基于半刚性四面体四齿配体构筑的Zn(Ⅱ)/Cd(Ⅱ)配位聚合物及其性质
唐文渊 任世斌*,,2 朱冬冬 韩得满 周馨慧
(1台州学院医药化工学院,台州317000)
(2南京大学化学化工学院生命分析化学国家重点实验室,南京210093)
(3南京邮电大学有机电子与信息显示国家重点实验室及先进材料研究所,南京210023)
基于半刚性四面体四齿配体构筑的Zn(Ⅱ)/Cd(Ⅱ)配位聚合物及其性质
唐文渊1任世斌*,1,2朱冬冬1韩得满1周馨慧*,3
(1台州学院医药化工学院,台州317000)
(2南京大学化学化工学院生命分析化学国家重点实验室,南京210093)
(3南京邮电大学有机电子与信息显示国家重点实验室及先进材料研究所,南京210023)
在水热的条件下,利用四(4-吡啶氧甲基)甲烷(L1)或四(3-吡啶氧甲基)甲烷(L2)、1,4-萘二甲酸(1,4-NDC)和d10金属离子发生自组装反应合成了2个化合物{[Cd2(L1)(1,4-NDC)2]·2H2O}n(1)和{[Zn2(L2)(1,4-NDC)2]·DMF·3H2O)}n(2)。单晶结构表明化合物1是通过L1配体与一维链[Cd(1,4-NDC)]n相连构建而成的三维骨架化合物,而化合物2是一对螺旋链与另外的一维链相互垂直交联而形成二维网络结构。更为重要的是,通过引入2种不同空间位阻的配体,研究了辅助配体对金属有机配位聚合物结构多样性的影响。另外,它们的荧光性质也做了相应的探讨。
羧酸配体;异构化;空间位阻;荧光
The study of coordination polymers(CPs)has received increasing interest owing to their fascinating architectures and topologies as well as many potential applications[1].The unique properties of CPs are closely related to their specific structures.In the context,the rational selection of metal ions and linker geometries are of great importance in controlling their desired architectures and functionalities[2].In particular,auxiliary linkers also play a marvelous role in the construction of CPs.Auxiliary aromatic carboxylates have great influence on the structural diversity by the postsynthetic modification or the change of the position and amounts of carboxylic groups[3].However,the systematic study on structural change of CPs helps us to predict and synthesize coordination polymers with expected structures,which enable us to well-tune the structure and function of CPs.
To a large extent,the structural diversity mainly depends on organic linkers,which offers the opportunity to functionalize porous frameworks.In recent years,a large number of studies have been reported that the CPs built from mixed ligands,especially from N-donor ligands and aromatic carboxylate,will exhibit more diverse and fascinating networks with potential applications[4].Additionally,it is well known that using mixed linkers will become more easy for the targeted synthesis of CPs than using a single ligand[5].However, as far as we know,the study on the effect of steric hindrance of an auxiliary linker on the structural diversity of CPs remains largely unexplored.
The incorporation of mixed linkers within CPs offers a significant opportunity to explore the influence factor of their structural change.Herein,we have chosen a couple of N-donor linkers,tetrakis(4-pyridyloxymethylene)methane(L1)and tetrakis(3-pyridyloxymethylene)methane(L2),due to their specific configurations and flexible conformers[4,6].Additionally,naphthalene-1,4-dicarboxylic acid(1,4-NDC)is used as an auxiliary linker to explore the effect of steric hindrance on structural diversity of CPs.We have prepared two complexes:one non-interpenetration network and another threefold interpenetration network,which are built from L1and L2,respectively[4a,6c].Herein,two complexes based on d10metal ions,L1or L2and 1,4-NDC have been successfully prepared under hydrothermal conditions(Scheme 1).They are also characterized by X-ray crystallography,elemental analysis, infrared spectra(IR),powder X-ray diffraction(PXRD), and thermogravimetric(TG)analyses.Besides,photoluminescence and the effect of steric hindrance of an auxiliary linker on the structural diversity of CPs have been discussed.
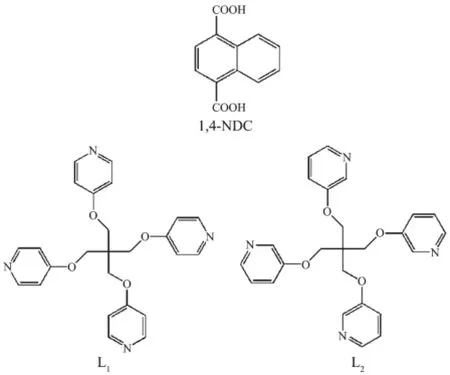
Scheme 1Structure of organic ligands
1 Experimental
1.1 Materials and methods
Ligand L1and L2was prepared according to the literature[6d].Reagents and solvents employed were commercially available.The C,H,and N microanalyses were carried out with a Perkin-Elmer PE 2400ⅡCHN elemental analyzer.Fourier transform infrared (FT-IR)spectra of the compounds were obtained in the 4 000~400 cm-1region on a Bruker Vector 22 spectrophotometer with KBr pellets.Powder X-ray diffraction(PXRD)measurements were obtained on a Bruker D8 Advance X-ray diffractometer with Cu Kα radiation.Thermal analyses(TG)were performed in a nitrogen stream using Seiko Extar 6000 TG equipment with a heating rate of 10℃·min-1.Luminescence spectra were recorded on a Hitachi 850 fluorescence spectrophotometer.
1.2 Synthesis of complexes
1.2.1 Synthesis of{[Cd2(L1)(1,4-NDC)2]·2H2O}n(1)
A mixture of L1(0.044 4 g,0.1 mmol),1,4-NDC(0.021 6 g,0.1 mmol)and Cd(NO3)2·4H2O(0.061 7 g, 0.20 mmol)in CH3CN and H2O(1∶1,V/V)was sealed into a 30 mL Teflon lined stainless steel vessel and heated to 90℃for 3 days.After the mixture was cooled to room temperature,brown rodlike crystals of 1 suitable for X-ray diffraction analyses were obtained in the yield of 25.7%.Elemental analysis Calcd.for C49H40Cd2N4O14(%):C,51.9;H,3.6;N,4.9.Found(%):C,5.20;H,3.7;N,4.8.Selected IR data(KBr,cm-1):3 250(m),2 427(w),1 685(s),1 600(m),1 559(s), 1 536(w),1 398(s),1 356(m),1 327(m),1 294(m), 1 258(w),1 194(w),933(w),795(s).
1.2.2 Synthesis of{[Zn2(L2)(1,4-NDC)2]·DMF·3H2O)}n(2)
A mixture of L2(0.044 6 g,0.1 mmol),1,4-NDC (0.021 6 g,0.1 mmol)and Zn(NO3)2·6H2O(0.059 5 g, 0.20 mmol)in DMF and H2O(1∶1,V/V)was sealed into a 30 mL Teflon lined stainless steel vessel and heated to 90℃for 3 days.After the mixture was cooled to room temperature,yellow block crystals of 2 suitable for X-ray diffraction analyses were obtained in the yield of 35.2%.Elemental analysis Calcd.for C52H49N5O16Zn2(%):C,55.2;H,4.4;N,6.2.Found (%):C,55.1;H,4.5;N,6.1.Selected IR data(KBr, cm-1):3 152(m),1 650(m),1 500(m),1 411(s),1 400 (s),1 359(s),1 194(m),1 103(m),929(m),788(w).
1.3 X-ray crystallography
The single-crystal X-ray data collection for 1 and 2 was performed on a Bruker SMART APEX CCD diffractometer with graphite-monochromated Mo Kα radiation(λ=0.071 073 nm)at room temperature.Data reductions and absorption corrections were performed using the SAINT and SADABS software packages, respectively.Raw frame data were integrated with the SAINT program[7a].The structure was solved by direct methods and refined by full-matrix least-squares on F2using SHELX-97[7b].An empirical absorption correction was applied with the program SADABS[7c].All nonhydrogen atoms were refined anisotropically.Hydrogen atoms were set in calculated positions and refined by a riding mode,with a common thermal parameter.The relevantcrystallographicdataarepresentedand selected bond lengths and angles are given in Table S1~2.
CCDC:1019510,1;1019511,2.

Fig.1(a)Coordination environments of Cd atoms in 1; (b)1D zigzag chain formed by Cd-1,4-NDC; (c)3D structure formed by the coordination of pyridyl N-donors of L1with Cd atoms in 2D chain;(d)Schematic view of the topological structure of 1
2 Results and discussion
2.1 Descriptions of crystal structures
2.1.1 Crystal structure of{[Cd2(L1)(1,4-NDC)2]·2H2O}n(1)
The crystal structure determination reveals that 1 crystallizes in the orthorhombic system and space group Pnnm.The asymmetric unit contains one Cd atom,a half of L1,one deprotonated 1,4-NDC and one coordinated water molecule.Each Cd atom coordinates to three oxygen atoms from two distinct 1,4-NDC and two nitrogen atoms from two distinct L1(Fig.1a).The rest of the coordination sites in each Cd atom are occupied by one water molecule.Thus,each Cd atom has adopted a distorted octahedral geometry.Cd-O and Cd-N distances lie in the ranges of 0.225 0(3)~0.237 8(2)nm and 0.229 4(3)~0.230 0(3)nm,respectively,and the corresponding bond angles around each Cd atom are in the range of 27.50(12)°~169.26(12)°. Each couple of L1shares two Cd atoms through its N-donors to form infinite chains parallel to each other. On the other hand,1,4-NDC adopts μ2-η1∶η1or μ2-η2∶η2-fashion alternatively to connect Cd atoms via itscarboxylate groups,resulting in a 1D zigzag chain [Cd(1,4-NDC)]n(Fig.1b).The 1D chain[Cd(1,4-NDC)]nis threaded with L1ligand via sharing the metal sites to yield a 3D framework(Fig.1c).Topologically,if L1and Cd atoms are viewed as(4,4)-connected nodes, respectively,the overall motif of 1 is a 2-nodal(4,4)-connected(5·62·72·8)(52·6·7·82)2network(Fig.1d).
2.1.2 Crystal structure of{[Zn2(L2)(1,4-NDC)2]·DMF· 3H2O)}n(2)
Complex 2 crystallizes in the monoclinic system with the P21/n space group.The single-crystal X-ray analysis of 2 revealed one Zn(Ⅱ)atom,one L2,two deprotonated 1,4-NDC,a lattice DMF and three free water molecules in the asymmetric unit.As shown in Fig.2a,each Zn atom is pent-coordinated by two nitrogen atoms from two L2and three oxygen atoms from two 1,4-NDC.The Zn-N and Zn-O bond distances are in range of 0.203 4(3)~0.208 7(3)nm and 0.193 8(3)~0.198 2(3)nm,respectively.The bond angles around the Zn atom vary from 94.47(12)°to 132.31(13)°, adopting the trigonal bipyramid geometry.Each L2acts as a tetradentate bridging ligand and joins four Zn atoms to form a 1D chain.Additionally,adjacent two 1,4-NDC are joined end to end by sharing Zn atoms to produce an arched chain,weak interactions betweenwhichacceleratetheformationofan unprecedented double helix(Fig.2b).As a result, these helix are extended by L2to afford a 2D sheet (Fig.2c).From a topological point of view,the central Zn atom and L2ligand are viewed as 4-connecting nodes,respectively,the overall motif of 2 is a 2-nodal (4,4)-connected(4·64·7)2(42·62·7·8)net(Fig.2d).
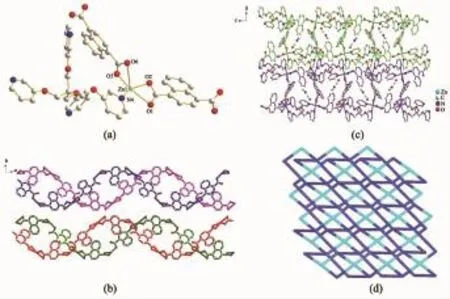
Fig.2(a)Coordination environment of Zn atoms in 2; (b)1D chain in 2;(c)2D layer of 2; (d)Schematic 2-nodal(4,4)-connected(4·64·7)2(42·62·7·8)net in 2
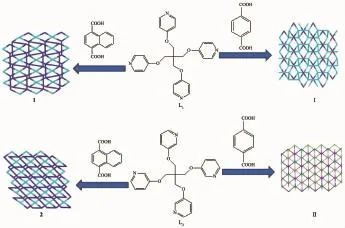
Fig.3Schematic view of control over the structural diversity by adjusting the steric hindrance of an auxiliary linker
2.2 Effect of steric hindrance of an auxiliary linker on the structural diversity
We tried to explore the effect of steric hindrance of an auxiliary linker on the structural diversity of CPs.The phenomenon can be explained by the introduction of an couple of 1,4-BDC and 1,4-NDC within the study system.For 1 andⅠ,the change of the auxiliary linker will bring about the variation of coordination modes,which vary from μ2-η1∶η1-fashion to μ2-η1∶η1and μ2-η2∶η2-fashion.The change of the auxiliary linker from 1,4-NDC to 1,4-BDC result in the change of their topology from 2-nodal(4,4)-connected(5·62·72·8)(52·6·7·82)2network to 2-nodal(4,4) -connected(62·84)network(1 andⅠin Fig.3).In contrast,for 2 andⅡ,they exhibit a 3D network and 2D layer structure,respectively.But it is worth nothing that the change of the auxiliary linker enable their structures to vary from 2-nodal(4,4)-connected (4·64·7)2(42·62·7·8)net to 3-nodal(4,6)-connected (3·4·52·62)(34,42,53·65·7)2(4·52·62·7)net(2 andⅡin Fig.3).As far as their component are concerned,the structure of 1 and I both contain the same componentsthe central d10ions and L1except for auxiliary linker.As a matter of fact,the difference in their structure is attributed to the significant steric hindrance of 1,4-NDC over those of BDC.Therefore,topological structure of 1 and 2 is a far cry from that of theircounterpartsⅠandⅡon account of distinct steric hindrance of auxiliary linkers.In conclusion,the effect of steric hindrance of an auxiliary linker will strongly affect the structural diversity of CPs.
2.3 X-ray powder diffraction and thermal analyses
To confirm whether the single crystal structure is truly representative of the bulk materials,powder X-ray diffraction measures were carried out for 1 and 2 at room temperature.As shown in Fig.S1,the peak positions of the experimental and simulated XRPD patterns are in agreement with each other,suggesting the presence of the phase purity in the corresponding samples 1 and 2.Thermal gravimetric analysis was performed for 1 and 2 under N2atmosphere with a heating rate of 10℃·min-1in the temperature range of 25~800℃(Fig.S2).The TGA curve of 1 shows a weight loss of 6.2%(Calcd.3.2%)from room temperature to 187℃corresponding to the release of the surface and included water molecules and then there is an intense weight loss from 491.8℃,which is attributed to the decomposition of the framework.For 2,the weight loss of 3.5%(Calcd.3.1%)attributed to the gradual release of the surface and included water molecules is observed in the range of 20.1~220.5℃, the framework retains in the temperature range of 220.5~491.8℃,and then decomposes.
2.4 Photoluminescence
Coordination complexes with d10metal ions have been investigated for luminescent properties as well as theirpotentialapplicationsinelectroluminescent displays,chemical sensors,and photochemistry[8].The changeable luminescent features are originated from adopting different coordination modes around d10metal ions,interaction with bridging linkers,and so on.The solid state luminescent properties of free L1,L2,1 and 2 were studied at room temperature,as shown in Fig. 4.The ligand L1or L2displays obvious luminescent intensity in the range of 400~600 nm under excitation at 370 nm at room temperature.The emission spectra for 1 and 2 exhibit similar emission peaks at 417 nm. However,the relatively different intensities of the two emissions could result from the variety of coordinating modes of the metal ion with the bridging linker. Additionally,in view of the structural characteristic of d10metal ion,the luminescent properties of 1 and 2 can be attributed to the mixture of ligand-centered emission and LMCT.
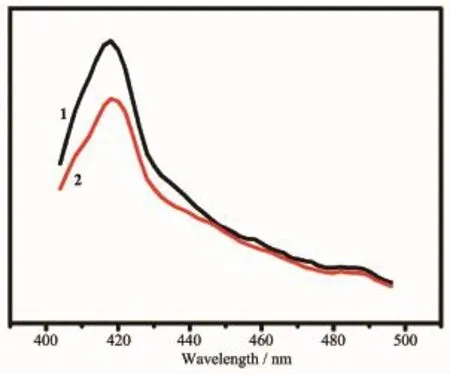
Fig.4Solid-state luminescent spectra of 1 and 2
3 Conclusions
In summary,we established an experimental diagram to study the effect of the steric hindrance of an auxiliary linker on the structural diversity of CPs by the incorporation of two kinds of auxiliary linkers withdifferentsterichindranceintoexperimental systems.Therelationshipbetweenthestructural diversity of CPs and steric hindrance of the auxiliary linker can be well illustrated by comparing the topological structure 1 and 2 with those ofⅠandⅡ. In particular,L1or L2is appropriate for constructing variousMOFsduetoitsspecifictetrahedral configuration and flexible conformation.All this will provide us a research way to explore the structureproperties correlation for other similar systems.
Supporting information is available at http://www.wjhxxb.cn
[1](a)Moulton B,Zaworotko M J.Chem.Rev.,2001,101:1629-1658
(b)James S L.Chem.Soc.Rev.,2003,32:276-288
(c)Ockwig N W,Delgado-Friedrichs O,O′Keeffe M,et al. Acc.Chem.Res.,2005,38:176-182
(d)Noro S,Kitagawa S,Kondo M,et al.Angew.Chem.Int.Ed.,2000,39:2081-2084
(e)Zhang J P,Lin Y Y,Huang X C,et al.J.Am.Chem. Soc.,2005,127:5495-5506
(f)Song X Z,Song S Y,Zhao S N,et al.Adv.Funct.Mater., 2014,24:4034-4041
[2](a)Perry J J 4th,Perman J A,Zaworotko M J,et al.Chem. Soc.Rev.,2009,38:1400-1417
(b)Li J R,Sculley J,Zhou H C.Chem.Rev.,2012,112:869-932
(c)FarhaOK,HuppJT.Acc.Chem.Res.,2010,43:1166-1175
[3](a)Ma T,Li M X,Wang Z X,et al.Cryst.Growth Des.,2014, 14:4155-4165
(b)Zhang X,Hou L,Liu B,et al.Cryst.Growth Des.,2013, 13:3177-3187
(c)Hua J A,Zhao Y,Liu Q,et al.CrystEngComm,2014,16: 7536-7546
(d)Lu X,Ye J,Li W,et al.CrystEngComm,2012,14:1337-1344
[4](a)Ren S B,Zhou L,Zhang J,et al.CrystEngComm,2010, 12:1635-1638
(b)Ren S B,Chen F J,Wu C L,et al.J.Mol.Struct.,2013, 1034:193-197
(c)Ren S B,Qiu Z J,Yan J,et al.J.Mol.Struct.,2013,1046: 15-20
[5](a)Paz F A A,Klinowski J,Vilela S M F,et al.Chem.Soc. Rev.,2012,41:1088-1110
(b)Liang X,Zhang F,Zhao H,et al.Chem.Commun.,2014, 50:6513-6516
(c)Zhou X H,Li L,Li H H,et al.Dalton Trans.,2013,42: 12403-12409
[6](a)Ren S B,Zhou L,Zhang J,et al.CrystEngComm,2009,11: 1834-1836
(b)Ren S B,Zhou L,Zhang J,et al.Inorg.Chem.,Commun., 2011,14:558-561
(c)Ren S B,Lin Y Q,Zhang Q,et al.Polyhedron,2014,83: 130-136
(d)Zhang Q,Bu X,Lin Z,et al.Inorg.Chem.,2008,47:9724-9726
[7](a)SAINT-Plus,Version 6.02,Bruker Analytical X-ray System, Madison,WI,1999.
(b)Sheldrick G M.SADABS,AnEmpiricalAbsorption Correction Program,Bruker Analytical X-ray Systems, Madison,WI,1996.
(c)Sheldrick G M.Acta Crystallogr.,2008,A64:112
[8](a)He K H,Song W C,Li Y W,et al.Cryst.Growth Des., 2012,12:1064-1068
(b)Ren S B,Yang X L,Zhang J,et al.CrystEngComm, 2009,11:246-248
(c)Lu Y B,Jian F M,Jin S,et al.Cryst.Growth Des.,2014, 14:1684-1694
Crystal Structures and Properties of Zn(Ⅱ)/Cd(Ⅱ)Coordination Polymers Based on Semirigid Tetrahedral Quadridentate Ligand
TANG Wen-Yuan1REN Shi-Bin*,1,2ZHU Dong-Dong1HAN De-Man1ZHOU Xin-Hui*,3
(1School of Pharmaceutical and Chemical Engineering,Taizhou University,Taizhou,Zhejiang 317000,China)
(2State Key Laboratory of Analytical Chemistry for Life Science,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210093,China)
(3Key Laboratory for Organic Electronics&Information Displays(KLOEID)and Institute of Advanced Materials(IAM),Nanjing University of Posts&Telecommunications,Nanjing 210023,China)
Two complexes,{[Cd2(L1)(1,4-NDC)2]·2H2O}n(1)and{[Zn2(L2)(1,4-NDC)2]·DMF·3H2O)}n(2)(L1=tetrakis (4-pyridyloxymethylene)methane,L2=tetrakis(3-pyridyloxymethylene)methane,1,4-NDC=naphthalene-1,4-dicarboxylic acid)are obtained by the self-assembly reaction of L1or L2,1,4-NDC,and d10metal ions under solvothermal conditions.The X-ray analysis reveals that 1 is constructed from a 1D zigzag chain[Cd(1,4-NDC)]nwhich is linked by L1ligand to yield a 3D framework.And yet in case of 2,one double helix is mutually interdigitated by another 1D chain into a 2D network.Above all,the effect of steric hindrance of the auxiliary linker on the structural diversity of CPs can be discussed in great detail by the introduction of two kinds of auxiliary linkers with different steric hindrance.Additionally,their luminescent properties are also observed.CCDC:1019510,1; 1019511,2.
carboxylate ligands;isomerization;steric hindrance;luminescence
O614.24+2;O614.24+1
A
1001-4861(2015)10-2073-06
10.11862/CJIC.2015.229
2015-04-13。收修改稿日期:2015-05-14。
国家自然科学基金(No.21471110,21375092),浙江省自然科学基金(No.LY12B01006),中国博士后基金(No.2013M531314),生命分析国家重点实验室开放课题(No.SKLACLS1206),浙江省大学生创新计划项目(No.2014R428014),台州市科技局项目(No.131KY03)及浙江省科技厅公益分析测试项目(No.2015C37034)资助项目。
*通讯联系人。E-mail:renshibin@tzc.edu.cn;iamxhzhou@njupt.edu.cn;会员登记号:S06N4804M1203。

