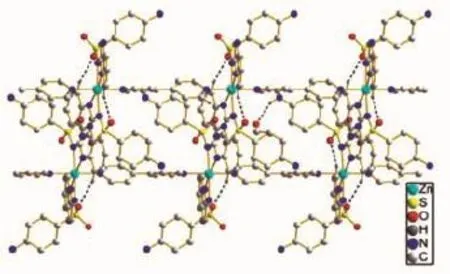
Fig.6 A view of 2D chain structure of complex 2
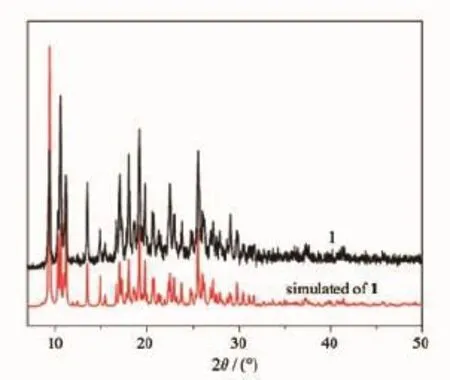
Fig.7 Experimental and simulated powder XRD patterns of 1

Fig.8 Experimental and simulated powder XRD patterns of 2
pattern of the complex and correlated the results with the simulated powder pattern obtained from the single crystal data of complex 1 and 2.As depicted in Fig.5 and Fig.6,the basic feature peaks of the PXRD patterns resemble the simulated from single crystal data for the complex,indicating that the bulk products obtained are homogenous for complex 1 and 2, respectively.
2.3 UV-Vis absorption and fluorescent spectra
The UV-Vis absorption spectra of compound 1 and 2 are shown in Fig.9 and Fig.10 and,respectively. The UV-Vis absorption of phen is at 230,264 and 347 nm,and the UV-Vis absorption of sulfaquinoxalinum is at 230 and 260 nm.Therefore, the high-energy peaks of 227 and 266 nm in 1 could come from fluorescent emissions of the phen,and peak of 366 nm from the fluorescent emissions of sulfaquinoxalinum ligand(Fig.9).The UV-Vis absorption of the compounds 2 is an overlay enhancement from the UV-Vis absorption of the bpy and the sulfaquinoxalinum ligands because these ligands have similar absorption bond(Fig.10).
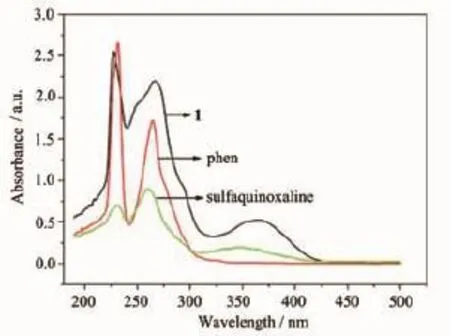
Fig.9 UV-Vis absorption spectra of compound 1,phen and sulfaquinoxaline in the dichloromethane at room temperature
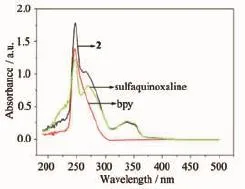
Fig.10 UV-Vis absorption spectra of compound 2,bpy and sulfaquinoxaline in the tetrahydrofuran solution at room temperature
2.4 Fluorescent em ission
Previous studies have shown that compounds containing zinc could exhibit fine photoluminescent properties[31].The solid-state fluorescent spectra of 1 and 2 at room temperature are displayed in Fig.11 and Fig.12,respectively.The phen shows the strong emission peaks at 361 and 379 nm upon the excitation at 400 nm and the free sulfaquinoxaline shows the strong emission peak at 448 nm upon the excitation at 419 nm.Compound 1 displays the strongemission peak at 542 nm,which may originate from the radiative transition of ligand-to-metal or metal-toligand charge transfer emission.
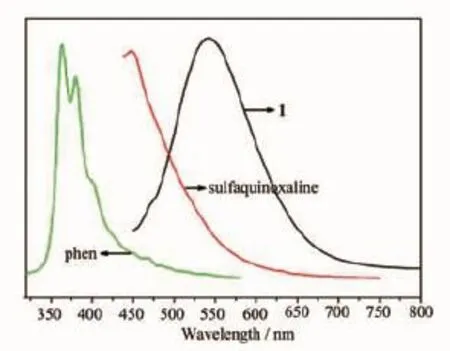
Fig.11 Fluorescent spectra of 1(λex=423 nm),phen (λex=400 nm)and sulfaquinoxaline(λex=419 nm)

Fig.12 Fluorescent spectra of 2(λex=400 nm),bpy (λex=342 nm)and sulfaquinoxaline(λex=419 nm)
The bpy shows the strong peak at 468 nm upon the excitation at 400 nm.In comparison,compound 2 displays the similarly fluorescent emission peak 469 nm,which is attributed to that the sulfaquinoxaline has no effect on the interaction between the metal and the ligand[32].
2.5 Thermal analysis
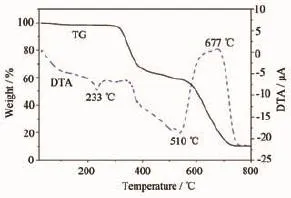
Fig.13 TG-DTA curve of compound 1

Fig.14 TG-DTA curve of compound 2
Thermal analyses are conducted to determine the thermal stability of the title complexes,which is an important aspect for coordination compounds[33-34].As shown in Fig.13,The DTA curve of 1 shows two weak endothermic peaks and one strong exothermic peak centered at 233,510 and 677℃,respectively, corresponding to the physical or chemical reactions[35]. The first weight loss of 1.9%(Calcd.2%)corresponds to removal of one water molecule from room temperature to 289℃.The second sharp weight loss of 33.5%(Calcd.34.83%)corresponds to removal of one sulfaquinoxaline molecule per formula unit from 289℃to 418℃,which exhibits that the framework stability of 1 is up to 289℃,the third sharp weight loss of 55.5%(Calcd.55.73%)corresponds to removal of one sulfaquinoxaline and one phen molecule per formula unit from 418℃to 774℃.The final decomposed product presumably is ZnO(Obsd. 10.14%,Calcd.8.9%,based on the remaining mass) upon 774℃.
Similarly,the DTA curve of 2 shows two weak endothermic peaks and one strong exothermic peak centered at 240,493 and 607℃,respectively(Fig. 14).The first drop of weight is observed from 153 to 231℃,with a weight loss of 9.3%(Calcd.10.1%) corresponding to elimination of two EtOH molecule. The second sharp drop of weight is observed from 231 to 485℃,with a weight loss of 49.4%(Calcd.50%) corresponding to elimination of one bpy molecule and one sulfaquinoxaline per formula unit,which indicatesthe stability of the framework of 2 up to 231℃.The third sharp weight decrease of 31.6%(Calcd.32.9%) upon 485℃can be attributed to loss of the other sulfaquinoxaline ligand per formula unit in 2.The final decomposed product presumably is ZnO(Obsd. 9.7%,Calcd.8.9%,based on the remaining mass) upon 683℃.
3 Conclusions
[1]Noro S,Kitagawa S,Kondo M,et al.Angew.Chem.Int.Ed., 2000,39:2082-2085
[2]Zaworotko M J.Chem.Soc.Rev.,1994,23:283-288
[3]Yaghi O M,Li H,Davis C,et al.Acc.Chem.Res.,1998,31: 474-484
[4]JIANG Jing(江晶),WANG Dong-Jie(王冬杰),LIU Zhi-Peng(刘志鹏),et al.Chinese J.Inorg.Chem.(无机化学学报),2013,29(9):1952-1956
[5]Li X,Wei D Y,Huang S J,et al.J.Solid State Chem.,2009, 182:95-101
[6]LIU Xian-Chun(刘羡春),XU Qing(徐清),WANG Li (王丽),et al.J.Ningbo Univ.(宁波大学学报),2009,22(2): 251-254
[7]Kremer E,Facchin G,Estévez E,et al.J.Inorg.Biochem., 2006,100:1167-1175
[8]Supuran C T,Casini A,Scozzafava A,et al.Curr.Cancer Drug Targets,2002,2:55-75
[9]Owa T,Nagasu T.Expert Opin.Ther.Patents,2000,10: 1725-1740
[10]Supuran C T,Scozzafava A.Expert Opin.Ther.Patents, 2000,10:575-600
[11]Saleh N,Khowdiary M,Badawi A F.Tenside Surfactants Deterg.,2001,1:61-97
[12]Lobb K L,Hipskind P A,Aikins J A,et al.Eur.J.Med. Chem.,2004,47:5367-5380
[13]Medina J C,Roche D,Shan B,et al.Bioorg.Med.Chem. Lett.,1999,9:1843-1846
[14]Shao B,Dong D,Wu Y,et al.Anal.Chim.Acta,2005,546: 173-181
[15]Harun A L P.J.Anim.Vet.Adv.,2008,7:1174-1178
[16]Nebot C,Regal P,Mirtinez B,et al.J.Food Drug Anal., 2010,18:191-201
[17]FangGZ,He JX,WangS.J.Chromatogr.A,2006,1127:12-17
[18]Wong E,Giandomenico C M.Chem.Rev.,1999,99:2451 -2466
[19]Milliam M.Martindale:The Extra Pharmacopoeia.London: Pharmaceutical Press,1996:31-35
[20]Sigel A,Sigel H.Metal Ions in Biological Systems,New York:CRC,1983:261-278
[21]Kocer S,Urus S,Cakir A,et al.Dalton Trans.,2014,43: 6148-6164
[22]Cabaleiro S,Castro J,Romero J,et al.Acta Crystallogr. Sect.C:Cryst.Struct.Commun.,2000,56:293-295
[23]Beloso I,Castro J,Garcia-Vazquez J A,et al.Polyhedron, 2006,25:2673-2682
[24]ZHAO Xiu-hua(赵秀华),ZHAO Ya-yun(赵亚云),ZHANG Jie(张洁),et al.Chinese J.Inorg.Chem.(无机化学学报), 2014,30(3):633-639
[25]Higashi T.ABSCOR,Rigaku Corporation,Tokyo,Japan, 1995.
[26]Sheldrick G M.SADABS 2.05,University of Göttingen, Germany,2002.
[27]RAN Fan-Min(冉繁敏),ZHAO Ya-Yun(赵亚云),ZHAO Xiu-Hua(赵秀华),et al.Chinese J.Inorg.Chem.(无机化学学报),2014,30(4):913-920
[28]Sheldrick G M.SHELXL97,Program for the Refinement of Crystal Structure,University of Göttingen,Germany,1997.
[29]Blasco F,Ortiz R,Perelló L,et al.J.Inorg.Biochem.,1994, 53:117-126
[30]JIANG Jing(江晶),WANG Dong-Jie(王冬杰),LI Xing (李星),et al.J.Ningbo Univ.(宁波大学学报),2013,26(1): 74-76
[31]Xue X,Wang X S,Wang L Z,et al.Inorg.Chem.,2002,41: 6544-6546
[32]WANG Qing-Qi(王清琪),XU Qing(徐清).J.Ningbo Univ. (宁波大学学报),2012,25(2):112-115
[33]HUANG You-Gui(黄有桂),ZHOU You-Fu(周有福),WU Ben-Lai(吴本来),et al.Chinese J.Struct.Chem.(结构化学),2005,24:1123-1128
[34]Guo H D,Guo X M,Batten S R,et al.Cryst.Growth Des., 2009,9:1394-1401
[35]Qi J L,Xu W,Zheng Y Q.Z.Naturforsch.,2012,67b:1185 -1190
Syntheses,Crystal Structures and Fluorescent Properties of Two ZnCom plexes Based on Sulfaquinoxalines
KANG Jing-Yan1HUANG Shi-Jie2ZHAO Xiu-Hua1ZHAO Ya-Yun1LI Xing*,1
(1Faculty of Science,Ningbo University,Ningbo,Zhejiang 315211,China)
(2Beilun entry-exit inspection and Quarantine Bureau of P.R.C,Ningbo,Zhejiang 315800,China)
Two Zncompounds,[Zn(L)2(phen)]·H2O(1)and{[Zn(L)2(bpy)]·2EtOH}n(2)(L=sulfaquinoxaline, phen=phenanthroline,bpy=4,4′-bipyridine)have been synthesized and characterized by single crystal X-ray diffraction,thermal analysis,elemental analysis,IR,UV-Vis and fluorescence.Single crystal X-ray diffraction reveals that complex 1 is a mononuclear structure and complex 2 is one-dimensional structure.Thermal analyses show that complex 1 and 2 have high thermal stability.CCDC:991934,1;991935,2.
sulfaquinoxaline;Zncompound;crystal structure;fluorescence
O614.24+1
A
1001-4861(2015)04-0798-09
10.11862/CJIC.2015.097
2014-10-12。收修改稿日期:2015-01-05。
国家自然科学基金(No.20971075),宁波市基金(No.2014A610106、2014C50037),浙江省新苗人才计划项目(No.2013R405068),浙江省省级重点企业研究院青年科学家培养计划(No.2013R40009),宁波大学研究生优秀学位论文培育基金(No.py2013001),宁波大学王宽诚幸福基金资助。
*通讯联系人。E-mail:lixing@nbu.edu.cn;会员登记号:S060014541M。

