合成黑索今中副产物3,5-二硝基-1-氧-3,5-二氮杂环己烷的晶体结构及热分解动力学
李 静 陈丽珍 王建龙 兰贯超 侯 欢 李 满
(中北大学化工与环境学院, 太原 030051)
合成黑索今中副产物3,5-二硝基-1-氧-3,5-二氮杂环己烷的晶体结构及热分解动力学
李 静 陈丽珍 王建龙*兰贯超 侯 欢 李 满
(中北大学化工与环境学院, 太原 030051)
直接法硝解乌洛托品制备黑索今的过程中合成了一种新型的环形副产物, 采用硅胶柱层析法分离得到3,5-二硝基-1-氧-3,5-二氮杂环己烷, 洗脱剂为: 丙酮/二氯甲烷, 梯度洗脱. 以丙酮为溶剂培养得到了3,5-二硝基-1-氧-3,5-二氮杂环己烷单晶, 用元素分析、傅里叶变换红外(FTIR)光谱、核磁共振氢谱(NMR)以及质谱(MS)对其结构进行了表征, 用X射线单晶衍射仪测定了其晶体结构. 结果表明, 晶体C3H6N4O5分子量为178.12,属于单斜晶系, 空间群P121/n1, 晶胞参数: a = 0.58128(13) nm, b = 1.72389(14) nm, c = 0.71072(6) nm, β = 112.056°, V = 0.66006(16) nm3, Z = 4, DC= 1.792 gcm–3, μ = 0.17 mm–1, F(000) = 368.0, 最终偏差因子R = 0.0397. 用同步热分析仪技术研究了3,5-二硝基-1-氧-3,5-二氮杂环己烷的热行为, DSC曲线上在383.15和519.05 K分别有一个尖锐的熔化吸热峰和分解放热峰. 另外, 根据Kissinger方程及Flynn-Wall-Ozawa方程和不同升温速率下的TG曲线计算得到了该化合物的热分解动力学参数(活化能和指前因子), 利用Coats-Redfern法研究了该物质的热分解机理. 结果表明: 3,5-二硝基-1-氧-3,5-二氮杂环己烷是一种低熔点、热稳定性好的化合物. Kissinger方程计算其活化能为212.32 kJmol–1, 指前因子为6.20 × 1020s–1, Flynn-Wall-Ozawa方程计算其活化能为210.39 kJmol–1, 该物质的热分解动力学方程为G(α) = (1―α)–1―1, 反应级数为2.
黑索今; 3,5-二硝基-1-氧-3,5-二氮杂环己烷; 晶体结构; 热分解动力学; 表观活化能; 机理
1 Introduction
RDX is a kind of preferable single explosive judging from combination property, which is of high energy, great brisance, and stable chemical property.1It is widely used in composite explosives and propellant. In the 1940s, the research of manufacturing method of RDX entered into a period of rapid development,2–4the most abundant research of the related field of RDX was in United States, Germany, Britain, and Canada countries.5,6But the current preparation method has shown enormous non-economic,7its application and development were restricted by environmental quality, production costs, and other factors. In order to improve the process of preparation of RDX by direct nitration, achieve green production process, its nitrolysis mechanism need to be fully grasped. Although the research literature of nitrolysis byproducts and mechanism had been largely reported,8–12the report of wastewater analysis during RDX preparation by direct nitration was rare. Mixed byproducts were obtained from wastewater treatment, and then separated, characterized. We could analyze the possible nitrolysis mechanism by correlating raw materials and various products. Although parts of byproducts had been reported by predecessors,13–153,5-dinitro-1-oxygen-3,5-diazacyclohexane was one of the byproducts which was never reported by literature. In addition, kinetics of thermal decomposition reaction is a field of great concern for explosives workers which plays an important role in evaluation of thermal behavior and study of reaction mechanism.16,17So the structure and thermal analysis of 3,5-dinitro-1-oxygen-3,5-diazacyclohexane provide reliable experimental data for nitrolysis mechanism of direct nitration.
2 Experimental section
2.1 Materials and measurements
Hexamethylenetetramine (HA, AR) and nitric acid (NA, 98%) were purchased from commercial sources of chemical plant in Changzhou and Institute of Chemical Reagents in Tianjin, respectively. In addition, the other materials used in experiments such as ethyl acetate, acetone, dichloromethane, and anhydrous magnesium sulfate were analytically pure and the methanol was chromatographically pure which purchased from Institute of Chemical Reagents in Tianjin. Distilled water was provided by self-prepared.
The elemental analyses for carbon, hydrogen, nitrogen, and oxygen were taken on an Elementar Vario EL cube produced by Elementar Company in Germany and melting point was determined using a Melting Point M-565 provided by BUCHI in Switzerland, respectively. High performance liquid chromatography (HPLC) was determined using a P230 manufactured by Elite in China. The FTIR spectra were measured on an IR-8400S produced by Analysis Plant of Instruments in Beijing, FTIR spectrophotometer (KBr pellet, ν [cm–1]).1H-NMR spectra were detected with an AVANCE IIITM 600 MHz manufactured by Bruker Company in Switzerland using (CD3)2CO as solvent. MS spectra were recorded by APEXIV produced by Bruker in America at a Fourier transform ion cyclotron resonance mass spectrometer with electrospray ionization (ESI) as the ionization method. Single-crystal X-ray diffraction data of title compound (CCDC 1403942) were collected on an Agilent Xcalibur & Gemini which made by Agilent in America and the main crystallographic data are listed in Table 1. Thermal analysis was carried out on a NETZSCH STA 409 PC/PG Germany instruments.
2.2 Separation of 3,5-dinitro-1-oxygen-3,5-diazacyclohexane
The HA and NA (98%) were used for synthesis of RDX with a molar ratio of 1 : 11, the byproduct of title compound was synthesized at the same time. Reaction was carried out in a four-necked flask which was first added to nitric acid (145 mL, 98%) in ice-water with the stirring rate of 800 rmin–1, the system temperature was maintained below 10 °C. Hexamethylenetetramine (20 g) was then added to the nitric acid at a constant rate, and the nitrolysis temperature was held at 14–16 °C. Next the reaction solution was incubated for 20 min, 3,5-dinitro-1-oxygen-3,5-diazacyclohexane was formed (Scheme 1) and then poured into ice-water, RDX was separated out. After RDX was filtered out, waster water was obtained for the following use. Byproducts were enriched from waste water by extraction of ethyl acetate, and then neutralized with sodium bicarbonate to neutral, dehydrated with anhydrous magnesium sulfate, concentrated, mixed byproducts were obtained, analyzed in thin layer chromatography (TLC) qualitatively, silicone column chromatography was applied to separate the stable byproducts, which eluted by a mixture of acetone and dichloromethane with a changed ratio. Yield: 0.12 g.
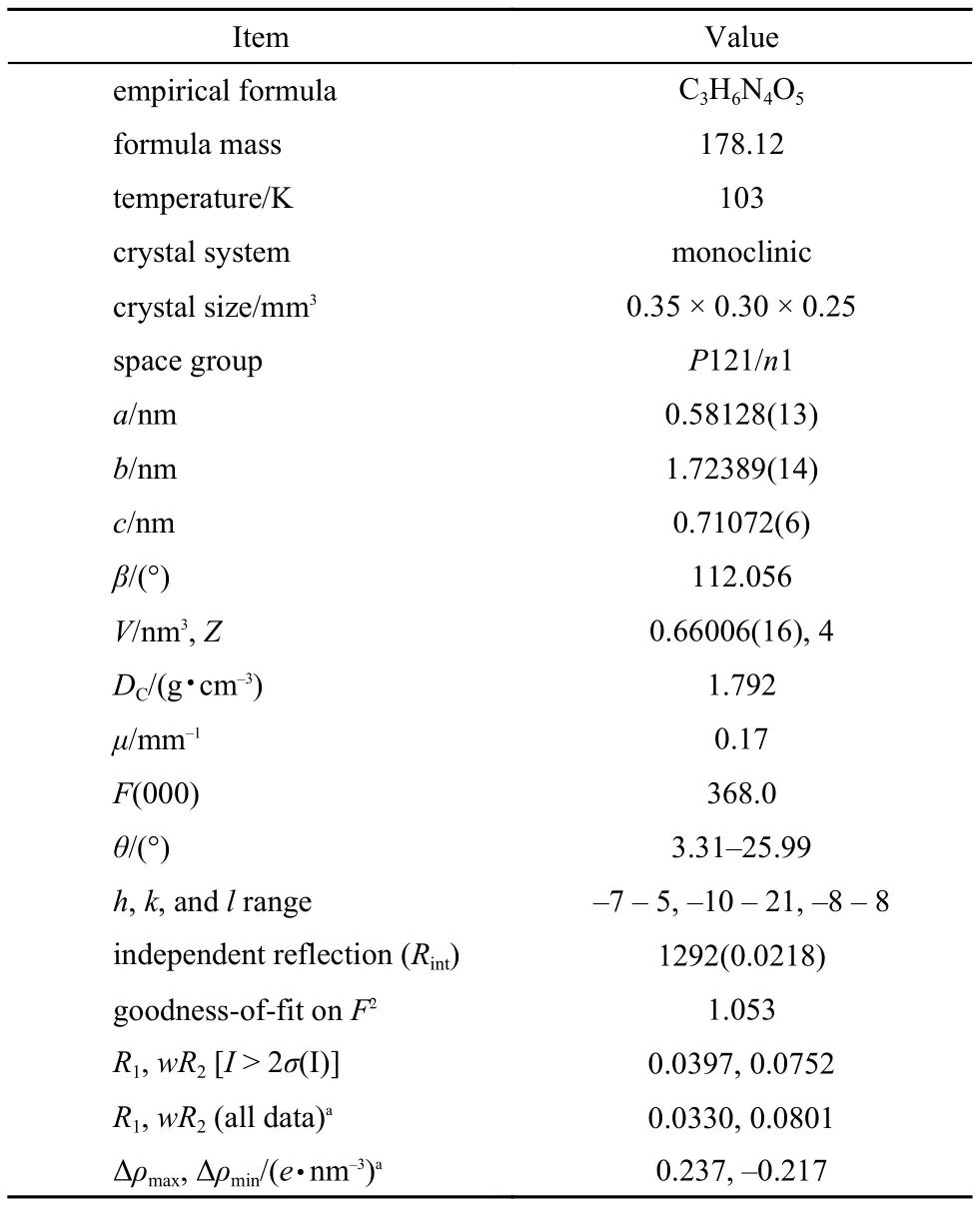
Table1 Crystal data and structure refinement for title compound
2.3 Purity test of 3,5-dinitro-1-oxygen-3,5-diazacyclohexane
High performance liquid chromatography (HPLC) was detected by P230 under the chromatographic conditions that mobile phase was a mixture of methanol and water with a volume ratio of 60 : 40 and the UV absorption wavelength was 252 nm. The injection volume and flow rate were 10 μL and 1 mLmin–1, respectively. The sample of 0.5% concentration was prepared by chromatography methanol.
The purity of title compound was determined by HPLC (Fig.1) under the optimal conditions, and the resultant mass fraction purity was 0.98, which was an acceptable purity for structure characterization measure.
2.4 Structure characterization of 3,5-dinitro-1-oxygen-3,5-diazacyclohexane
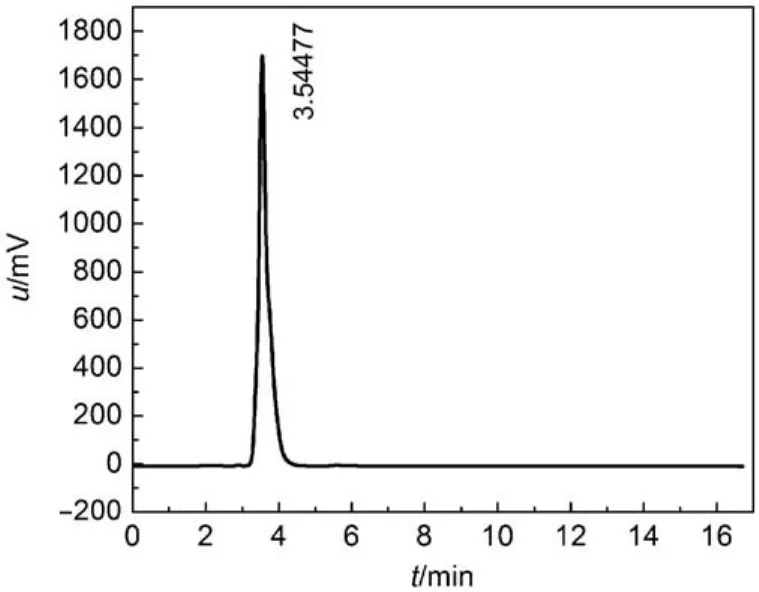
Fig.1 HPLC curve of the title compound
Melting point was determined using a Melting Point M-565. The short melting range of 100–103 °C indicated that the title compound was pure. Anal. Calcd. for C3H6N4O5(%): C 21.21, H 3.45, N 30.61, O 44.73; found (%) : C 20.23, H 3.40, N 31.46, O 44.92. IR data (KBr pellet, ν/cm–1): 3065.6, 2945.5(CH2); 1559.0, 1292.2 (N―NO2); 1183.2 (C―O―C).1H-NMR ((CD3)2CO): 5.591 (4H), 6.207 (2H). MS (ESI) m/z: 178.01 (M+).
2.5 Crystal structure determination of 3,5-dinitro-1-oxygen-3,5-diazacyclohexane
The single crystal of the size of 0.35 mm × 0.30 mm × 0.25mm was selected to determine its structure by an X-ray single crystal diffractometer. A graphite monochromator ray of Mo Kαradiation (λ = 0.07107 nm) at a temperature of 103 K and the θ within the range of 3.31°–25.99° was applied to scan. The total number of diffraction spots (–7 ≤ h ≤ 5, –10 ≤ k ≤ 21, –8 ≤ l ≤8) was 2543, among which 1292 independent diffraction spots (Rint= 0.0218) and 1104 observable points [I > 2σ(I)] were used for the crystal structure analysis. All the data were corrected by Lpfactor and experience absorption, and solved by direct method and Fourier synthetics. The matrix least square method was used to modify F2, the whole calculation was completed by SHELX97 program.18,19
2.6 Thermal experimental instruments and conditions
Thermal analysis was carried out on a NETZSCH STA 409PC/PG Germany instruments at heating rates of 10, 15, 20, 25 Kmin–1,20in a nitrogen atmosphere with a flow rate of 50 mLmin–1. The sample was 13.070–15.088 mg.
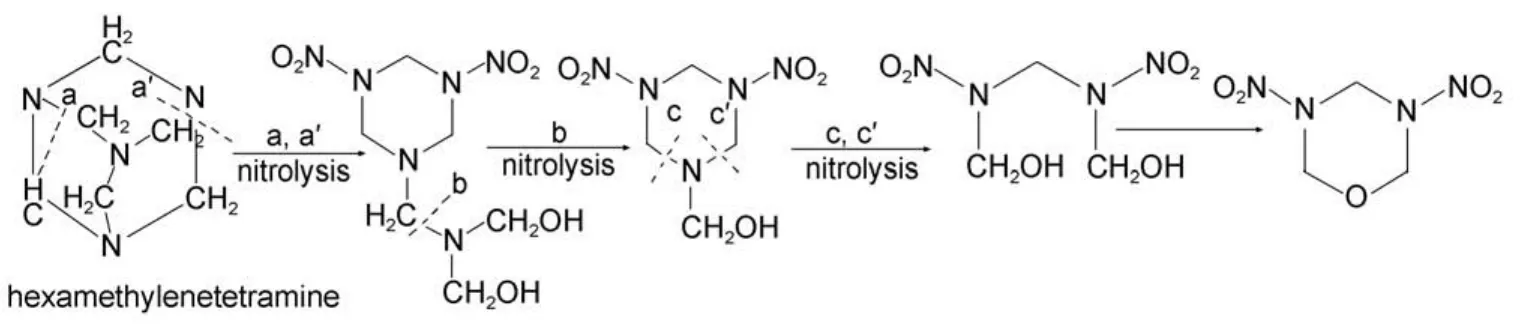
Scheme1 Formation route of 3,5-dinitro-1-oxygen-3,5-diazacyclohexane
3 Results and discussion
3.1 IR,1H-NMR and MS analysis of the title compound
The IR data show that there are strong absorption peaks in 3065.6 and 2945.5 cm–1which are the stretching vibration absorption peaks of C―H on the ring. 1559.0 and 1292.2 cm–1arethe asymmetric stretching vibration peak and symmetric stretching vibration peak of N―NO2, respectively. Finally, 1183.2 cm–1is the stretching vibration absorption peak of C―O―C which the scope of absorption peak is from 1250 to 1000 cm–1. The1H-NMR data of the title compound we obtained in our study show two kinds of hydrogen. Chemical shift δ = 5.591 (4H) indicates the hydrogen of ―CH2―O―. There are four hydrogen atoms because of its symmetrical structure. Meanwhile, chemical shift δ = 6.207 (2H) demonstrates the hydrogen of N―CH2―N. The MS is positive spectrum detected with ESI as the ionization method. The peak of 201.01 is [M+Na], so the mass of title compound is 178.01. To sum up, the IR,1H-NMR, and MS of the product are all in good agreement with the assumed structure.
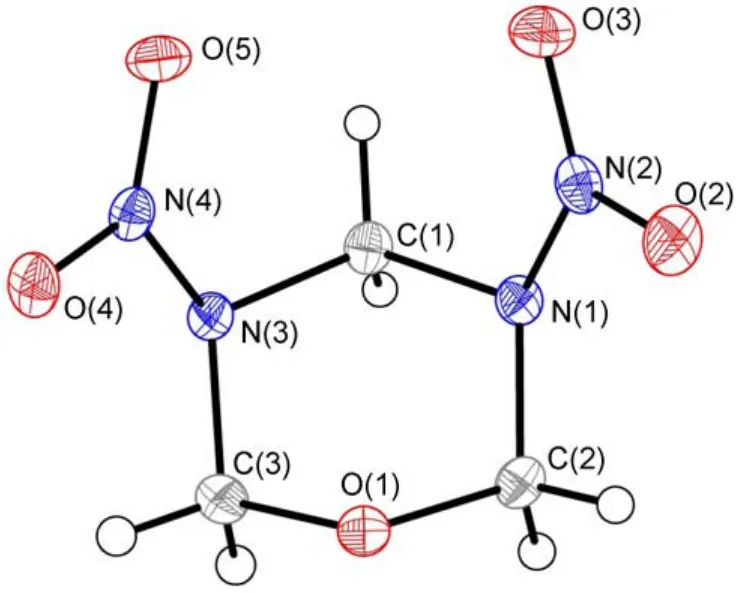
Fig.2 Molecular structure of the title compound
3.2 Crystal structure description
Molecular structure of title compound and packing of the title compound molecule in crystal lattice are shown in Fig.2 and Fig.3.
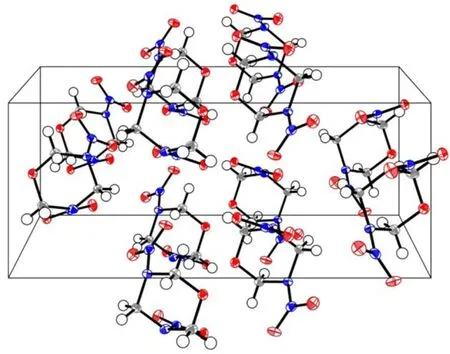
Fig.3 Packing of the title compound molecule in crystal lattice
X-ray structural data show that the title compound is composed of a hexatomic ring with two ―NNO2on N(1) and N(3) (Fig.2). The dihedral angles of C(1)―N(3)―C(3)―O(1), C(1)―N(1)―C(2)―O(1), C(2)―O(1)―C(3)―N(3), C(2)―N(1)―C(1)―N(3), C(3)―O(1)―C(2)―N(1) are 56.03(16)°, –52.13(16)°, –60.96(15)°, 45.97(16)°, 58.96(15)°, respectively (Table 2). The data indicate that the coplanarity of six atoms of the ring is relatively poor. The biggest dihedral angle is 60.96(15)°. In addition, C(1) and O(1) as the axle wire, the bond angles of O(1)―C(3)―N(3) and O(1)―C(2)―N(1) are ca 111°, C(3)―N(3)―C(1) and C(2)―N(1)―C(1) are ca 113°, N(4)―N(3)―C(1) and N(2)―N(1)―C(1) are ca 116°, N(4)―N(3)―C(3) and N(2)―N(1)―C(2) are ca 116°, respectively (Table 2). It demonstrates that the structure of title compound has certain stability because of its symmetry.
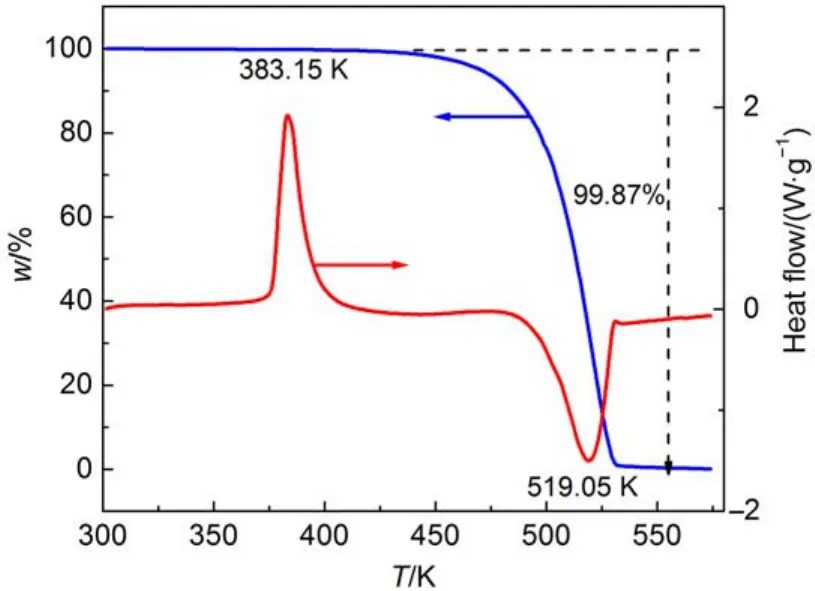
Fig.4 DSC-TG curve of the title compound
Title compound is synthesized during RDX preparation by direct nitration which has a definite attribution on synthetic route with RDX. The bond lengths of N―N in both ―NNO2are 0.13883(18) and 0.13996(18) nm (Table 2) which are the same as RDX with 0.1392(2) nm and 0.1398(2) nm,21respectively.
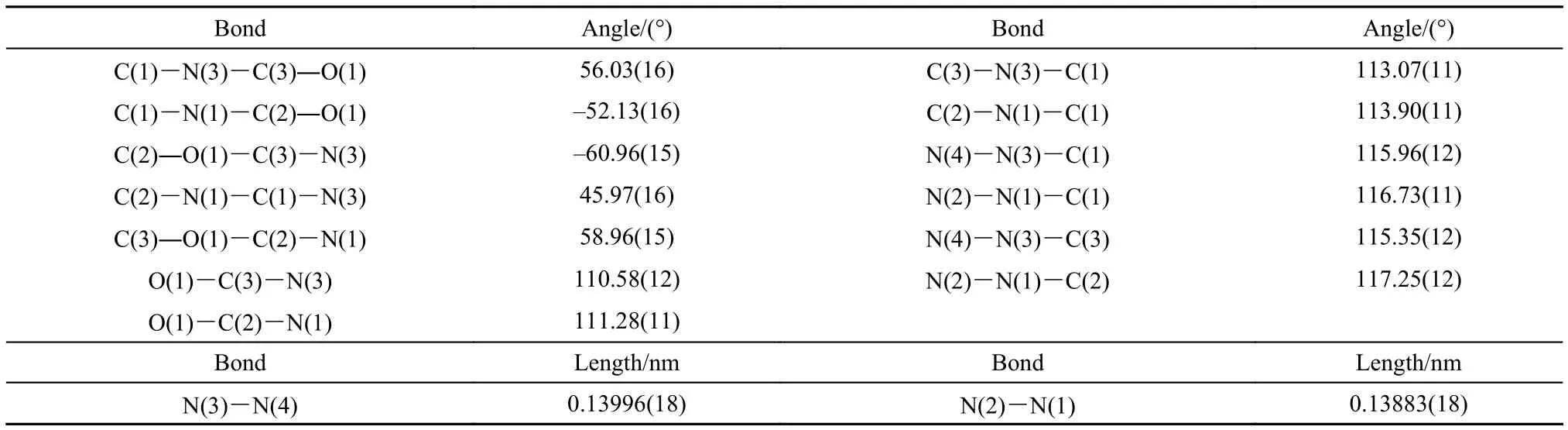
Table2 Selected dihedral angles, bond angles and lengths of the title compound
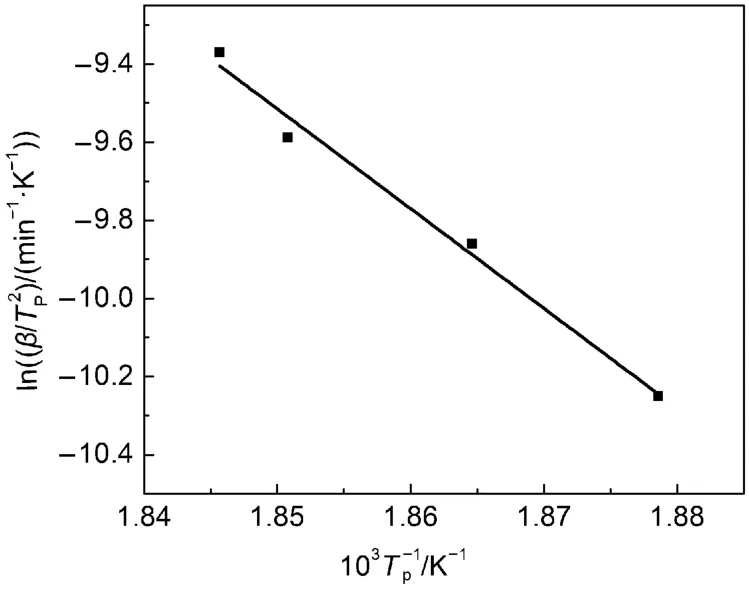
Fig.5 Plots of ln(β/Tp2)–1/Tpby Kissinger method
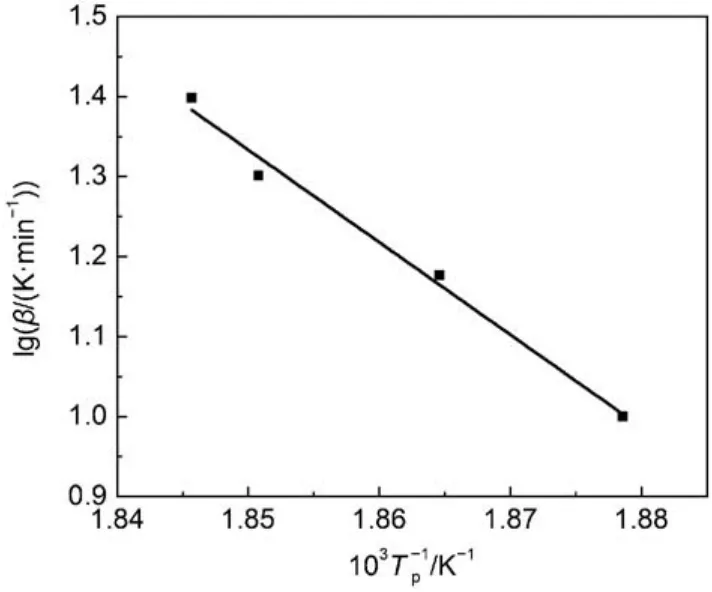
Fig.6 Plots of lgβ–1/Tpby FWO method
3.3 Thermal behavior of 3,5-dinitro-1-oxygen-3,5-diazacyclohexane
3.3.1 TG/DSC curve of 3,5-dinitro-1-oxygen-3,5-diazacyclohexane
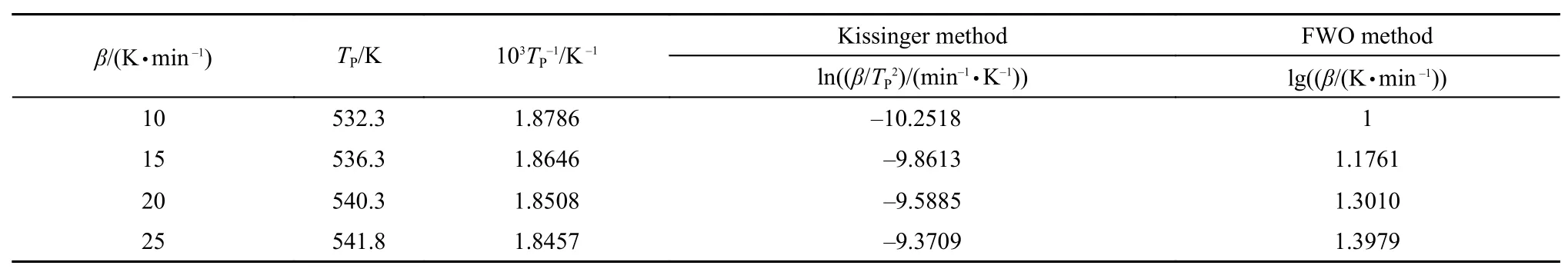
Table3 Kinetics basic data of TG

Table4 Kinetics parameters of title compound
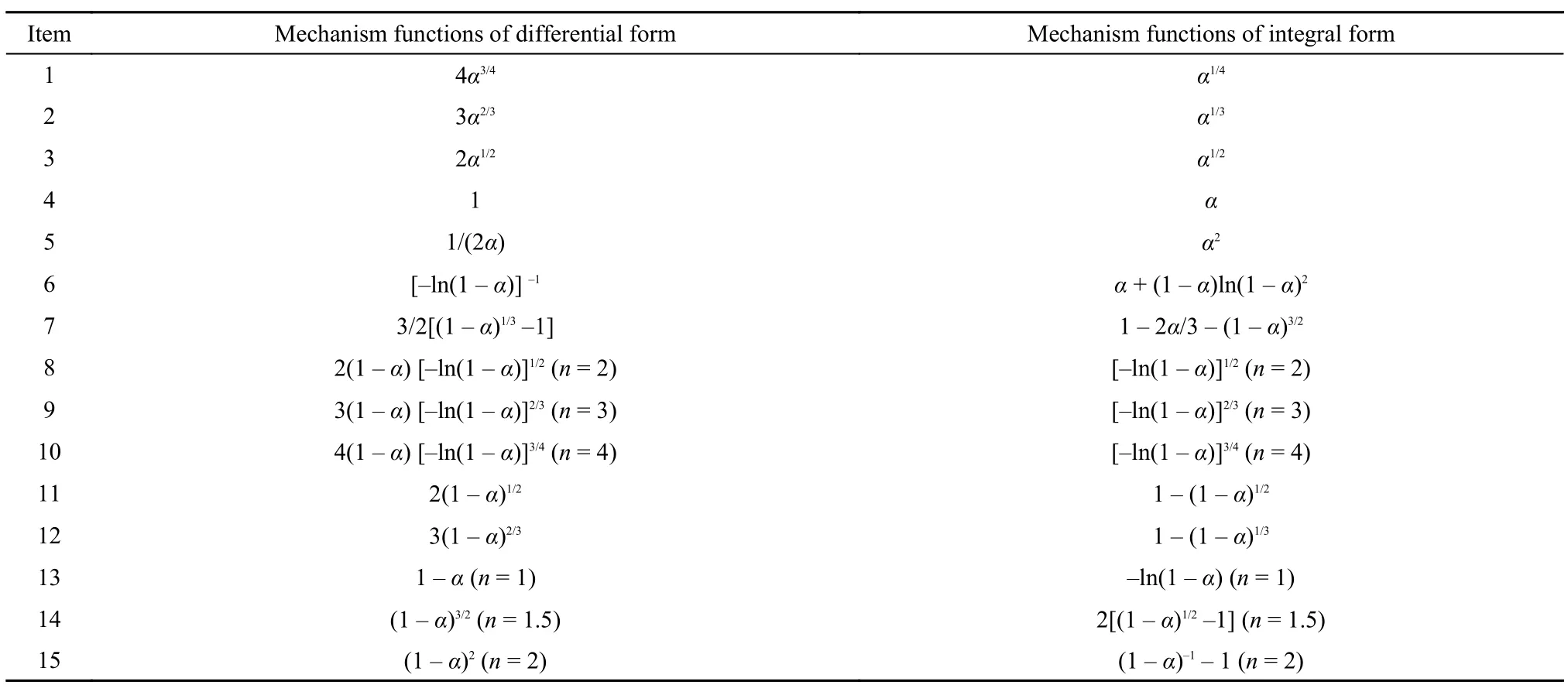
Table5 15 types of thermal decomposition mechanism functions
The typical DSC-TG curve (Fig.4) indicates that the thermal behaviors of title compound can be described in two stages which contain an endothermic and an exothermic processes. The first stage is an obvious endothermic process starting from 372.52 K and the temperature of top is 383.15 K. The second phase on the DSC curve is shown a sharp exothermic process, of which peak temperature is 519.05 K and extrapolation starting temperature is 485.71 K. In addition, the ΔHdof the two processes were 119.0 and 144.9 Jg–1, respectively. Corresponding to the second stage, there is one strongly decomposed mass loss of 99.87% in TG curve from 485.71 to 531.36 K,which indicates that the title compound is almost completely broken down. According to the DSC-TG curve, the endothermic process is almost consistent with melting process from 373.15 to 376.15 K and the thermal decomposition temperature is relatively high showing a good stability. So it can stably exist in the temperature system of the synthesis reaction of RDX which is beneficial for us to separate and do further study of nitrolysis mechanism of RDX.
3.3.2 Non-isothermal decomposition kinetics
In order to obtain the kinetics parameters (the apparent activation energy (E) and pre-exponential constant (A)) of the main exothermic decomposition reactions for the title compound, a multiple heating method (Kissinger method22and Flynn-Wall-Ozawa method23) was employed. The Kissinger (1) and Flynn-Wall-Ozawa (2) equations are as follows:
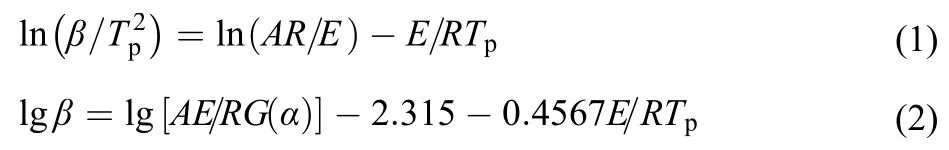
where β is the linear heating rate (Kmin–1), TPis the peak temperature (K), A is pre-exponential constant (s–1), R is the gas constant (Jmol–1K–1), E is the apparent activation energy (kJmol–1), G(α) is the integral form of reaction mechanism function. Ecand Ac, Eoand Ao, Ekand Akare the apparent activation and pre-exponential factor obtained using Coats-Redfern method, FWO method, Kissinger method, respectively.
Corresponding to the Kissinger equation and Flynn-Wall-Ozawa equation, peak values were obtained from the TG carves of different heating rates, the parameters and data processing results are shown in Table 3.
According to the kinetics basic data in Table 3, Fig.5 shows the fitting curve correlated by Kissinger equation (1), which graphically illustrates the function between lnand 1/Tp. From the Fig.5 we can obtain that the slope and intercept of the line are –25.53661 and 37.72806, respectively. Meanwhile we get the correlation coefficient (r2) of 0.98358. Then the kinetics parameter values (E and A) are calculated.24
Table 3 also contains values of the Flynn-Wall-Ozawa equation. The relationship between lgβ and 1/Tpis graphically illustrated in Fig.6. The slope and intercept of the line are–11.55618 and 22.71238, respectively. Moreover the correlation coefficient (r2) of 0.98485 is obtained from Fig.6. Finally, the apparent activation (E) is calculated.
The kinetics parameter values (E and A) determined by the Kissinger method and Flynn-Wall-Ozawa method and linear correlation coefficients (r2) are listed in Table 4. We can see that the apparent activation (E) obtained by Kissinger method agrees well with that obtained by the FWO method, and the correlation coefficients (r2) are all close to 1, indicating the satisfactory validity of the results. The high apparent activation (Table 4) of title compound indicates that more energy is required in its thermal decomposition, so it can exist stably which is benefit of capture and separation. There is no doubt to discuss its kinetics of thermal decomposition because the study of byproducts is of great concern in organic reaction mechanisms. 3.3.3 Mechanism speculation
Coats-Redfern25(3) is a method that we are supposed to get the points of TG curves upon different heating rates to find the counterpart fragment (W) of the sample in different temperatures to calculate the degree of reaction (α) and we use the thermal decomposition mechanism function G(α) to deal with the data obtained. Then the data are substituted into 15 kinds of thermal decomposition mechanism functions (Table 5), respectively.
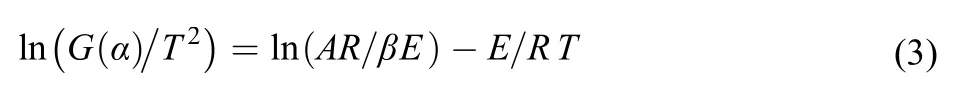
The kinetics parameter values (E and A) can be acquired from the relationship of ln[G(α)/T2] and 1/T. The best mechanism is in keeping with the closest values to E and A through comparing the results with the Kissinger method and Flynn-Wall-Ozawa method.
The kinetics parameter values (Ecand Ac) in Table 6 are obtained based on the 15 kinds of thermal decomposition mechanism functions and the values of TG curves of different heating rates. Comparing these Ecwith Eothat calculated by FWO method, we find the Ecwhich meets the conditions of |(Eo–Ec)/Eo| ≤ 0.1. Then lgAkwhich calculated by Kissinger method and lgAcare compared to find the appropriate lgActhat meets the conditions of |(lgAc―lgAk)/lgAk| ≤ 0.2. We find that function 15 is the most qualified through calculation. The average values of |(Eo―Ec)/Eo| and |(lgAc―lgAk)/lgAk| are 0.1011 and 0.1364 that are satisfied with the conditions. Moreover the correlation coefficients (r2) are all greater than 0.98 indicating that the function 15 has good liner relationship. So we regard the G(α) = (1―α)–1―1 (n = 2) as the thermal decomposition kinetics equation.
4 Conclusions
In summary, 3,5-dinitro-1-oxygen-3,5-diazacyclohexane as a key byproduct for the synthesis of energetic material RDX was successfully separated and structurally characterized and the crystal of title compound was obtained by slow evaporation of an acetone solution with low boiling point at room temperature. The results indicate that the crystal molecular weight is 178.12. It belongs to monoclinic system with space group P121/n1, a = 0.58128(13) nm, b = 1.72389(14) nm, c = 0.71072(6) nm, β = 112.056°, V = 0.66006(16) nm3, Z = 4, DC= 1.792 gcm–3, μ = 0.17 mm–1, F(000) = 368.0, the final deviation factor R is 0.0397. The DSC-TG technique was used to investigate the thermal behavior of title compound, showing that it is a low melting point compound with good stability. Moreover the Kissinger method, Flynn-Wall-Ozawa method, and Coats-Redfern method were used to study the apparent activation energy (E), pre-exponential constant (A), and the thermal decomposition kinetics of the 3,5-dinitro-1-oxygen-3,5-diazacyclohexane. The results show that the E obtained by Kissinger equa-tion and FWO equation are 212.32 and 210.39 kJmol–1, respectively. The A is 6.20 × 1020s–1. What is more, we get the most qualified mechanism function G(α) = (1―α)–1―1 (n = 2) by Coats-Redfern method which is useful to study the thermal decomposition process of title compound. What we have done may provide a useful approach for the design and conclusions of other byproducts which can further help us to analyze the nitrolysis mechanism during RDX preparation by direct nitration.
Supplementary data: CCDC-1403942 (103 K) contains the supplementary crystallographic data for this paper. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre.
(1)Ren, T. S. Chemistry and Technology of Nitramine and Nitrate Explosives; Ordnance Industry Press: Beijing, 1994; pp 27–41. [任特生. 硝胺及硝酸酯炸药化学与工艺学. 北京: 兵器工业出版社, 1994: 27–41.]
(2)Bachmann, W. E. J. Am. Chem. Soc. 1949, 71 (5), 1842. doi: 10.1021/ja01173a092
(3)Duiming, W. J.; Millard, B.; Nutt, C. W. J. Chem. Soc. 1952, 1264.
(4)Willson, F. G.; Forster, A.; Rorberts, E. Cyclo-Trimethylenetrinitramine. US, 2525252, 1950-10-10.
(5)Agrawal, J. P.; Hodgson, R. D. J. Hazard. Mater. 2007, 1–2 (146), 431.
(6)Wang, Q. L. Ammonium Nitrate Explosive; National Defense Industry Press: Beijing, 1984; p 150. [王起来. 硝铵炸药. 北京:国防工业出版社, 1984: 150.]
(7)Yu, X. H. Discussion about Nitrolysis Method of RDX. In Conference Proceedings on China Material Seminar, China Material Seminar, Beijing, 2002. [余咸旱. 关于黑索今硝解方法的探讨. 2002年中国材料研讨会论文集. 北京: 中国材料研究会, 2002.]
(8)Gilpin, V.; Winkler, C. A. Can. J. Chem. 1952, 10 (30), 743.
(9)Bell, J. A.; Dunstan, I. J. Chem. Soc. 1969, 11 (5), 1559.
(10)Fang, Z. J.; Wang, S. F.; Chen, J.; Li, F. P. Chin. J. Explos. Propell. 1992, No. 1, 45. [方志杰, 王绍芳, 陈 驹, 李福平. 火炸药学报, 1992, No. 1, 45.]
(11)He, Z. Y.; Luo, J.; Lü, C. X.; Wang, P.; Xu, R.; Li, J. S. J. Energ. Mater. 2012, 20 (1), 5. [何志勇, 罗 军, 吕春绪, 汪 平, 徐蓉, 李金山. 含能材料, 2012, 20 (1), 5.]
(12)Song, H. Y.; Wang, P.; Qin, G. M.; Ge, Z. X.; Wang, B. Z.; Meng, Z. H.; Li, Q. X. Chin. J. Org. Chem. 2010, 30 (3), 414. [宋红燕, 王 鹏, 覃光明, 葛忠学, 王伯周, 孟子晖, 李清霞. 有机化学, 2010, 30 (3), 414.]
(13)Yu, B. Study of Nitrolysis Mechanism during RDX Synthesis by Direct Nitration in Acid Ionic Liquid. M. S. Dissertation, Nanjing University of Science & Technology, Nanjing, 2009. [郁 波. 酸性离子液体催化下直接法合成黑索金及硝解机理研究[D]. 南京: 南京理工大学, 2009.]
(14)Shen, Y.; Li, Y. X.; Gao, Z. Q.; Tan, Q. Q.; Wang, J. L.; Cao, D. L. Chem. Ind. Eng. Prog. 2014, 33 (4), 1041. [沈 勇, 李永祥,高志强, 谭情请, 王建龙, 曹端林. 化工进展, 2014, 33 (4), 1041.]
(15)Fang, Z. J.; Wang, S. F.; Chen, J.; Chen, L.; Li, F. P. Chin. J. Explos. Propell. 1992, No. 3, 1. [方志杰, 王绍芳, 陈 驹,陈 里, 李福平. 火炸药学报, 1992, No. 3, 1.]
(16)Zuo, J. Q. Solving and Analysis of Activation Energy in Thermal Analysis. M. S. Dissertation, Nanjing University of Science & Technology, Nanjing, 2006. [左金琼. 热分析中活化能的求解与分析[D]. 南京: 南京理工大学, 2006.]
(17)Liu, Z. H. Introduction to Thermal Analysis, 1st ed.; Chemical Industry Press: Beijing, 1991. [刘振海. 热分析导论[M]. 第一版.北京: 化学工业出版社, 1991.]
(18)Sheldrick, G. M. SHELXS-97, Program for X-ray Crystal Structure Refinement; Göttingen University: Germany, 1997.
(19)Sheldrick, G. M. SHELXS-97, Program for X-ray Crystal Structure Solution; Göttingen University: Germany, 1997.
(20)Gotor, F. J.; Criado, J. M. Thermochim. Acta 2002, 383 (1–2), 53. doi: 10.1016/S0040-6031(01)00658-X
(21)Choi, C. S.; Prince, E. Acta Crystallogr. B 1972, No. 28, 2857.
(22)Kissinger, H. E. Anal. Chem. 1957, 29 (11), 1702. doi: 10.1021/ac60131a045
(23)Ozawa, T. Bull. Chem. Soc. Jpn. 1965, 38 (11), 1881. doi: 10.1246/bcsj.38.1881
(24)Schawe, J. E. K. Thermochim. Acta 2002, 388 (1–2), 299. doi: 10.1016/S0040-6031(02)00041-2
(25)Coats, A. W.; Redfern, J. P. Nature 1964, 201 (4914), 68. doi: 10.1038/201068a0
Crystal Structure and Thermal Decomposition Kinetics of Byproduct of Synthesis of RDX: 3,5-Dinitro-1-oxygen-3,5-diazacyclohexane
LI Jing CHEN Li-Zhen WANG Jian-Long*LAN Guan-Chao HOU Huan LI Man
(School of Chemical Engineering and Environment, North University of China, Taiyuan 030051, P. R. China)
A new cyclic byproduct was formed during hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) preparation by direct nitration. Silicone column chromatography with acetone and dichloromethane in various ratios as the eluent was used to separate 3,5-dinitro-1-oxygen-3,5-diazacyclohexane from the product mixture. A single crystal of 3,5-dinitro-1-oxygen-3,5-diazacyclohexane was grown from acetone, and characterized using elemental analysis, Fourier-transform infrared (FTIR) spectroscopy,1H nuclear magnetic resonance (NMR) spectroscopy, and mass spectrometry (MS). Its structure was determined using an X-ray single-crystal diffractometer. The results indicate that the crystal molecular weight is 178.12. It belongs to the monoclinic system with the space group P121/n1, a = 0.58128(13) nm, b = 1.72389(14) nm, c = 0.71072(6) nm, β = 112.056°, V = 0.66006(16) nm3, Z = 4, DC= 1.792 gcm–3, μ = 0.17 mm–1, and F(000) = 368.0; the final deviation factor R is 0.0397. Differential scanning calorimetrythermogravimetry (DSC-TG) was used to investigate the thermal behavior of the title compound. Sharppeaks were observed at 383.15 K (melting) and 519.05 K (decomposition). The kinetic parameters were obtained using the Kissinger and Flynn-Wall-Ozawa methods and the TG data at different heating rates. The Coats-Redfern method was used to study the thermal decomposition mechanism of 3,5-dinitro-1-oxygen-3,5-diazacyclohexane. The results show that the title compound is a low-melting-point compound with good stability; its apparent activation energy and pre-exponential factor, calculated using the Kissinger equation, are 212.32 kJmol–1and 6.20 × 1020s–1, respectively. The apparent activation energy, calculated using the Flynn-Wall-Ozawa equation, is 210.39 kJmol–1. G(α) = (1―α)–1―1 (n = 2) obtained using Coats-Redfern method is regarded as the most appropriate thermal decomposition kinetic equation.
RDX; 3,5-Dinitro-1-oxygen-3,5-diazacyclohexane; Crystal structure; Thermal decomposition kinetics; Apparent activation energy; Mechanism
O641; TJ55
10.3866/PKU.WHXB201510092
Received: July 16, 2015; Revised: October 8, 2015; Published on Web: October 9, 2015.
*Corresponding author. Email: 619379961@qq.com.
The project was supported by the National Natural Science Foundation of China (11447219) and Defense Product Innovation Project, China.
国家自然科学基金(11447219)和国防产品创新项目资助
©Editorial office of Acta Physico-Chimica Sinica

