SIRT3与细胞代谢及心血管疾病的相关性
曹丽娟,刘昕訸,查晴,宋倩,杨克,刘艳
SIRT3与细胞代谢及心血管疾病的相关性
曹丽娟,刘昕訸,查晴,宋倩,杨克,刘艳
上海交通大学医学院附属瑞金医院心血管内科,上海 200025
蛋白去乙酰化酶在细胞生理过程中发挥着极为重要的作用。人蛋白去乙酰化酶包括HDACⅠ、HDACⅡ、HDACⅢ和HDACⅣ4个家族。其中第Ⅲ类即Sir2(Silent information regulator 2)家族包括7个成员——SIRT1~ SIRT7,每个成员都具有不同的细胞定位,并且发挥不同的生物学功能。作为主要定位于线粒体的组蛋白去乙酰化酶,SIRT3不仅调节细胞的能量代谢,并在细胞凋亡、肿瘤生长和一些疾病中发挥作用。文章综述了SIRT3在细胞代谢中的生物学功能以及其在心血管疾病中的研究进展。
SIRT3;组蛋白去乙酰化酶;细胞代谢;心血管疾病
蛋白质乙酰化/去乙酰化是一种非常重要的翻译后修饰,也是调节酶活性以适应细胞代谢和能量改变的一个重要方式。在酵母(Meyen ex Hansen)中,蛋白去乙酰化酶分为Rpd3(Reduced potassium dependency 3)、Hda1(Histone deacetylase 1)和Sir2(Silent information regulator 2)共3个家族,根据对酵母种系发育中组蛋白去乙酰化酶(Histone deacetylases, HDACs)结构的同源性分析[1],真核生物HDACs可分为4类,即HDACⅠ~Ⅳ类[2]。Ⅰ类HDACs包括HDAC1~3和HDAC8,与酵母Rpd3蛋白具有同源性,主要分布于细胞核中,并在各类组织细胞中广泛表达。Ⅱ类HDACs分为Ⅱa和Ⅱb,与酵母Hda1蛋白同源,其中Ⅱa包括HDAC4、HDAC5、HDAC7和HDAC9,Ⅱb包括HDAC6和HDACl0。Ⅱ类HDACs在细胞核和细胞质均有分布,主要在心脏、肺、骨骼肌中表达。Ⅱ类HDACs在细胞核、细胞质的分布受肌细胞增强因子2(Myocyte enhancer factor 2, MEF2)和14-3-3蛋白调节[3]。正常情况下,MEF2与Ⅱ类HDACs结合并以复合物的形式定位于细胞核以维持细胞的稳态;而在病理状况下,Ⅱ类HDACs可被磷酸化并与伴侣分子14-3-3蛋白结合而滞留在细胞浆内,同时MEF2可被磷酸化并调控相应基因的表达,导致肌细胞增生[3~5]。HDAC11兼有Ⅰ类和Ⅱ类HDACs特性,单独归为第Ⅳ类。上述3类HDACs均为金属蛋白酶,其酶催化中心均含有Zn2+。而Ⅲ类HDACs与酵母Sir2蛋白同源,其活性依赖于尼克酰胺腺苷酸(NAD+),而非Zn2+[6,7]。Sir2是一类在进化中高度保守的蛋白质,存在于原核和真核生物中,具有NAD+蛋白活性。
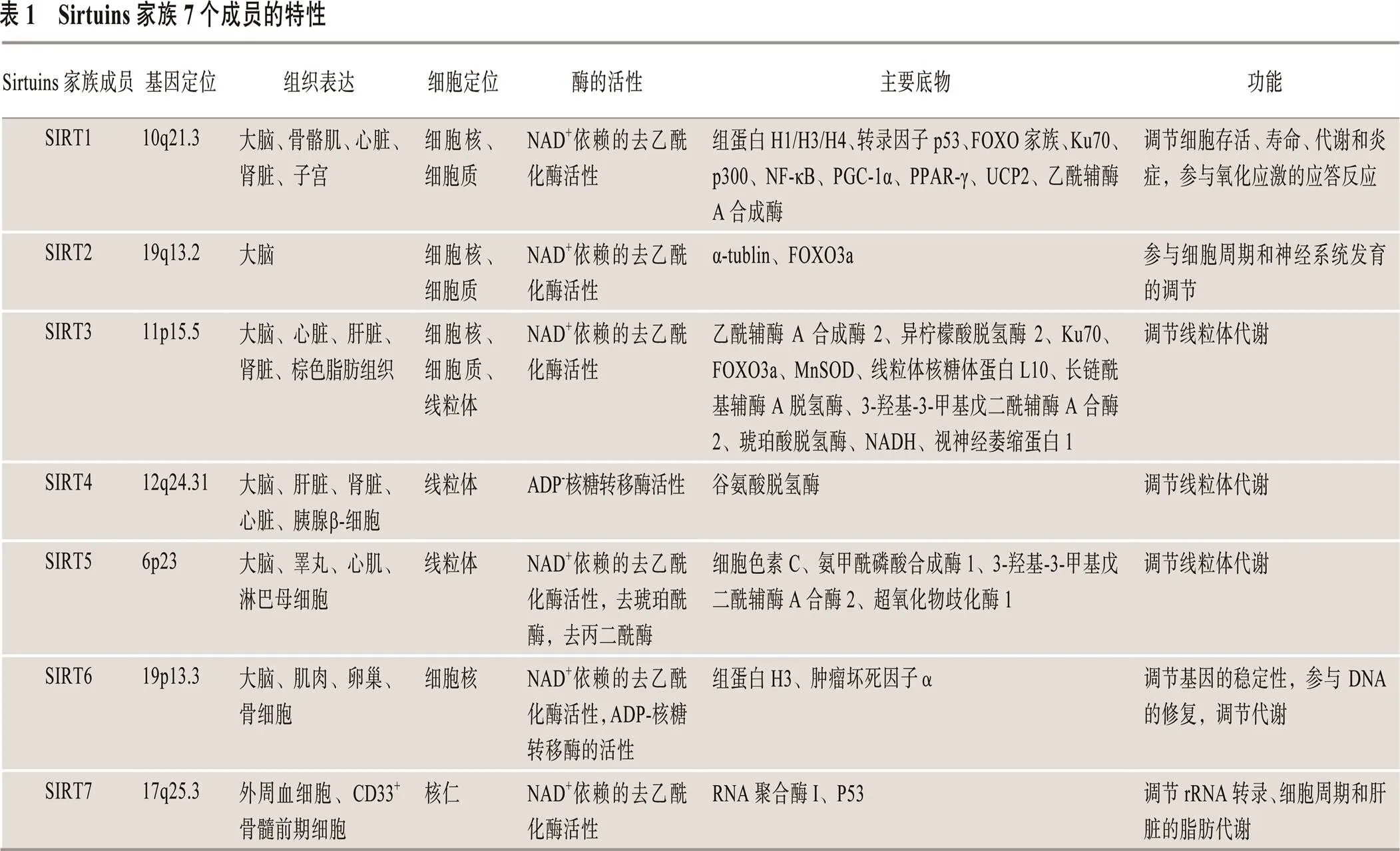
Sirtuins是一类NAD+依赖性组蛋白去乙酰化酶,其去乙酰化作用无特异性,除组蛋白底物外,还可使非组蛋白底物去乙酰化[8~10]。哺乳动物Sirtuins家族有7个成员(SIRTl~SIRT7),其基因定位、组织表达、细胞定位、酶活性、主要作用底物及功能方面各不相同(表1)[11~49]。SIRT1~SIRT7家族成员在衰老、基因沉默、细胞代谢、细胞生长和凋亡以及昼夜节律等方面起重要的调节作用[11]。
以往针对Sirtuins家族的研究主要集中于SIRT1,主要与细胞生存[16,22,42]、细胞代谢[47]、炎症[48]、氧化应激[14]、衰老及衰老相关性疾病(如动脉粥样硬化及糖尿病等)[28,37]密切相关。目前,其他成员的研究亦成为热点,特别是家族重要成员SIRT3,其分布于人体多个脏器,且作用底物广泛,主要调控细胞能量代谢、生物合成及细胞凋亡等,并与心血管疾病密切相关[17,18]。本文就SIRT3的结构、定位、主要功能及其在心血管疾病中的临床意义等研究进展进行了综述。
1 SIRT3定位与分布
人基因位于11号染色体p15.5(11p15.5),由21 902个碱基构成。基因编码蛋白由399个氨基酸构成,分子量约44 kDa。其N端包含一个由25个氨基酸残基组成的线粒体定位信号肽,在线粒体基质中被肽酶识别剪切后,形成一个28 kDa大小的成熟酶蛋白[50,51]。两个可变剪接转录变体编码两种不同蛋白亚型,分别为1~399氨基酸(全长型SIRT3)和142~399氨基酸(成熟型SIRT3)。
Cooper等[52]认为,全长型SIRT3仅定位于线粒体。但随着研究的不断深入,Iwahara等[53]发现,细胞在应激刺激后,全长型SIRT3可被酶剪切为成熟SIRT3[53,54]。由于缺乏线粒体定位信号,成熟型SIRT3非特异性地分布于线粒体或细胞质中,甚至出现于细胞核中。研究发现,无论是定位在线粒体、细胞质还是细胞核,SIRT3均具去乙酰化酶活性[41]。因此,SIRT3的去乙酰化功能可能与其细胞定位无关。
但是,SIRT3分布具有明显的组织特异性,并与器官代谢活性相关。代谢活性越强的器官,如肝脏、棕色脂肪组织、心脏及肾脏等,其SIRT3表达也越高。研究表明,在低温环境中,机体通过增强棕色脂肪细胞中的SIRT3表达,去乙酰化并激活解偶联蛋白活性,提高机体代谢率以维持体温[51]。同样,运动、节食和能量限制等均可增强SIRT3表达活性;而长期高脂饮食会使其表达降低[7,17,21]。虽然SIRT3组织特异性分布的具体机制尚不清楚,但是其高表达于高能量代谢器官,提示其与细胞能量代谢调控密切相关。
2 SIRT3功能
线粒体的主要功能为能量合成,同时还具有调节活性氧(Reactive oxygen species, ROS)生成、细胞代谢(如胆固醇、激素合成等)及细胞凋亡等生物活性。SIRT3作为线粒体中主要的去乙酰化酶,可调节细胞能量合成和线粒体生理活性,同时参与调控细胞生存维持所需生物分子(如蛋白质及脂质等)合成等作用[17,55]。SIRT3参与几乎所有与机体细胞代谢相关信号通路的调控,如ROS产生和清除[56]、三羧酸循环(Tricarboxylic acid cycle, TAC)[17,18,57~59]、脂肪酸氧化(Fatty acid metabolism, FAM)[21,51,60]、尿素循环(Urea cycle, UC)[21]、酮体生成[24,61]、蛋白质合成[7,8]及细胞生长和凋亡[20]等(图1)。
研究表明,SIRT3表达水平及其生物活性受多种因素影响,包括:外界因素如能量限制、禁食、体温、衰老及运动等[17,27,62];内部因素如NAD+/NADH活性变化、过氧化物酶体增值物激活受体-γ共激活因子1-α(Peroxisome proliferator-activated receptor γ coactivator-1α, PGC-1α)的表达及激活、雌激素受体反应元件(Estrogen receptor responsive element, ERRE)的激活及羟化作用和瘦素表达水平等[8,17,35,38](图1)。
中英文对照缩表见附表1。
环境因素(昼夜、气温及应激等)对机体的各项生命活动具有非常重要的影响,往往会造成机体中脏器、细胞乃至分子的波动,而这些影响往往与能量密切相关。但是,各项生命活动均需一个相对稳定的内部环境,因此需要一系列基因参与其内维持能量网络的稳态。正是基于这样一种生命维持模式,SIRT3的表达对于外界及细胞内多种刺激因素产生应答,同时,其下游调控基因也多与能量代谢密切相关。SIRT3作为能量代谢网络中一个重要环节调节机体自身能量代谢平衡,从而使得机体能够应对环境的变化。
2.1 SIRT3与能量代谢
能量代谢是细胞生存的基础活动,当细胞能量需求或供给发生变化时,细胞代谢就会发生一系列调整以维持细胞生存。当能量供应减少(如能量限制,即在保证机体正常营养需求情况下,减少机体30%~40%能量摄入)或能量需求增加(如寒冷暴露)时,一方面线粒体SIRT3通过去乙酰化线粒体核糖体蛋白L10,降低50%~60%线粒体核糖体内蛋白质(如细胞色素C氧化酶亚基II)合成,降低葡萄糖代谢速率及与其相关的氧化磷酸化水平[8]。
另一方面,线粒体SIRT3还通过去乙酰化作用增强长链酰基辅酶A脱氢酶、烯酰辅酶A水合酶、3-羟酰基辅酶A脱氢酶及亲环素D等蛋白活性,促进细胞脂肪酸氧化;激活羟甲戊二酰辅酶A合成酶2,促进酮体生成;提高谷氨酸脱氢酶、乙酰辅酶A合成酶2、苹果酸脱氢酶及琥珀酸脱氢酶等酶活性,加速细胞三羧酸循环;激活氨甲酰基磷酸合成酶Ⅰ和鸟氨酸氨甲酰基转移酶,加速尿素循环,促进氨基酸氧化[13,14,21,24,29]。
在能量缺乏时,机体通过SIRT3上述调节作用,减少葡萄糖氧化和蛋白质合成,提高脂肪酸、氨基酸氧化及酮体生成等过程,维持机体能量供给。同时,上述调节还能保证一些特殊器官(如大脑等)在机体葡萄糖水平降低的情况下,可通过消耗乙酰乙酸和b-羟丁酸来维持细胞的能量平衡[14,18,25,40,63]。
由此可见,SIRT3参与调节能量代谢的各个方面,对机体能量代谢各项机能起着不可替代的调控作用,通过维持机体内能量代谢平衡,从而保护应激条件下器官生理活性。
2.2 SIRT3与ROS
氧化应激所导致的细胞内ROS累积与心肌肥厚[20,44,64,65]、冠状动脉粥样硬化[66,67]、高脂血症[21,68]、2型糖尿病[69]、胰岛素抵抗[70]、脂肪肝[7,71]、神经退行性疾病(如年龄相关的听力损伤)[9,72]、阿尔兹海默病[73,74]及肿瘤[63,75~78]等疾病密切相关。
Bell等[79]认为,线粒体SIRT3可直接去乙酰化并激活抗氧化因子锰超氧化物歧化酶(Manganese superoxide dismutase, MnSOD)和异柠檬酸脱氢酶2(Isocitrate dehydrogenase 2, IDH2),启动抗氧化过程,防止ROS累积;Jacobs等[56]还发现,细胞核内SIRT3可通过去乙酰化从而增加转录因子叉形头转录因子的O亚型(Forkhead box O3, FOXO3a)表达,而后者则进一步促进MnSOD和IDH2表达减轻氧化应激对细胞的损伤,延缓相关疾病的发生和发展[56,79]。SIRT3通过直接和间接作用下调ROS水平,促进一氧化碳合成酶(Endothelial nitric oxide synthase, eNOS)生成,改善血管内皮功能,从而延缓动脉粥样硬化形成和进展[80]。
但是,当细胞内SIRT3表达水平下降时,线粒体中的MnSOD和IDH2等抗氧化因子活性也会随之下降,造成ROS累积,导致线粒体功能紊乱,从而诱发胰岛素抵抗、2型糖尿病及恶性肿瘤等疾病[43,63,79,81]。其导致胰岛素抵抗的可能机制为:伴随SIRT3水平降低,细胞内ROS清除作用削减,ROS在细胞内大量累积,从而激活氧化应激相关信号通路蛋白PKC (Protein kinase C)、S6激酶和JNK(Jun N-terminal kinase)等,下调胰岛素受体底物-1(Insulin receptor substrate, IRS-1)酪氨酸磷酸化水平,降低PI3K及Akt磷酸化水平,抑制胰岛素信号通路,诱发胰岛素抵抗,从而导致与胰岛素抵抗密切相关的2型糖尿病发生和发展。
SIRT3通过ROS信号通路还与肿瘤的发生发展密切相关。SIRT3表达上升可降低ROS水平,使低氧诱导因子-1α(Hypoxia inducible factor-1, HIF-1α)稳定性下降,通过抑制ROS累积和HIF-1α活性从而抑制肿瘤细胞生长。但是,当SIRT3水平降低时,ROS累积增加,HIF-1α表达上调,稳定性及活性增加,进而诱导缺氧反应蛋白(如血管内皮生长因子)的表达,加速糖酵解代谢,促进营养物质氧化和ATP产生[63],增加肿瘤细胞在缺氧环境下的适应性,促进肿瘤细胞增殖、新生血管生成、侵袭和迁移[43,79]。
ROS是导致大部分疾病发生发展的重要因素,而调节ROS累积对于疾病的发生和发展具有决定性作用,SIRT3的表达水平与ROS累积具有负相关性。在氧化应激时,其表达具有应激性升高的特点,起到保护细胞对抗氧化应激的作用。但是,当应激反应进一步放大时,其表达大幅下降,原有的平衡调节作用丧失,相反促进氧化应激反应,最终加速疾病进展。调节SIRT3表达可有效对抗氧化应激从而逆转病程。
2.3 SIRT3与细胞凋亡和生长
在细胞凋亡和生长过程中,SIRT3具有非常复杂的生物效应。一方面,SIRT3可通过调节Bcl-2或JNK2活性,促进细胞凋亡[82];另一方面,SIRT3又可通过核转录因子FOXO3a和Ku70信号通路,参与维持细胞生存[53,82,83]。
Allison等[82]在研究肿瘤发病机制时发现,敲除Bcl-2可引发细胞凋亡,但是共敲除SIRT3后,反而延缓了细胞的凋亡进程[82,84],提示肿瘤细胞中的SIRT3参与Bcl-2/p53凋亡信号通路。Allison等[82]研究还表明,JNK1可通过促进SIRT3转录,加速JNK-2/JNK1相关的非P53依赖性凋亡。
在生理状况下,SIRT3还可通过去乙酰化底物AceCS2参与Bcl-2/p53诱导的细胞生长停滞反应。研究发现,敲除Bcl-2可诱导细胞停滞在G1期,此过程依赖于SIRT3/AceCS2,推测SIRT3/AceCS2是细胞生长中不可或缺的调节因素。SIRT3可能通过该途径延长机体寿命。因此,SIRT3可能是一个长寿因子[85]。
在慢性炎症刺激等应激情况下,SIRT3可通过以下方式参与细胞凋亡的调控:(1)MnSOD和IDH2途径。SIRT3去乙酰化FOXO3a,提高其底物MnSOD和IDH2活性,后两者可减少细胞内ROS含量。线粒体SIRT3还能直接使MnSOD和IDH2蛋白去乙酰化,增强其抗氧化活性,减少ROS累积,从而降低ROS对细胞的损伤。(2)Ku70途径。SIRT3去乙酰化Ku70,使后者与促凋亡蛋白Bax紧密结合,阻止Bax由细胞核转运至线粒体,从而抑制细胞凋亡[85,86]。SIRT3通过这些途径来维持线粒体的内环境稳定,以保护线粒体的正常生理功能,抑制细胞凋亡和坏死。
在生理情况下,大多数离子和溶质都不能通过线粒体屏障,这有利于保持细胞膜内、外两侧Ca2+离子平衡和正常的细胞膜电位以维持细胞正常生理功能。然而,随着年龄的增长,细胞内环境的改变,如ROS和Ca2+水平升高,可诱导线粒体膜表面通透性转换孔(Mitochondrial permeability transition pore, mPTP)的形成。在病理情况下,慢性低水平的应激刺激可诱导线粒体上mPTP的形成,如糖尿病病人中蛋白高糖基化或高血脂氧化脂蛋白刺激,均可促进线粒体膜去极化,造成线粒体肿胀,从而诱发细胞凋亡。反之,SIRT3可通过去乙酰化亲环素D减少ROS累积及促进线粒体氧化磷酸化,延迟mPTP的形成和开放,保证线粒体基质中与代谢和生存相关信号传导的正常进行,进而延缓细胞凋亡,维持细胞生长[20,87]。
SIRT3除了参与能量代谢平衡及抗氧化应激作用,其在细胞内还调控细胞周期,通过抑制抑癌基因活性、保护线粒体完整性及控制Ca2+水平,从而抑制细胞凋亡,保护细胞活性,延长细胞寿命。
综上所述,SIRT3在功能方面主要表现为能量代谢平衡、抗氧化应激及调控细胞周期。在SIRT3参与的整个生命过程中,不难发现其与应激状态下细胞自身恢复内稳态有着密切相关性。可以认为,SIRT3是机体在进化过程中衍生出来适应外界快速变化的调控模式,其通过多个层面(能量代谢、氧化应激、细胞信号通路等)发挥作用,从而维持正常生命活动。
3 SIRT3与心血管疾病
SIRT3在细胞代谢、生长、凋亡和生长中具有重要的调控作用,已成为多种疾病(如2型糖尿病、神经退行性变、炎症和癌症等)的治疗靶点,具有非常重要的科学研究价值和临床应用前景[88]。
近年来,越来越多的研究开始关注SIRT3与心血管疾病的关系。Wu等[89]在研究动脉粥样硬化发病机制时发现,在生理状态下SIRT3在血管平滑肌中表达稳定;但在高血压及交感神经兴奋等情况下,随着血管紧张素Ⅱ(Angiotensin II, Ang II)水平升高,血管平滑肌细胞中SIRT3表达增加,进而抑制血管平滑肌细胞增殖。而敲除基因后,平滑肌细胞增殖明显加快,诱发动脉粥样硬化的发生发展。但是,该过程的具体作用机制尚不明确。Winnik等[90]研究发现,敲除SIRT3并没有影响动脉粥样硬化病变进展及斑块稳定性。因此,SIRT3在动脉粥样硬化中的作用还有待于进一步的深入研究。
一项间隔有氧训练的实验表明,相比于对照组,急性心肌损伤(如心肌梗死)组大鼠心肌细胞中SIRT3表达较低,而间隔有氧呼吸可上调心肌细胞中SIRT3基因的表达,进而改善心肌受损情况[8,91]。当心肌细胞发生缺血再灌注损伤时,SIRT3缺失加剧心肌损伤,其可能机制是:SIRT3缺失后导致其底物如MnSOD等不能被正常去乙酰化,心肌细胞抗氧化活性减弱,不能拮抗缺血所致的ROS累积,从而加剧缺血性心肌能量不足及与再灌注相关的氧化应激、凋亡、炎症及微循环应激等反应[92,93]。
最近,Michael等正在开展一项正常饮食和间断快速饮食(Intermittent fasting diet)与SIRT3表达的临床观察研究。该研究将观察控制饮食3周,通过比较两组受试者外周血单个核细胞中基因表达水平的差异,探讨SIRT3与心血管疾病的相关性及其潜在的调控机制(网络资料来源:http://clinicaltrials. gov/ct2/show/NCT02122575?term=SIRT3&rank=4.)。
而在一项临床Ⅳ期研究中,将SIRT3作为一个重要的心血管疾病调控因素,以评估Omega-3和维生素E调节抗氧化酶活性的作用。研究者通过检测基因的表达水平探讨其对冠状动脉粥样硬化性心脏病产生的影响(网络资料来源:http://clinicaltrials. gov/ct2/show/NCT02011906?term=SIRT3&rank=1)。
由于SIRT3在细胞能量代谢、细胞生长及凋亡等生理活动中发挥着极为重要的调控作用,SIRT3与心血管疾病,如冠状动脉粥样硬化、心肌肥厚和心肌梗死等疾病也密切相关,可能成为一种新的心血管疾病药物治疗靶点[4]。目前,SIRT3在心血管疾病中的作用越来越受到人们的关注,其在心血管疾病中的作用机制也亟待进一步的研究。
www.Chinagene.cn
[1] Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis., 2004, 338(1): 17–31.
[2] Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men., 2008, 9(3): 206–218.
[3] Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang SR, Ling HY, Bers DM, Maier LS, Olson EN, Brown JH. CaMKIIδ isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses., 2007, 282(48): 35078–35087.
[4] Nebbioso A, Manzo F, Miceli M, Conte M, Manente L, Baldi A, De Luca A, Rotili D, Valente S, Mai A, Usiello A, Gronemeyer H, Altucci L. Selective classⅡ HDAC inhibitors impair myogenesis by modulating the stability and activity of HDAC-MEF2 complexes., 2009, 10(7): 776–782.
[5] Lu J, McKinsey TA, Zhang CL, Olson EN. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class Ⅱ histone deacetylases., 2000, 6(2): 233–244.
[6] Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase., 2002, 158(4): 647–657.
[7] Kendrick AA, Choudhury M, Rahman SM, McCurdy CE, Friederich M, Van Hove JL, Watson PA, Birdsey N, Bao J, Gius D, Sack MN, Jing E, Kahn CR, Friedman JE, Jonscher KR. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation., 2011, 433(3): 505–514.
[8] Yang YJ, Cimen H, Han MJ, Shi T, Deng JH, Koc H, Palacios OM, Montier L, Bai YD, Tong Q, Koc EC. NAD+-dependent deacetylase SIRT3 regulates mitochondrial protein synthesis by deacetylation of the ribosomal protein MRPL10., 2010, 285(10): 7417–7429.
[9] Kim SH, Lu HF, Alano CC. Neuronal Sirt3 protects against excitotoxic injury in mouse cortical neuron culture., 2011, 6(3): e14731.
[10] Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria., 2002, 99(21): 13653–13658.
[11] Jin L, Wei WT, Jiang YB, Peng H, Cai JH, Mao C, Dai H, Choy W, Bemis JE, Jirousek MR, Milne JC, Westphal CH, Perni RB. Crystal structures of human SIRT3 displaying substrate-induced conformational changes., 2009, 284(36): 24394–24405.
[12] Bao J, Lu Z, Joseph JJ, Carabenciov D, Dimond CC, Pang L, Samsel L, McCoy JP Jr, Leclerc J, Nguyen P, Gius D, Sack MN. Characterization of the murine SIRT3 mitochondrial localization sequence and comparison of mitochondrial enrichment and deacetylase activity of long and short SIRT3 isoforms., 2010, 110(1): 238–247.
[13] Buler M, Aatsinki SM, Izzi V, Uusimaa J, Hakkola J. SIRT5 is under the control of PGC-1α and AMPK and is involved in regulation of mitochondrial energy metabolism., 2014, 28(7): 3225–3237.
[14] Das S, Mitrovsky G, Vasanthi HR, Das DK. Antiaging properties of a grape-derived antioxidant are regulated by mitochondrial balance of fusion and fission leading to mitophagy triggered by a signaling network of Sirt1-Sirt3- Foxo3-PINK1-PARKIN., 2014, 2014: Article ID 345105.
[15] Dolle C, Rack JG, Ziegler M. NAD and ADP-ribose metabolism in mitochondria., 2013, 280(15): 3530–3541.
[16] Du G, Song YL, Zhang T, Ma L, Bian N, Chen XM, Feng JY, Chang Q, Li ZC. Simvastatin attenuates TNF-α-inducedapoptosis in endothelial progenitor cells via the upregulation of SIRT1., 2014, 34(1): 177–182.
[17] Fernandez-Marcos PJ, Jeninga EH, Canto C, Harach T, de Boer VC, Andreux P, Moullan N, Pirinen E, Yamamoto H, Houten SM, Schoonjans K, Auwerx J. Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis., 2012, 2: Article number: 425.
[18] Finley LW, Haas W, Desquiret-Dumas V, Wallace DC, Procaccio V, Gygi SP, Haigis MC. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity., 2011, 6(8): e23295.
[19] Gertler AA, Cohen HY. SIRT6, a protein with many faces., 2013, 14(6): 629–639.
[20] Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy., 2010, 2(12): 914–923.
[21] Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, Shortreed MR, Prolla T, Markley JL, Smith LM, Zhao S, Guan KL, Denu JM. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction., 2011, 41(2): 139–149.
[22] Herbert KJ, Cook AL, Snow ET. SIRT1 inhibition restores apoptotic sensitivity in p53-mutated human keratinocytes., 2014, 277(3): 288–297.
[23] Hirahatake KM, Slavin JL, Maki KC, Adams SH. Associations between dairy foods, diabetes, and metabolic health: potential mechanisms and future directions., 2014, 63(5): 618–627.
[24] Hirschey MD, Shimazu T, Capra JA, Pollard KS, Verdin E. SIRT1 and SIRT3 deacetylate homologous substrates: AceCS1, 2 and HMGCS1, 2., 2011, 3(6): 635–642.
[25] Inoue T, Nakayama Y, Li YZ, Matsumori H, Takahashi H, Kojima H, Wanibuchi H, Katoh M, Oshimura M. SIRT2 knockdown increases basal autophagy and prevents postslippage death by abnormally prolonging the mitotic arrest that is induced by microtubule inhibitors., 2014, 281(11): 2623–2637.
[26] Jin L, Galonek H, Israelian K, Choy W, Morrison M, Xia Y, Wang XH, Xu YH, Yang YC, Smith JJ, Hoffmann E, Carney DP, Perni RB, Jirousek MR, Bemis JE, Milne JC, Sinclair DA, Westphal CH. Biochemical characterization, localization, and tissue distribution of the longer form of mouse SIRT3., 2009, 18(3): 514–525.
[27] Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, Koya D. Sirtuins and renal diseases: relationship with aging and diabetic nephropathy., 2013, 124(3): 153–164.
[28] Koka S, Aluri HS, Xi L, Lesnefsky EJ, Kukreja RC. Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: potential role of NO/SIRT1/PGC-1αsignaling., 2014, 306(11): H1558–H1568.
[29] Kugel S, Mostoslavsky R. Chromatin and beyond: the multitasking roles for SIRT6., 2014, 39(2): 72–81.
[30] Lin ZF, Xu HB, Wang JY, Lin Q, Ruan Z, Liu FB, Jin W, Huang HH, Chen X. SIRT5 desuccinylates and activates SOD1 to eliminate ROS., 2013, 441(1): 191–195.
[31] Liu B, Che W, Xue J, Zheng C, Tang K, Zhang J, Wen J, Xu Y. SIRT4 prevents hypoxia-induced apoptosis in H9c2 cardiomyoblast cells., 2013, 32(3): 655–662.
[32] Liu B, Che W, Zheng C, Liu W, Wen J, Fu H, Tang K, Zhang J, Xu Y. SIRT5: a safeguard against oxidative stress-induced apoptosis in cardiomyocytes., 2013, 32(4): 1050–1059.
[33] Mortuza R, Chen SL, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway., 2013, 8(1): e54514.
[34] Osborne B, Cooney GJ, Turner N. Are sirtuin deacylase enzymes important modulators of mitochondrial energy metabolism?, 2014, 1840(4): 1295– 1302.
[35] Pirinen E, Lo Sasso G, Auwerx J. Mitochondrial sirtuins and metabolic homeostasis., 2012, 26(6): 759–770.
[36] Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, Danielson SR, Guo A, Gut P, Sahu AK, Li B, Uppala R, Fitch M, Riiff T, Zhu L, Zhou J, Mulhern D, Stevens RD, Ilkayeva OR, Newgard CB, Jacobson MP, Hellerstein M, Goetzman ES, Gibson BW, Verdin E. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks., 2013, 18(6): 920–933.
[37] Rehan L, Laszki-Szczachor K, Sobieszczanska M, Polak-Jonkisz D. SIRT1 and NAD as regulators of ageing., 2014, 105(1–2): 1–6.
[38] Sack MN, Finkel T. Mitochondrial metabolism, sirtuins, and aging., 2012, 4(12):pii: a013102, doi: 10.1101/cshperspect.a013102.
[39] Sadi G, Bozan D, Yildiz HB. Redox regulation of antioxidant enzymes: post-translational modulation of catalase and glutathione peroxidase activity by resveratrol in diabetic rat liver., 2014, 393(1–2): 111–122.
[40] Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, Archer SL, Chan DC, Gupta MP. SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress., 2014, 34(5): 807–819.
[41] Scher MB, Vaquero A, Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress., 2007, 21(8): 920–928.
[42] Sin TK, Yu AP, Yung BY, Yip SP, Chan LW, Wong CS, Ying M, Rudd JA, Siu PM. Modulating effect of SIRT1 activation induced by resveratrol on Foxo1-associated apoptotic signalling in senescent heart., 2014, 592(Pt 12): 2535–2548.
[43] Sun HL, Wu YR, Fu DJ, Liu YC, Huang C. SIRT6 regulates osteogenic differentiation of rat bone marrow mesenchymal stem cells partially via suppressing the nuclear factor-κB signaling pathway., 2014, 32(7): 1943–1955.
[44] Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice., 2009, 119(9): 2758–2771.
[45] Tan MJ, Peng C, Anderson KA, Chhoy P, Xie ZY, Dai LZ, Park J, Chen Y, Huang H, Zhang Y, Ro J, Wagner GR, Green MF, Madsen AS, Schmiesing J, Peterson BS, Xu GF, Ilkayeva OR, Muehlbauer MJ, Braulke T, Mühlhausen C, Backos DS, Olsen CA, McGuire PJ, Pletcher SD, Lombard DB, Hirschey MD, Zhao YM. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5., 2014, 19(4): 605–617.
[46] Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction., 2007, 6(4): 505–514.
[47] Wilking MJ, Singh C, Nihal M, Zhong W, Ahmad N. SIRT1 deacetylase is overexpressed in human melanoma and its small molecule inhibition imparts anti-proliferative response via p53 activation., 2014, 563: 94–100.
[48] Yao H, Sundar IK, Ahmad T, Lerner C, Gerloff J, Friedman AE, Phipps RP, Sime PJ, McBurney MW, Guarente L, Rahman I. SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism., 2014, 306(9): L816–L828.
[49] Yoshizawa T, Karim MF, Sato Y, Senokuchi T, Miyata K, Fukuda T, Go C, Tasaki M, Uchimura K, Kadomatsu T, Tian Z, Smolka C, Sawa T, Takeya M, Tomizawa K, Ando Y, Araki E, Akaike T, Braun T, Oike Y, Bober E, Yamagata K. SIRT7 controls hepatic lipid metabolism by regulating the ubiquitin-proteasome pathway., 2014, 19(4): 712–721.
[50] Smith BC, Settles B, Hallows WC, Craven MW, Denu JM. SIRT3 substrate specificity determined by peptide arrays and machine learning., 2011, 6(2): 146–157.
[51] Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes., 2005, 280(14): 13560–13567.
[52] Cooper HM, Spelbrink JN. The human SIRT3 protein deacetylase is exclusively mitochondrial., 2008, 411(2): 279–285.
[53] Iwahara T, Bonasio R, Narendra V, Reinberg D. SIRT3 functions in the nucleus in the control of stress-related gene expression., 2012, 32(24): 5022–5034.
[54] Shulga N, Wilson-Smith R, Pastorino JG. Sirtuin-3 deacetylation of cyclophilin D induces dissociation of hexokinase II from the mitochondria., 2010, 123(Pt 6): 894–902.
[55] Hendrickson SL, Lautenberger JA, Chinn LW, Malasky M, Sezgin E, Kingsley LA, Goedert JJ, Kirk GD, Gomperts ED, Buchbinder SP, Troyer JL, O'Brien SJ. Genetic variants in nuclear-encoded mitochondrial genes influence AIDS progression., 2010, 5(9): e12862.
[56] Jacobs KM, Pennington JD, Bisht KS, Aykin-Burns N, Kim HS, Mishra M, Sun L, Nguyen P, Ahn BH, Leclerc J, Deng CX, Spitz DR, Gius D. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression., 2008, 4(5): 291–299.
[57] Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis., 2008, 105(38): 14447–14452.
[58] Chen YH, Zhang JY, Lin Y, Lei QY, Guan KL, Zhao SM, Xiong Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS., 2011, 12(6): 534–541.
[59] Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, Haigis MC. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization., 2011, 19(3): 416–428.
[60] Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation., 2010, 464(7285): 121–125.
[61] Shimazu T, Hirschey MD, Hua L, Dittenhafer-Reed KE, Schwer B, Lombard DB, Li Y, Bunkenborg J, Alt FW, Denu JM, Jacobson MP, Verdin E. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production., 2010, 12(6): 654–661.
[62] Lu Z, Bourdi M, Li JH, Aponte AM, Chen Y, Lombard DB, Gucek M, Pohl LR, Sack MN. SIRT3-dependent deacetylation exacerbates acetaminophen hepatotoxicity., 2011, 12(8): 840–846.
[63] Li HQ, Feng ZQ, Wu WZ, Li J, Zhang JQ, Xia TY. SIRT3 regulates cell proliferation and apoptosis related to energy metabolism in non-small cell lung cancer cells through deacetylation of NMNAT2., 2013, 43(5): 1420–1430.
[64] Park ES, Kang JC, Jang YC, Park JS, Jang SY, Kim DE, Kim B, Shin HS. Cardioprotective effects of rhamnetin in H9c2 cardiomyoblast cells under H2O2-induced apoptosis., 2014, 153(3): 552–560.
[65] Pillai VB, Sundaresan NR, Gupta MP. Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging., 2014, 114(2): 368–378.
[66] Dong C, Della-Morte D, Wang L, Cabral D, Beecham A, McClendon MS, Luca CC, Blanton SH, Sacco RL, Rundek T. Association of the sirtuin and mitochondrial uncoupling protein genes with carotid plaque., 2011, 6(11): e27157.
[67] Li LF, Zeng H, Hou XW, He XC, Chen JX. Myocardial injection of apelin-overexpressing bone marrow cells improves cardiac repair via upregulation of Sirt3 after myocardial infarction., 2013, 8(9): e71041.
[68] Giralt A, Hondares E, Villena JA, Ribas F, Diaz-Delfin J, Giralt M, Iglesias R, Villarroya F. Peroxisome proliferator- activated receptor-gamma coactivator-1α controls transcription of the Sirt3 gene, an essential component of the thermogenic brown adipocyte phenotype., 2011, 286(19): 16958–16966.
[69] Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1α in skeletal muscle., 2009, 1(9): 771–783.
[70] Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stančáková A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RV Jr, Kahn CR, Verdin E. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome., 2011, 44(2): 177–190.
[71] Fritz KS, Galligan JJ, Smathers RL, Roede JR, Shearn CT, Reigan P, Petersen DR. 4-Hydroxynonenal inhibits SIRT3 via thiol-specific modification., 2011, 24(5): 651–662.
[72] Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction., 2010, 143(5): 802–812.
[73] Lutz MI, Milenkovic I, Regelsberger G, Kovacs GG. Distinct patterns of sirtuin expression during progression of Alzheimer's disease., 2014, 16(2): 405–414.
[74] Weir HJ, Murray TK, Kehoe PG, Love S, Verdin EM, O'Neill MJ, Lane JD, Balthasar N. CNS SIRT3 expression is altered by reactive oxygen species and in Alzheimer's disease., 2012, 7(11): e48225.
[75] Chen Y, Fu LL, Wen X, Wang XY, Liu J, Cheng Y, Huang J. Sirtuin-3 (SIRT3), a therapeutic target with oncogenic and tumor-suppressive function in cancer., 2014, 5: e1047.
[76] Desouki MM, Doubinskaia I, Gius D, Abdulkadir SA. Decreased mitochondrial SIRT3 expression is a potential molecular biomarker associated with poor outcome in breast cancer., 2014, 45(5): 1071–1077.
[77] Huang KH, Hsu CC, Fang WL, Chi CW, Sung MT, Kao HL, Li AF, Yin PH, Yang MH, Lee HC. SIRT3 expression as a biomarker for better prognosis in gastric cancer., 2014, 38(4): 910–917.
[78] Papa L, Hahn M, Marsh EL, Evans BS, Germain D. SOD2 to SOD1 switch in breast cancer., 2014, 289(9): 5412–5416.
[79] Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1α and tumor growth by inhibiting mitochondrial ROS production., 2011, 30(26): 2986–2996.
[80] Xue L, Xu F, Meng L, Wei S, Wang J, Hao P, Bian Y, Zhang Y, Chen Y. Acetylation-dependent regulation of mitochondrial ALDH2 activation by SIRT3 mediates acute ethanol-induced eNOS activation., 2012, 586(2): 137–142.
[81] Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, Kahn CR. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production., 2011, 108(35): 14608–14613.
[82] Allison SJ, Milner J. SIRT3 is pro-apoptotic and participates in distinct basal apoptotic pathways., 2007, 6(21): 2669–2677.
[83] Cooper HM, Huang JY, Verdin E, Spelbrink JN. A new splice variant of the mouse SIRT3 gene encodes the mitochondrial precursor protein., 2009, 4(3): e4986.
[84] Kawamura Y, Uchijima Y, Horike N, Tonami K, Nishiyama K, Amano T, Asano T, Kurihara Y, Kurihara H. Sirt3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest., 2010, 120(8): 2817– 2828.
[85] Li S, Banck M, Mujtaba S, Zhou MM, Sugrue MM, Walsh MJ. p53-induced growth arrest is regulated by the mitochondrial SirT3 deacetylase., 2010, 5(5): e10486.
[86] Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70., 2008, 28(20): 6384–6401.
[87] Shulga N, Pastorino JG. Ethanol sensitizes mitochondria to the permeability transition by inhibiting deacetylation of cyclophilin-D mediated by sirtuin-3., 2010, 123(Pt 23): 4117–4127.
[88] Shih J, Donmez G. Mitochondrial sirtuins as therapeutic targets for age-related disorders., 2013, 4(3–4): 91–96.
[89] Wu XN, Bu PL, Liu JN, Zhao LX, Wang X, Li N. Inhibitory effect of Sirt3 on proliferation of vascular smooth muscle cells induced by angiotensinⅡ., 2013, 29(3): 237–241.
[90] Winnik S, Gaul DS, Preitner F, Lohmann C, Weber J, Miranda MX, Liu YL, van Tits LJ, Mateos JM, Brokopp CE, Auwerx J, Thorens B, Luscher TF, Matter CM. Deletionof Sirt3 does not affect atherosclerosis but accelerates weight gain and impairs rapid metabolic adaptation in LDL receptor knockout mice: implications for cardiovascular risk factor development., 2014, 109(1): 399.
[91] Jiang HK, Miao Y, Wang YH, Zhao M, Feng ZH, Yu XJ, Liu JK, Zang WJ. Aerobic interval training protects against myocardial infarction-induced oxidative injury by enhancing antioxidase system and mitochondrial biosynthesis., 2014, 41(3): 192– 201.
[92] Pantazi E, Zaouali MA, Bejaoui M, Folch-Puy E, Ben Abdennebi H, Rosello-Catafau J. Role of sirtuins in ischemia-reperfusion injury., 2013, 19(43): 7594–7602.
[93] Porter GA, Urciuoli WR, Brookes PS, Nadtochiy SM. SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts., 2014, 306(12): H1602–H1609.
(责任编委: 张博)
The relationship of SIRT3 with cellular metabolism and cardiovascular diseases
Lijuan Cao, Xinhe Liu, Qing Zha, Qian Song, Ke Yang, Yan Liu
Protein deacetylases play an extremely crucial role in cellular biological processes and have been categorized into four families (HDACⅠ, HDACⅡ, HDACⅢ and HDACⅣ) in human. Of them, HDACⅢ, also known as the Sir2 (Silent information regulator 2) family, contains seven members, SIRT1-7, each exhibiting different cellular localization and biological function. As a major mitochondrial deacetylase, SIRT3 not only modulates cellular metabolism, but also plays important roles in apoptosis, tumor growth, aging and a number of other diseases. In this review, we summarize recent findings related to SIRT3 with an emphasis on its biological functions in regulating cell metabolism and its possible roles in cardiovascular diseases.
SIRT3; HDAC; cell metabolism; cardiovascular diseases
2014-08-26;
2014-10-10
国家自然科学基金项目(编号:81200204,81470547)资助
曹丽娟,硕士研究生,专业方向:动脉粥样硬化。Tel:021-64370045;E-mail:caolijuanqq@163.com
刘艳,博士,主任医师,硕士生导师,研究方向:动脉粥样硬化。E-mail:liuyan_ivy@126.com
10.16288/j.yczz.14-283
网络出版时间: 2014-9-24 14:14:22
URL: http://www.cnki.net/kcms/detail/11.1913.R.20140926.1342.005.html

