Association of schizophrenia with the rs821633 polymorphism in the DISC1 gene among Han Chinese
Guoqin HU, Chengqing YANG, Jing ZHAO, Minghuan ZHU, Xiangqing GUO, Chenxi BAO, Si JIA,Ahong XU, Yong JIE, Zuowei WANG, Chen ZHANG, Yongguang HE,*, Qinyu LV,*, Shunying YU,Zhenghui YI
•Original research article•
Association of schizophrenia with the rs821633 polymorphism in the DISC1 gene among Han Chinese
Guoqin HU1, Chengqing YANG1, Jing ZHAO1, Minghuan ZHU1, Xiangqing GUO1, Chenxi BAO1, Si JIA1,Ahong XU2, Yong JIE2, Zuowei WANG2, Chen ZHANG1, Yongguang HE1,*, Qinyu LV1,*, Shunying YU1,Zhenghui YI1
schizophrenia;DISC1gene; transmission disequilibrium test; single nucleotide polymorphism; age of onset; China
1. Introduction
Schizophrenia is a common mental illness of unknown etiology that usually starts in young adulthood and includes various combinations of positive psychotic symptoms, negative symptoms, and cognitive impairment in attention and information processing.[1]In 2000 Millar and colleagues[2]first reported an association of the Disrupted-in-Schizophrenia 1 (DISC1) gene with schizophrenia in a large Scottsh genealogy. Subsequent studies by Paunio,[3]Burdick,[4]and Cannon[5]showed that theDISC1gene is associated with brain structure and cognitive function. More recent studies confirmed that theDISC1gene is one of the genes most closely associated schizophrenia[6]and that the three primary risk alleles in theDISC1gene are rs1538979(T),rs821577(G), and rs821633(C).[7,8]Moreover, Tomppo and colleagues[7]reported that social anhedonia – a symptom that often occurs before the onset of the core psychotic symptoms of schizophrenia[9,10]– is more prominent in carriers of the risk allele for rs821633 than among carriers of the risk alleles for rs1538979 and rs821577.
There have been several previous studies ofDISC1gene polymorphisms among individuals with schizophrenia in China,[11-16]but none of them have considered the relationship of the polymorphisms to the age of onset of the illness. The current study assesses the relationship between the rs821633(C) risk allele in theDISC1gene and the occurrence and age of onset of schizophrenia in the main ethnic group of Chinese individuals (i.e., Han Chinese). The goals of the study are to (a) confirm the relationship between the rs821633 polymorphism and schizophrenia reported in other racial groups among Han Chinese, and (b)assess whether or not the relationship of this risk allele with schizophrenia is different in genetically more homogeneous early-onset schizophrenia (defined as those who developed the illness prior to 19 years of age[17-18]) than in genetically more heterogeneous lateonset schizophrenia (those who develop the illness at 19 years of age or older).
2. Methods
2.1 Sample
The recruitmen t process is shown in Figure 1.Participants with schizophrenia were recruited from among outpatients and inpatients treated at theShanghai Mental Health Center from April 2008 to April 2013. All recruited patients met the following criteria: (a) belonged to the Han Chinese ethnic group;(b) met the diagnostic criteria for schizophrenia of the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)[19]as determined by two senior clinicians who independently evaluated the patient using the Chinese version of the Structured Clinical Interview for DSM-IV Axis I Disorders[20](the two clinicians’ inter-rater reliability was excellent:Kappa=0.87); (c) were not in the first episode of illness(i.e., had one or more prior episodes of illness); (d) had been taking antipsychotic medication and been clinically stable for at least 6 months prior to recruitment; (e) did not have any other co-morbid mental disorder, a history of suicidal behavior, or a serious somatic illness; and (f)were not pregnant.

Figure 1.Enrollment of participants in the study
The age of onset was defined as the age when obvious positive symptoms (i.e., hallucinations or delusions) first appeared, based on information obtained during the clinical exam or as provided by family members. Individuals with an age of onset prior to becoming 19 were classified as ‘early-onset’ (n=315);those whose age of onset was after they turned 19 were classified as ‘late-onset’ (n=407).
During the same period we recruited 482 adult participants (i.e., 18 years of age or older) for the healthy control group via advertisements and social media. They were all from the Han Chinese ethnic group, had no mental illness (as assessed by the research psychiatrists), had no family history of mental illness, had no serious medical illnesses, and were not pregnant.
All subjects signed an informed consent form at the time of recruitment. The ethics committee of the Shanghai Mental Health Center approved the study(approval number: 2012-26R).
2.2 Genetic assessment
We considered single nucleotide polymorphism (SNP)markers smaller than +/- 500 bp with a minor allele frequency (MAF) equal to or above 20% in the Han Chinese ethnic group using data contained in the National Center for Biotechnology Information (NCBI)Database of Short Genetic Variation (dbSNP).[21]Based on previous findings in non-Han populations about the association of the rs821633 marker with schizophrenia,[7]we decided to make this the target SNP for the current study.
We obtained 2 ml of venous blood from all participants using 2% ethylenediamine tetraacetic acid(EDTA) anticoagulants. The Tiangen DNA extraction technique (Tiangen Biotech Co., Ltd, Beijing) was used to isolate leukocytes and extract DNA which was then stored at -80oC. TaqMan probes were used to examine polymorphism in the rs821633 SNP of theDISC1gene. The polymerase chain reaction (PCR) steps were conducted using Applied Biosystems 17900 (ABI7900)real-time quantitative PCR equipment. All reagents were provided by Life Technologies Corporation, USA.The PCR buffer used on the 384 pore plate was a total of 5 ul, including 20 ng of DNA primer, 2.0 ul of 2 × TaqMan PCR Master Mix, and 0.05 ul of 40 × SNP Assay (including primers and FAM/VIC probes [a 6-carboxyfluorescein reporter dye probe specific for allele “T” and a fl uorescent reporter dye probe specific for allele “C”]).The PCR amplif i cation started with 10 min denaturation at 95°C, followed by 50 cycles of 15 s at 92°C and 90 s at 60°C, and subsequent storage at 25°C. After the PCR amplification, we examined the distribution of alleles on the 384 pore plate using the Allele Discrimination program which assesses the signal strength of the FAM/VIC reporter dye probes. The sequence detection system (SDS) graphic analysis software was then used to genotype and save the results.
The two probes used in the TaqMan method used two different reporter dyes, FAM and VIC. In the process of the PCR, the probes combined with matched DNA templates and were decomposed by Ampli Taq Gold DNA Polymerase which strengthened the fl uorescence of the affected probe. Analysis of the different signal strengths of the two types of fluorescence in the 384 pores determined the pattern of SNP alleles: if there were only samples of enhanced fluorescence of FAM or VIC, the SNP was classified as homozygote; if the fl uorescence of both FAM and VIC were strengthened,the SNP was classified as heterozygote. We rechecked the samples in which the fluorescence signal strength showed no significant increase to confirm the result. We randomly chose 10 samples with increased fl uorescence and re-tested the sample; the good test-retest concordance (0.99) indicated that the use of TaqMan methods for SNP genotyping produced reliable results.
2.3 Statistical analysis
We used SPSS 13.0 software to compare the gender and age distribution in the three groups of subjects.A Tukey-type multiple comparison method based on an arcsin transformation of the original proportions[22]was used to conduct a multiple comparison test of the gender distribution between the three groups. To confirm the homogeneity of the genetic backgrounds of the subjects, we used SHEsisPlus software (http://analysis.bio-x.cn/myAnalysis.php) to conduct Hardy-Weinberg equilibrium goodness-of-fit tests among the allele frequencies in each of the three groups of subjects. SHEsisPlus software was also used to assess the association of age and gender with the risk allele of interest (rs821633(C)) and to calculate the odds ratios(OR) and the associated 95% conf i dence intervals of this risk allele for the early-onset group versus the control group, for the late-onset group versus the control group,and for the early-onset group versus the late-onset group. The power of the association analysis to detect significant differences was assessed using the Quanto 1.2.4 statistical package.[23]We conducted three separate survival analyses using the Kaplan-Meier statistical test in the SPSS statistical package to assess the relationship between the presence of the rs821633(C) risk allele(i.e., individuals with the C/C genotype or the C/T genotype versus individuals with the T/T genotype) and the age of onset of schizophrenia in individuals with early-onset schizophrenia, in individuals with late-onset schizophrenia, and in all individuals with schizophrenia(combining early-onset and late-onset patients). All statistical analyses used two-tailed tests with the level of statistical significance set atp<0.05.
3. Results
The age of onset among the 315 individuals in the earlyonset group ranged from 7 to 18 years of age; their mean age of onset was 16.4 (2.1) years of age. The age of onset among the 407 individuals in the late-onset group ranged from 19 to 71 years of age; their mean age of onset was 37.5 (8.8) years of age.
Among the early-onset patients, late-onset patients, and normal control subjects, 53.0%, 53.6%,and 40.5% were females, respectively (X2=21.91,df=2,p<0.001); multiple comparison assessment showed that individuals in the early-onset and late-onset groups were significantly (p<0.01) more likely to be female than those in the control group. The mean (sd)age at the time of enrollment in the three groups was 46.2 (16.6), 61.4 (15.7) and 32.6 (9.0) years of age,respectively (F=3960.59,df1=2,df2=1201,p<0.001);post-hoc Tukey multiple comparison tests found that early-onset patients were significantly younger than late-onset patients (p<0.05) and that both early-onset and late-onset patients were significantly older than healthy controls (p<0.05). Despite these significant differences in age and gender between the three groups, analyses using the SHEsisPlus software package found no significant correlation between age at thetime of recruitment or gender and the prevalence of the risk allele of interest (rs821633(C)), so it is unlikely that these differences between groups confounded the main results.
The genotype frequencies of rs821633 for all three groups satisf i ed the Hardy-Weinberg equilibrium goodness-of-fit test: in the early-onset groupX2=0.00,df=1,p=0.980; in the late-onset groupX2=2.08, df=1,p=0.150; and in the control groupX2=0.03, df=1,p=0.860.
As shown in Table 1, the C/C genotype and the C allele of rs821633 were significantly more prevalent in the early-onset group and in the late-onset group than in the control group, but there were no significant differences in the prevalence of the genotypes or of the alleles between the early-onset group and the lateonset group.
The results of the Kaplan–Meier survival analyses are shown in Figures 2-4. There was no statistically significant relationship between the presence of the rs821633(C) risk allele and the age of onset of schizophrenia in the 315 early-onset patients (X2=1.81,p=0.183), in the 407 late-onset patients (X2=0.11,p=0.740), or in the combined group of 722 patients(X2=0.18, p=0.672).
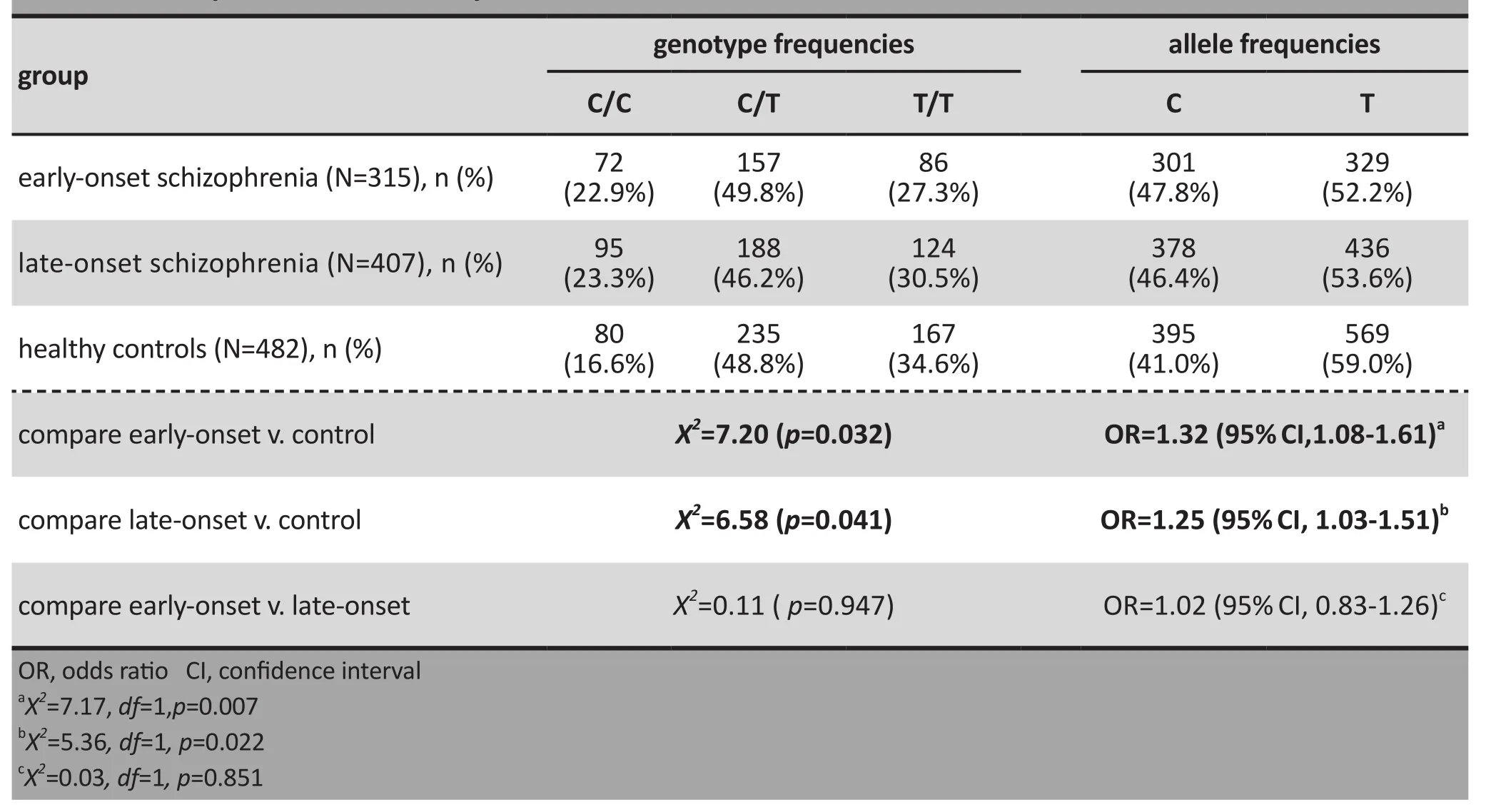
Table 1. Comparisons of allele and genotype frequencies for the rs821633 single nucleotide polymorphism in the DISC1 gene between individuals with early-onset schizophrenia, individuals with late-onset schizophrenia, and healthy controls

Figure 2. Relationship between the rs821633(C)risk allele and age of onset among 315 individuals with early-age-of-onset schizophrenia

Figure 3. Relationship between the rs821633(C)risk allele and age of onset among 407 individuals with late-age-of-onset schizophrenia
4. Discussion
4.1 Main findings
We found significant differences between the genotype frequencies and allele frequencies of the rs821633 SNP of theDISC1gene in Han Chinese individuals with schizophrenia compared to those in healthy control subjects. This result confirms findings in other racial groups which indicate that polymorphism in theDISC1gene is associated with schizophrenia. Tomppo and colleagues[7]assessed the relationship between different psychotic symptoms and 41 markers in theDISC1gene among 4651 individuals in Finland and found significant correlations between the rs821633 marker and social anhedonia. Chakirova and colleagues[24]used functional magnetic resonance imaging (fMRI)to compare the association between three markers of theDISC1gene (rs1538979, rs821577, and rs821633)and brain activation when completing the Hayling Sentence Completion Task (HSCT) in 33 healthy controls,20 individuals with schizophrenia, and 36 individuals with bipolar disorders; they found that presence of the risk alleles was associated with significant differences in location-specific brain activation when completing the task in both healthy controls and in the patient groups, confirming the relationship betweenDISC1polymorphism and the neurocognitive pathology in schizophrenia and bipolar disorder. We know of no studies that have failed to identify an association between schizophrenia and polymorphism of the rs821633 SNP of theDISC1gene.
The present study is the first to investigate the relationships between the rs821633 marker of theDISC1gene and the age-of-onset of schizophrenia. Using both a dichotomous classificationof age of onset (earlyonset versus late-onset schizophrenia) in a standard association analysis and a continuous measure of age of onset in Kaplan Meier survival analyses, we did not find a significant association between age of onset in schizophrenia and the prevalence of the different genotypes and alleles of rs821633. Failure to identify a relationship ofDISC1polymorphism and age of onset in schizophrenia may be due to several reasons;two likely reasons are that multiple genes may affect age of onset[25]and that age of onset may be strongly inf l uenced by both genetic and environmental factors.[26]

Figure 4. Relationship between the rs821633(C)risk allele and age of onset among 722 individuals with schizophrenia
4.2 Limitations
There are several limitations in the present study. (a)The statistical power for comparing patients to healthy controls was sufficient (>80%), but the power for comparing early-onset versus late-onset patients was low (<80%), so failure to identify differences between the two groups of individuals with schizophrenia may have been due to Type II errors (i.e., small sample size). (b) All the patients included in this study were treated in a single hospital in Shanghai, so they may not be representative of all Han Chinese individuals with schizophrenia. (c) We did not consider the many environmental factors that could confound the relationship between age of onset andDISC1polymorphism, including parents’ reproductive age,a history of stress and infection during the maternal gestational period, and a positive family history of schizophrenia.[27](d) We did not investigate the functions of the rs821633 SNP of theDISC1gene, so we were unable to assess the mechanism via which the rs821633(C) risk allele is associated with the onset of schizophrenia.
4.3 significance
These results provide further evidence about the association of theDISC1gene with schizophrenia.Previous studies have shown that theDISC1gene is a candidate gene for mental illness that plays several roles in brain development. In a large Scottish family study, Blackwood and colleagues[28]found that the P300 amplitude of event-related potentials (ERP) was significantly decreased among family members with a specific genetic variant of theDISC1gene, which suggests that variations inDISC1can inf l uence the core processing ability of the brain. Millar and colleagues[29]found that theDISC1protein interacts with other proteins (such as ataxin-1 and phosphodiesterase 4B[PDE4B]) which participate in neurite extension and arborization, and in neuronal proliferation, transport,and signal transmission. TheDISC1protein is present in several parts of the brain, including the hippocampus,cerebellum, cerebral cortex, hypothalamus, and thalamic nuclei.[30]The specific mechanism(s) via which polymorphism inDISC1is associated with schizophrenia remains unknown, but several studies[24,31]suggest that different expressions of theDISC1protein in specific brain regions can influence perceptual and motor function – functions that are often abnormal in individuals with schizophrenia. Further work is needed to clarify these mechanisms, but our study has shown that the reported associations in other racial groups are probably equally evident in Han Chinese.
Funding
This study was supported by the National Natural Science Foundation of China (81171272, 81301159), the Shanghai Training Plan for Excellent Academic Leaders in Public Health (GWDTR201227), the National Key Clinical Disciplines of the Office of Medical Affairs of the Chinese Ministry of Health at the Shanghai Mental Health Center (OMA-MH, 2011-873), the Training Plan for Excellent Academic Leaders of the Shanghai Health System (XBR2013087), and the Shanghai Mental Health Center Research Grant (2014-YJ-04, 2015-YJGJ-03).
Conflict of interest statement
The authors declare no conflicts of interest.
Informed consent
Every participant in this study signed a consent form at the beginning of the study.
Ethics approval
The ethics committee of the Shanghai Mental Health Center approved the study (approval number: 2012-26R).
Authors’ contributions
GH participated in the design and data collection for the study and drafted the manuscript. CY performed the statistical analysis and critically reviewed the manuscript. JZ, YF, and CZ carried out the clinical diagnosis and critically reviewed the manuscript. MZ,XG, CB, SJ, AX, YJ, ZW, and CZ helped enroll the subjects.All authors read and approved the fi nal manuscript.
1. Hennah W, Thomson P, Peltonen L, Porteous D. Genes and schizophrenia: beyond schizophrenia: the role of DISC1 in major mental illness.Schizophr Bull. 2006; 32(3): 409-416.doi: http://dx.doi.org/10.1093/schbul/sbj079
2. Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia.Hum Mol Genet. 2000; 9(9): 1415–1423. doi: http://dx.doi.org/10.1093/hmg/9.9.1415
3. Paunio T, Tuulio-Henriksson A, Hiekkalinna T, Perola M, Varilo T, Partonen T, et al. Search for cognitive trait components of schizophrenia reveals a locus for verbal learning and memory on 4q and for visual working memory on 2q.Hum Mol Genet. 2004; 13(16): 1693–1702. doi: http://dx.doi.org/10.1093/hmg/ddh184
4. Burdick KE, Hodgkinson CA, Szeszko PR, Lencz T, Ekholm JM, Kane JM, et al. DISC1 and neurocognitive function in schizophrenia.Neuroreport.2005; 16(12): 1399–1402
5. Cannon TD, Hennah W, van Erp TGM, Thompson PM,Lonnqvis, J, Huttunen M, et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory.Arch Gen Psychiatry. 2005; 62(11): 1205–1213. doi: http://dx.doi.org/10.1001/archpsyc.62.11.1205
6. Hennah W, Thomson P, McQuillin A, Bass N, Loukola A,Anjorin A, et al. DISC1 association, heterogeneity and interplay in schizophrenia and bipolar disorder.Mol Psychiatry. 2008; 14(9): 865-873. doi: http://dx.doi.org/10.1038/mp.2008.22
7. Tomppo L, Hennah W, Miettunen J, Järvelin M, Veijola J, Ripatti S, et al. Association of variants in DISC1 with psychosis-related traits in a large population cohort.Arch Gen Psychiatry. 2009; 66(2): 134-141. doi: http://dx.doi.org/10.1001/archgenpsychiatry.2008.524
8. Porteous DJ, Millar JK, Brandon NJ, Sawa A. DISC1 at 10:connecting psychiatric genetics and neuroscience.Trends Mol Med. 2011; 17(12): 699–706. doi: http://dx.doi.org/10.1016/j.molmed.2011.09.002
9. Lee JS, Jung S, Park IH, Kim JJ. Neural basis of anhedonia and amotivation in patients with schizophrenia: the role of reward system.Curr Neuropharmacol. 2015; 13(6):750-759. doi: http://dx.doi.org/10.2174/157015 9X13666150612230333
10. Zou LQ, Geng FL, Liu WH, Wei XH, Jiang XQ, Wang Y, et al.The neural basis of olfactory function and its relationship with anhedonia in individuals with schizotypy: An exploratory study.Psychiatry Res. 2015; 234(2): 202-207.doi: http://dx.doi.org/10.1016/j.pscychresns.2015.09.011
11. Zhang ZM. [Association study of disrupted-in-schizophrenia-1 gene single nucleotide polymorphism with schizophrenia in Han Chinese population].Yi Yao Lun Tan Za Zhi. 2014; 35(11):122-123. Chinese. doi: http://dx.chinadoi.cn/10.3760/cma.j.issn.1674-6554.2012.04.016
12. Ge GC, Guo SQ, Shao RR, Li WQ, Guo F, Zhang HM, et al.[Neurological soft signs in childhood-onset schizophrenia and its association with DISC1 rs4658971 polymorphism].Zhongguo Shen Jing Jing Shen Ji Bing Za Zhi. 2014; 40(5):263-268. Chinese
13. Ma XY, He YQ, Li X, Xun GL, Xia K, Hu ZM, et al. [Association study of disrupted in schizophrenia 1 gene polymorphisms with autism in Chinese Han children].Zhong Hua Shi Yong Er Ke Lin Chuang Za Zhi. 2014; 29(11): 857-861. Chinese
14. Zhang YW. [Study on schizophrenia gene 1 single nucleotide polymorphisms of paranoid schizophrenia patients].Hei Longjiang Yi Xue. 2014; 38(1): 74-75. Chinese. doi: http://dx.chinadoi.cn/10.3969/j.issn.1004-5775.2014.01.042
15. Guo WY, Li WQ, Zhang HX, Guo W, Lv LX. [Association study of disrupted-in-schizophrenia-1 gene single nucleotide polymorphism with schizophrenia in Han Chinese population].Zhong Hua Xing Wei Yi Xue Yu Nao Ke Xue Za Zhi. 2012; 21(4): 337-339. Chinese. doi: http://dx.chinadoi.cn/10.3760/cma.j.issn.1674-6554.2012.04.016
16. Zhou JG, Chen M, Su ZH, Li W, Yu Q, Li J, et al. [Association study between disrupted in schizophrenia 1(DISC1) gene polymorphism and schizophrenic and different subtype depressive patients].Zhong Hua Xing Wei Yi Xue Yu Nao Ke Xue Za Zhi. 2011; 20(7): 605-607. Chinese. doi: http://dx.chinadoi.cn/10.3760/cma.j.issn.1674-6554.2011.07.010
17. Clemmensen L, Vernal DL, Steinhausen HC. A systematic review of the long-term outcome of early onset schizophrenia.BMC Psychiatry. 2012; 12: 150. doi: http://dx.doi.org/10.1186/1471-244X-12-150
18. Uezato A, Yamamoto N, Iwayama Y, Hiraoka S, Hiraaki E, Umino A, et al. Reduced cortical expression of a newly identified splicing variant of the DLG1 gene in patients with early-onset schizophrenia.Transl Psychiatry. 2015; 5: e654. doi: http://dx.doi.org/10.1038/tp.2015.154
19. American Psychiatric Association.Diagnostic and Statistical Manual-Text Revision, 4th ed (DSM-IV-TR, 2000). American Psychiatric Association; 2000
20. Phillips MR, Liu XH.Translated and Adapted Chinese version of Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P)by Michael B First, Robert L. Spitzer, Miram Gibbon, and Janet B.W. Willams. Shanghai: Suicide Research and Prevention Center, Shanghai Mental Health Center; 2011
21. Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation.Nucleic Acids Res. 2001; 29(1): 308-311
22. Zar HG.Biostatistical Analysis (4th edition). Prentice Hall:New Jersey. 1999; p: 563-565
23. Gauderman W, Morrison J.QUANTO Documentation. (Technical report no. 157). Los Angeles, CA: Department of Preventive Medicine, University of Southern California; 2001
24. Chakirova G, Whalley HC, Thomson PA, Hennah W,Moorhead TW, Welch KA, et al. The effects of DISC1 risk variants on brain activation in controls, patients with bipolar disorder and patients with schizophrenia.Psychiatry Res. 2011; 192(1): 20-28. doi: http://dx.doi.org/10.1016/j.pscychresns.2011.01.015
25. Kamiya A. Animal models for schizophrenia via in utero gene transfer: understanding roles for genetic susceptibility factors in brain development.Prog Brain Res. 2009; 179: 9-15. doi:http://dx.doi.org/10.1016/S0079-6123(09)17902-5
26. Ayhan Y, McFarland R, Pletnikov MV. Animal models of geneenvironment interaction in schizophrenia: a dimensional perspective.Prog Neurobiol. 2015; 136: 1-27. doi: http://dx.doi.org/10.1016/j.pneurobio.2015.10.002
27. van Os J, Ruten BP, Poulton R.Gene-environment interactions in schizophrenia:review of epidemiological findings and future directions.Schizophr Bull. 2008; 34(6):1066-1082. doi: http://dx.doi.org/10.1093/schbul/sbn117
28. Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorderscosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family.Am J Hum Genet. 2001; 69 (2): 428–433
29. Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling.Science. 2005;310(5751): 1187–1191. doi: http://dx.doi.org/10.1126/science.1112915
30. Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK.The DISC locus in psychiatric illness.Mol Psychiatry. 2008; 13(1): 36–64. doi: http://dx.doi.org/10.1038/sj.mp.4002106
31. Borgwardt SJ, Picchioni MM, Ettinger U, Toulopoulou T,Murray R, et al. Regional gray matter volume in monozygotic twins concordant and discordant for schizophrenia.Biological Psychiatry. 2010; 67(10): 956–964. doi: http://dx.doi.org/10.1016/j.biopsych.2009.10.026
(received, 2015-11-03; accepted, 2015-12-10)

Dr. Guoqin Hu graduated with a bachelor’s degree in medicine from Anhui Medical University in 2012 and obtained a master’s degree in psychiatry and mental health from the Shanghai Jiao Tong University School of Medicine in 2015. She has been working in the Shanghai Mental Health Center as a resident doctor since July 2015. Her main research interest is the molecular genetics of schizophrenia.
DISC1基因rs821633位点多态性与中国汉族人群精神分裂症的相关性研究
胡国芹,杨程青,赵静,朱明环,郭向晴,鲍晨曦,贾思,徐阿红,介勇,汪作为,张晨,何永光,吕钦谕,禹顺英,易正辉
精神分裂症;DISC1基因;传递不平衡检验;单核苷酸多态性;首发年龄;中国
Background: Previous studies report that various single nucleotide polymorphisms (SNP) in the Disrupted-in-Schizophrenia 1 (DISC1) gene are closely associated with schizophrenia, but there are no studies that assess the relationship of age of onset of schizophrenia with these SNPs.Objective:Investigate the relationship between the rs821633 SNP in theDISC1gene and the occurrence and age of onset of schizophrenia in Han Chinese.Methods:We used the TaqMan genotyping technology to examine the rs821633 SNP in theDISC1gene among 315 individuals who developed schizophrenia prior to 19 years of age (‘early-onset’), 407 individuals who developed schizophrenia when 19 years of age or older (‘late-onset’), and 482 healthy controls. We used survival analyses to investigate the relationship between the rs821633(C) risk allele and the age of onset of schizophrenia.Results:Compared to the prevalence in healthy controls, the prevalence of the C/C genotype of rs821633 and of the C allele in rs821633 were significantly greater in individuals with early-onset schizophrenia (X2=7.17,df=1,p=0.007;X2=7.20,df=2,p=0.032) and significantly greater in individuals with late-onset schizophrenia(X2=5.36,df=1,p=0.022;X2=6.58,df=2,p=0.041). However, there were no significant differences in the prevalence of the C/C genotype or the C allele between individuals with early-onset and late-onset schizophrenia. Kaplan-Meier survival analyses found no significant association between the rs821633(C) risk allele and age of onset in schizophrenia.Conclusion:We confirm the association of polymorphism in the rs821633 SNP in theDISC1gene with schizophrenia among Han Chinese, but we found no association between the rs821633(C) risk allele and the age of onset in individuals with schizophrenia.
[Shanghai Arch Psychiatry. 2015, 27(6): 348-355.
http://dx.doi.org/10.11919/j.issn.1002-0829.215120]
1Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
2Mental Health Center of Hongkou District, Shanghai, China
*correspondence: 18616550357@163.com; hyg_512@126.com
A full-text Chinese translation of this article will be available at http://dx.doi.org/10.11919/j.issn.1002-0829.215120 on April 25, 2016.
背景:既往研究报告显示精神分裂症断裂基因1(Disrupted-in-Schizophrenia 1 gene,DISC1) 中不同的单核苷酸多态性 (single nucleotide polymorphisms, SNP) 与精神分裂症密切相关,但目前尚无研究评估了SNP与精神分裂症发病年龄之间的关系。目的:探讨DISC1基因中rs821633位点的SNP和中国汉族精神分裂症患者的发病及首发年龄的相关性。方法我们采用TaqMan基因分型技术对315例19岁之前发病的精神分裂症患者(即“早发性”)、407例19岁后发病的精神分裂症患者(即“非早发性”)和482名健康对照进行DISC1基因rs821633位点的SNP检测。我们使用生存分析研究rs821633(C)位点的危险等位基因与精神分裂症患者首发年龄之间的关系。结果相比健康对照组,rs821633位点C/C基因型和C等位基因型频率分布在早发性 (X2=7.17,df=1,p=0.007;X2=7.20,df=2,p=0.032) 和晚发性 (X2=5.36,df=1,p=0.022;X2=6.58,df=2,p=0.041) 精神分裂症患者中显著较高。然而,C/C基因型或C等位基因型的携带率在早发和晚发性精神分裂症患者中没有显著差异。Kaplan-Meier生存分析发现rs821633(C)危险等位基因与精神分裂症首发年龄之间没有显著相关性。结论我们证实了DISC1基因rs821633位点多态性的SNP与中国汉族人群精神分裂症之间存在相关性,但未发现rs821633(C)危险等位基因与精神分裂症首发年龄之间的相关性。
本文全文中文版从2016年4月25日起在
http://dx.doi.org/10.11919/j.issn.1002-0829.215120可供免费阅览下载
- 上海精神医学的其它文章
- Metformin for treatment of clozapine-induced weight gain in adult patients with schizophrenia: a meta-analysis
- Comparison of the density of gamma-aminobutyric acid in the ventromedial prefrontal cortex of patients with first-episode psychosis and healthy controls
- Comorbid anxiety and depression in school-aged children with attention deficit hyperactivity disorder (ADHD) and selfreported symptoms of ADHD, anxiety, and depression among parents of school-aged children with and without ADHD
- Psychopharmacological treatment for schizophrenia: less is more
- Case report of eosinophilia induced by quetiapine
- Panic attacks 10 years after heart transplantation successfully treated with low-dose citalopram: a case report

