两个以3-硝基邻苯二甲酸为配体构筑的锌和钴配位聚合物的合成、晶体结构和性质
尹卫东李桂连刘广臻辛凌云 李晓玲 马录芳
两个以3-硝基邻苯二甲酸为配体构筑的锌和钴配位聚合物的合成、晶体结构和性质
尹卫东李桂连刘广臻*辛凌云 李晓玲 马录芳
(洛阳师范学院化学化工学院,洛阳471022)
通过水热法合成了2种配位聚合物{[Zn(3-Nbdc)(bpmp)]·H2O}n(1)和{[Co(3-Nbdc)(bpmp)(H2O)]·H2O}n(2)(3-Nbdc2-=3-硝基邻苯二甲酸根,bpmp=1,4-二(4-吡啶甲基)哌嗪),并通过X-射线单晶衍射、元素分析和红外光谱对其结构进行了表征。配合物1和2均为二维(4,4)格子层结构,其中1含有双核单元,2含有螺旋金属羧酸链。此外,对它们的热重、粉末X-射线衍射、固体荧光和磁性性能进行了考察。1显示与游离的3-硝基邻苯二甲酸相似的荧光性质,锌离子的配位环境和配体之间的耦合作用对其荧光性能几乎没有影响。2在低温区表现出铁磁耦合作用,高温区的χMT值降低主要是由于八面体钴的自旋轨道耦合导致的。
水热合成;钴;光致发光;磁性;配合物
Much attention has been paid to the design and assembly of metal-organic frameworks or porous coordination polymers in recent years not only because of their intriguing structures[1-3],but also due to their potential applications in various areas such as gas adsorption,separation,heterogeneous catalysis,and photoluminescence[4-9].In this field,the organic bridging ligands play a crucial role in the design and construction of desirable metal-organic frameworks (MOF),because changes in flexibility,length,and symmetry of organic ligands can result in materials bearing diverse architectures and functions.The rigid aromatic benzenedicarboxylic acid and its derivatives (such as 1,n-benzenedicarboxylic acid,n=2,3,4)are widely used as building blocks to link metal ions to produce metal-organic frameworks with interesting structures and properties because of having strong coordination ability and diverse coordination modes[10-14]. Recently,our group chose the nitro-1,2-benzenedicarboxylic acid(NbdcH2)as organic bridging ligands to self-assemble and produce versatile 1D,2D and 3D metal-organic frameworks with the diverse properties[15-18].Though the nitro group does notparticipate in coordination,NbdcH2may provide the potential to construct unpredictable and interesting network structures due to the existence of an electron-withdrawing nitro-group on the aromatic backbone[19-21].
On the other hand,the N-donor ligand is also very important for the design and assembly of diverse coordination polymers.Throughout the multitudinous N-donor ligands,the bis-pyridyl-type ligand,such as bpe(1,2-bis(4-pyridyl)ethene),bpa(1,2-bis(4-pyridyl) ethane)and bpp(1,3-bis(4-pyridyl)propane),is one of the most common auxiliary ligands to combine with polycarboxylates main ligands,and has obtained a series of MOFs with different structures and properties[22-24].In this article,We use 1,4-bis(4-pyridylmethyl)piperazine(bpmp)as new bis-pyridyl-type coligand to construct coordination polymers not only because bpmp has twisted conformation,but also its four nitrogen atoms may improve the structures and properties of coordination polymers[25-26].We obtained two coordination polymers under similar hydrothermal reaction conditions,namely,{[Zn(3-Nbdc)(bpmp)]· H2O}n(1)and{[Co(3-Nbdc)(bpmp)(H2O)]·H2O}n(2). The synthesis,structures,stabilities,fluorescence and magnetic property for complexes 1 and 2 are given in this paper.
1 Experimental
1.1 Materials and methods
All reagents were commercially available and used as received withoutfurtherpurification.Elemental analyses were determined with on a Flash 2000 organic elemental analyzer.Infrared spectra(4 000~600 cm-1) were recorded on powdered samples using a NICOLET 6700 FT-IR spectrometer.Thermogravimetric analyses (TGA)were performed on a SII EXStar 6000 TG/ DTA6300 analyzer with a heating rate of 10℃·min-1up to 900℃under N2atmosphere.Powder X-ray diffraction(PXRD)patterns were taken on a Bruker D8-ADVANCE X ray diffractometer with Cu Kα radiation(λ=0.154 18 nm).Luminescence spectra were performed on an Aminco Bowman Series 2 luminescence spectrometer at room temperature. Variable temperature magnetic susceptibilities were measured by using a MPMS-7 SQUID magnetometer.
1.2 Synthesis of{[Zn(3-Nbdc)(bpmp)]·H2O}n(1)
A mixture ofZn(OAc)2·2H2O(0.021 g,0.10 mmol), 3-NbdcH2(0.042 g,0.20 mmol),bpmp(0.027 g,0.10 mmol),KOH(0.006 g,0.10 mmol),EtOH(4 mL)and H2O(3 mL)was placed in a 23 ml Teflon-lined autoclave at 120℃for 4 days,then cooled to room temperature.Colourless block crystals were obtained in 71%yield.Elemental analysis Calcd.(%)for C24H25N5O7Zn:C,51.39;H,4.49;N,12.49.Found(%): C,51.35;H,4.51;N,12.43.IR(KBr,cm-1):2 800~3 600(m),1 639(s),1 620(s),1 597(s),1 563(m),1 523 (s),1 452(m),1 433(m),1 341(s),1 291(m),1 230(m), 1 156(w),1 145(w),1 129(w),1 069(m),1 032(m),1 000 (w),921(w),847(m),825(s),798(s),779(s),755(s),713 (s),696(s),667(w).
1.3 Synthesis of{[Co(3-Nbdc)(bpmp)(H2O)]· H2O}n(2)
A mixture ofCo(OAc)2·4H2O(0.025 g,0.10 mmol), 3-NbdcH2(0.021 g,0.10 mmol),bpmp(0.054 g,0.20mmol),KOH(0.006 g,0.10 mmol),and H2O(7 mL) was placed in a 23 mL Teflon-lined autoclave.The vesselwas heated to 120℃for 4 days,then cooled to room temperature.Pink block crystals were obtained in 87%yield.Elemental analysis Calcd.(%)for C24H27N5O8Co:C,50.36;H,4.75;N,12.23.Found(%):C, 50.32;H,4.92;N,12.21.Selected IR(KBr,cm-1): 2 800~3 600(w),1 600(s),1 577(s),1 554(s),1 525 (s),1 450(w),1 427(m),1 392(s),1 341(s),1 329(s), 1 295(m),1 155(m),1 133(m),1 069(w),1 013(m), 943(m),922(m),877(w),834(s),826(s),785(m),761 (m),754(w),736(s),713(w),668(s).
1.4 X-ray crystallography
The crystallographic data collections for complexes 1 and 2 were recorded at room temperature on a Bruker SMART APEX IICCD diffractometer equipped with graphite-monochromated Mo Kαradiation(λ= 0.071 073 nm).All structures were solved by direct methods with SHELXS-97 and refined on F2by fullmatrix least-squares using the SHELXL-97 program package[27-28].All hydrogen atoms were placed in calculated positions and refined isotropically with a riding model except for water H atoms,which were initially located in a difference Fourier map and included in the final refinement by use of geometrical restraints with d(O-H)=0.085 nm and Uiso=1.5Ueq(O).The details of the structure solutions and final refinements for two complexes are summarized in Table 1.Selected bond distances and angles and hydrogen bonds are listed in Table S1 and Table S2.
CCDC:1042678,1;1042679,2.

Table 1 Crystal and structure refinement data for compounds 1 and 2
2 Results and discussion
2.1 Structural description of{[Zn(3-Nbdc) (bpmp)]·H2O}n(1)
X-ray crystallographic analysis reveals that 1crystallizes in triclinic crystal system,space group P1 and features a 2D(4,4)grid layer.The asymmetry unit contains one crystallographically unique Zn2+cation, one completely deprotonated 3-Nbdc2-,one bpmp molecule and one guest water,as shown in Fig.1(a).The Zn2+center is coordinated by two carboxylate O atoms from two 3-Nbdc2-and two N atoms from two bpmp molecules to form a four-coordinated distorted pyramid [ZnN2O2]geometry.The Zn-O bond lengths are 0.192 98(17)and 0.194 88(17)nm,and the Zn-N bond lengths are 0.203 5(2)and 0.204 9(2)nm,respectively.

Fig.1(a)View of the asymmetric unit showing the local coordination environments of Zn ion in 1; (b)Polyhedral view of the 2D(4,4)grid layer; (c)View of 3D packing of two adjacent layers
The adjacent Zn2+centers are bridged by the 3-Nbdc2-ligands with two carboxyl groups adopting a monodentate coordination mode to form binuclear units with the Zn…Zn separation of 0.511 36(7)nm, as indicated in Fig.1(b).The binuclear units are interlinked together with bpmp coligands to form a(4,4) grid layer with parallelogram cavities.Individual layers stack together in an-ABAB-motif forming its entire three-dimensional supramolecular structure via weak Van der Waals interaction between the interlayers(Fig.1(c)).The parallelogram cavities in single layerare almostcompletely covered due to the stacking of multilayers.There exist intralayer H-bond interactions between carboxylate O atom and free water O atom(O8W-H2W…O1i:0.319 8(3)nm,136.1°; O8W-H2W…O3i:0.313 5(3)nm,144.7°).No significantπ…πinteractions are observed between all the aromatic rings of all the ligands due to the terraced arrangement of the adjacentlayers.
2.2 Structuraldescription of{[Co(3-Nbdc) (bpmp)(H2O)]·H2O}n(2)
X-ray crystallographic analysis reveals that 2 crystallizes in monoclinic crystal system,space group P21/c and features a 2D(4,4)grid layer.The asymmetry unit contains one crystallographic Co2+ion,one deprotonated 3-Nbdc2-,one bpmp molecule,one coordination water and one guest water,as shown in Fig.2(a). The Co2+center is a distorted octahedral[CoN2O4]geometry by four oxygen atoms in the equatorial plane, three carboxylic oxygen atoms from two 3-Nbdc2-ligands and one oxygen atoms from the coordination water,and two nitrogen atoms from two bpmp coligands at the axial positions(Fig.2(a)).The Co-O bond distances fall in the range of 0.205 88(14)~0.214 30(15)nm,and the Co-N bond lengths are 0.213 76(17)and 0.214 77(17)nm,respectively.
The two carboxyl groups of a 3-Nbdc2-adopt abidentate-bridging and a monodentate coordination mode,respectively.The adjacent Co2+cations are connected by twoμ2-carboxylates forming 1D carboxylate-metal zigzag chain along b direction with the Co…Co distance of0.527 22(4)nm,as indicated in Fig.2(b).Each zigzag chain connects with throughligand Co…Co separation of 1.626 03(6)nm across bpmp ligands to form a 2D(4,4)grid layer(Fig.2(c)). The adjacent layers are stacked in a parallel mode along c direction with the 3-Nbdc2-anions occupying the interlayer regions to form a three-dimensional supramolecular framework by interlayer H-bond interactions between the guest water O atom and the carboxylate O atom of 3-Nbdc2-anions(O9W-H4W…O4:0.286 2(2)nm,171.5°;O9W-H3W…O4v:0.286 4(2) nm,169.7°),between coordination water O atom and free water O atom(O8W-H1W…O9Wiii:0.276 8(2) nm,165.1°).There also exist intralayer H-bond interactions between the coordination water O atom and the carboxylate O atoms of 3-Nbdc2-anions(O8WH2W…O2ii:0.301 5(2)nm,123.7°;O8W-H2W…O1i: 0.286 3(2)nm,154.4°;Symmetry codes:ix,y-1,z;ii-x+2,y-1/2,-z+1/2;iii-x+2,-y+1,-z+1;v-x+2,-y +2,-z+1)(Fig.S1).There exists the weak face to face π…πinteraction between phenyl rings of 3-Nbdc2-and pyridyl rings of bpmp coligands within the layer (centroid distance:0.395 17(1)nm,dihedral angle: 22.103°)(Fig.S2).It is obvious that the H-bonding bonds andπ…πinteractions among the coordination polymers play importantroles in the self-assembly and enhanced stability ofthe resultantstructure.

Fig.2(a)View of the coordination environments of Co ion for 2;(b)View of 1D Co-carboxylate chain along b direction;(c)Polyhedral view of the 2D grid layer
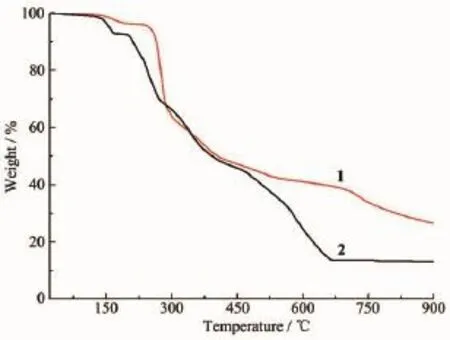
Fig.3 TGA curves of complexes 1 and 2
2.3 Thermogravimetric analyses and powder X-ray diffractions
The thermogravimetric analyses(TGA)of 1 and 2 performed by heating the polycrystalline samples display significantly different thermal degradations between room temperature and 900℃under N2atmosphere,as shown in Fig.3.The TGA curve for 1 suggests that the first weight loss of 3.34%from 85 to 190℃corresponds to the loss of one lattice water (Calcd.3.21%).The residual framework starts to decompose above 250℃with a series of complicated weight losses and does not stop until heating ends. The observed finalmass remnantof25.75%cannotbe specifically identified and may be the mixtures of metal oxide and carbonaceous material,because the theoretical remaining mass of 14.44%is calculated by assuming a final phase ZnO.The TGA curve of 2 shows that the weight loss of about 6.45%in the range of80~165℃is corresponded to one coordinationwater and one guest water per formula unit(Calcd. 6.30%),and the framework can keep stability up to about 200℃.The observed final mass remnant of 13.18%likely representing deposition of CoO is agreementwith the calculated value of13.09%.
The powder X-ray diffractions of two compounds are in good agreement with the patterns simulated from the respective single-crystal data,implying their good phase purity(Fig.S3 and Fig.S4).
2.4 Fluorescence property
The photoluminescent properties of 1 and powdered free 3-NbdcH2ligand were investigated in the solid state at room temperature,as illustrated in Fig.4.Upon excitation at 300 nm,it is observed that the emission spectra of complex 1 is very similar to the free 3-NbdcH2ligand in rough shape,displaying a wide range of the emissions with maximum peaks at~433 nm,~472 nm and~548 nm.Since the Zn2+ions are difficult to oxidize or reduce due to their d10configuration,the emissions of the compound is neither metal-to-ligand charge transfer(MLCT)nor ligand-to-metal charge transfer(LMCT)in nature.It can probably be assigned to the ligand-centered charge transfer(n→π*orπ→π*)[17-18,29]based on 3-NbdcH2ligand because a similar emission is observed for the free 3-NbdcH2ligand,whereas the bpmp coligand shows almost no contribution to the emission ofthe compound 1 due to its weak fluorescentemission in the high energy emission region[25-26].All the results suggest little contribution from the Zn-O inorganic motifs to the emission and very little degree change of interligand coupling upon metalcoordination[30].
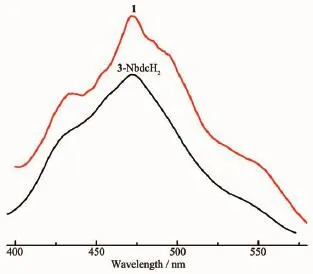
Fig.4 Solid-state emission spectra of compound 1 and the free 3-NbdcH2at room temperature
2.5 Magnetic property
The magnetic susceptibility(χM)of 2 was measured in the 2~300 K temperature range in a 2 000 Oe applied field,and shown asχMT andχM-1versus T plots in Fig.5.From a magnetic point of view,it is clear that the much larger Co…Co separation across bridging bpmp(more than 1.2 nm)when compared to the shorter value across carboxylate bridge(0.527 22(4) nm)allow us to discard the exchange pathways through N-donor ligands.So complex 2 can be considered as pseudo-1D metal-carboxylate chain polymers with the nonplanar syn-anti coordination mode,in which adjacent Co2+ions are linked by carboxylbridges since couplings through bpmp are almostnegligible.

Fig.5 Plot of theχM T(■)andχM-1(□)versus T in compound 2
TheχMT value at room temperature in 2 of 2.91 cm3·K·mol-1is significantly greater than the spin-only value(1.88 cm3·K·mol-1,S=3/2)expected for an isolated high-spin Co2+ion.The productχMT firstly decreases smoothly to a rounded minimum of 2.23 cm3·K·mol-1at 18.9 K,then increases rapidly to a high maximum of 3.47 cm3·K·mol-1at 2.0 K, indicating the ferrimagnetic-like behavior in complex 2[31-32].The temperature dependence of magnetic susceptibilities above 18.9 K follows the Curie-Weiss law χM=C/(T-θ)with a Weiss constantθ=-16.6 K and a Curie constant C=3.24 cm3·K·mol-1,which agrees well with those given in the literature for the Curie constant(C=2.8~3.5 cm3·K·mol-1)[33].The Curieconstant is much larger than the expected spin-only value,indicating that the orbital contribution of Co2+ions exists in complex 2.Thereby the negative value ofθcannot unambiguously confirm the existence of antiferromagnetic coupling between Co2+ions because of the strong spin-orbital coupling through the4T1gstate of the octahedral Co2+centers[33-34].All these data indicate that the ferromagnetic coupling between Co2+ions occurs in complex 2 and is sufficient to compensate the single-ion behavior resulting from spin-orbital coupling.The magnetic property of Co compound is very complicated by the fact that the orbital moment,spin-orbit coupling,distortion from regular stereochemistry,electron delocalization,and crystal field anisotropy can influence the magnetic susceptibility.Therefore,the magnetic mechanism of compound 2 will be further investigated.
3 Conclusions
In summary,two Zn/Co coordination polymers were successfully synthesized based on 3-nitrophthalic acid and 1,4-bis(4-pyridylmethyl)piperazine coligands. Both complexes 1 and 2 exhibit(4,4)grid layers including the dinuclear units in 1 and metalcarboxylate chains in 2.The solid state luminescence of compound 1 is attributed to intraligand n→π*or π→π*charge transfer since it shows similar emission spectra to the free 3-NbdcH2ligand.The magnetic property of complex 2 was also investigated and showed a ferromagnetic coupling between the Co2+centers in the low-temperature region,and the decrease ofχMT value in the high-temperature region may be attributed to the strong spin-orbit coupling,which is remarkable for the4T1gstate of Co2+in an octahedral ligand field.The magnetic mechanism of compound 2 will be further investigated.
Acknowledgments:This work was supported by the National Natural Science Foundation of China(No.20971064 and 21271098),the Program for Science&Technology Innovation Talents in Universities of Henan Province(No. 14HASTIT017),and the Program for Innovative Research Team (in Science and Technology)in University of Henan Province (No.14IRTSTHN008).
Supporting information is available athttp://www.wjhxxb.cn
[1]O′Keeffe M.Chem.Soc.Rev.,2009,38:1215-1217
[2]Xuan W M,Zhu C F,Liu Y,et al.Chem.Soc.Rev.,2012, 41:1677-1695
[3]Férey G.Chem.Soc.Rev.,2008,37:191-214
[4]Guo H,Zhu G,Hewitt I J,et al.J.Am.Chem.Soc.,2009, 131:1646-1647
[5]Huang A,Bux H,Steinbach F,et al.Angew.Chem.Int.Ed., 2010,49:4958-4961
[6]Du M,Li C P,Chen M,et al.J.Am.Chem.Soc.,2014,136: 10906-10909
[7]Zhao Y,Deng D S,Ma L F,et al.Chem.Commun.,2013,49: 10299-10301
[8]Li L C,Matsuda R,Tanaka I,et al.J.Am.Chem.Soc.,2014, 136:7543-7546
[9]Bauer C A,Timofeeva T V,Setterstten T B,et al.J.Am. Chem.Soc.,2007,129:7136-7144
[10]Bourne S A,Lu J,Mondal A,et al.Angew.Chem.Int.Ed., 2001,40:2111-2113
[11]Zou R Q,Bu X H,Zhang R H.Inorg.Chem.,2004,43:5382 -5386
[12]Ye B H,Ding B B,Weng Y Q,et al.Cryst.Growth Des., 2005,5:801-806
[13]GONG Teng-Fei(龚腾飞),ZHU Cheng-Feng(朱成峰),YE Cheng-Cheng(叶诚诚),et al.Chinese J.Struct.Chem.(结构化学),2013,8:1222-1228
[14]Li W,Barton P T,Burwooda R P,et al.Dalton Trans.,2011, 40:7147-7152
[15]Li G L,Liu G Z,Ma L F,et al.Chem.Commun.,2014,50: 2615-2617
[16]Li G L,Yin W D,Liu G Z,et al.J.Solid State Chem.,2014, 220:1-8
[17]Li G L,Yin W D,Liu G Z,et al.Inorg.Chem.Commun., 2014,43:165-168
[18]Li G L,Liu G Z,Huang L L,et al.J.Inorg.Organomet. Polym.,2014,24:617-623
[19]Zhang J,Zhu L G.CrystEngComm,2011,13:553-560
[20]Wang X L,Mu B,Lin H Y,et al.CrystEngComm,2012,14: 1001-1009
[21]Qu H,Qiu L,Leng X K,et al.Inorg.Chem.Commun.,2011, 14:1347-1352
[22]Liu G Z,Li S H,Li X L,et al.CrystEngComm,2013,15: 4571-4580
[23]Xin L Y,Liu G Z,Li X L,et al.Cryst.Growth Des.,2012, 12:147-157
[24]Li X L,Liu G Z,Xin L Y,et al.CrystEngComm,2012,14: 5757-5760
[25]Banisafar A,Martin D P,Lucas J S,et al.Cryst.Growth Des.,2011,11:1651-1661
[26]Xu B,LüJ,Cao R.Cryst.Growth Des.,2009,9:3003-3005
[27]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structure,University of Göttingen,Germany,1997.
[28]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structure,University of Göttingen,Germany,1997.
[29]LIU Xiu-Xiu(刘秀秀),CHENG Mei-Ling(程美令),REN Yan-Qiu(任艳秋),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31:611-618
[30]Bordiga S,Lamberti C,Ricchiardi G,et al.Chem.Commun., 2004,20:2300-2301
[31]Zeng M H,Zhang W X,Sun X Z,et al.Angew.Chem.Int. Ed.,2005,44:3079-3082
[32]Zhang X M,Hao Z M,Zhang W X,et al.Angew.Chem.Int. Ed.,2007,46:3456-3459
[33]Fang Z L,Yu R M,He J G,et al.Inorg.Chem.,2009,48: 7691-7697
[34]Su Z,Fan J,Chen M,et al.Cryst.Growth Des.,2011,11: 1159-1169
Syntheses,Structures and Properties of Two Coordination Polymers Constructed by 3-Nitrobenzene-1,2-dicarboxylate Acid and Zn/Co
YIN Wei-Dong LI Gui-Lian LIU Guang-Zhen*XIN Ling-Yun LIXiao-Ling MA Lu-Fang
(College of Chemistry and Chemical Engineering,Luoyang Normal University,Luoyang,Henan 471022,China)
Two coordination polymers{[Zn(3-Nbdc)(bpmp)]·H2O}n(1)and{[Co(3-Nbdc)(bpmp)(H2O)]·H2O}n(2)(3-Nbdc2-=3-nitrobenzene-1,2-dicarboxylate,bpmp=1,4-bis(4-pyridylmethyl)piperazine)were synthesized hydrothermally and characterized structurally by single-crystal X-ray diffractions,elemental analysis and infrared spectroscopy(IR).Both complexes 1 and 2 display(4,4)grid layers containing the dinuclear units in 1 and helix metalcarboxylate chains in 2.Thermogravimetric Analyses(TGA),powder X-ray diffractions(PXRD),fluorescence property and magnetic property for compounds 1 and 2 are also investigated.The solid state fluorescence indicates thatthe complex 1 shows similar emission spectra to the free 3-NbdcH2ligand due to little contribution from the Zn-O inorganic motifs to the emission and very little degree change of interligand coupling upon metal coordination.The complex 2 exhibits a ferromagnetic coupling between the metal centers in the low-temperature region,and the decrease ofχMT value in the high-temperature region may be attributed to the strong spin-orbit coupling through the4T1gstate ofthe octahedral Co2+centers.CCDC:1042678,1;1042679,2.
hydrothermal synthesis;cobalt;photoluminescence;magnetic property;coordination polymer
O614.24+1;O614.81+2
A
1001-4861(2015)07-1439-08
10.11862/CJIC.2015.202
2015-03-29。收修改稿日期:2015-06-08。
国家自然科学基金(No.20971064,21271098),河南省大学科技创新人才(No.14HASTIT017),河南省大学科技创新团队(No.14IRTSTHN008)资助项目。
*通讯联系人。E-mail:gzliuly@126.com

