Effects of Sub-chronic Aluminum Exposure on Renal Structure in Rats
Li Yan-fei, Liu Jian-yu, , and Cao Zheng
1 College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China
2 Police Dog Technical School of Ministry of Public Security, Shenyang 110034, China
Effects of Sub-chronic Aluminum Exposure on Renal Structure in Rats
LiYan-fei1,LiuJian-yu1,2,andCaoZheng1
1CollegeofVeterinaryMedicine,NortheastAgriculturalUniversity,Harbin150030,China
2PoliceDogTechnicalSchoolofMinistryofPublicSecurity,Shenyang110034,China
Toinvestigatetheeffectsofaluminum(Al)exposureonrenalstructureofrats,60Wistarratswererandomlydividedinto fourtreatmentgroupsandwereorallyexposedto0(controlgroup,GC),64.18(low-dosegroup,GL),128.36(middle-dosegroup, GM),and256.72(high-dosegroup,GH)mg•kg-1BWAlCl3indrinkingwaterfor120days.Thebodyweightofdifferentratswas recorded,thekidneypathologicstructureandtheultrastructurewereobserved.Theresultsshowedthatthebodyweightofdifferent ratswasmarkedlylowerinAl-treatedratsthanthoseinGC(P<0.05;P<0.01).Aftermassonstaining,thecollagenwasdeposited intherenalinterstitiumandaggravatedwithAldoseincreasesinAl-treatedrats.Underelectronmicroscope,theinfoldingofthe plasmamembranewasslightswollen,themitochondrionwasabundantwithdifferentsizes,themitochondrioncristaewasfused,the microvilluswasswollenandfusedinGH.Ourfindingsindicatedthatsub-chronicAlexposureslowedtheweightofratsandcaused thekidneypathologicdamageinrats.
sub-chronic,aluminumexposed,rat,renalstructure
Introduction
Aluminum(Al)isthethirdmostabundantelement inearthcrustandiswidelyusedindailylife.Alcan accumulateinkidney,brain,bone,liver,etc(Kumar andGill,2009;Linardakiet al.,2013).Kidneyis theimportantexcretoryorganthatexcretesmetabolic waste,generatesurine,andregulatesthebalancesof electrolyteandacid-base(Bolignano et al.,2014). SomeresearcheshaddemonstratedthatAlaccumulatedinanimalbodiescouldinjureandsuppressthe renalstructureandfunction(Bohreret al.,2008),but mechanismhadn'tbeencleared.Inthisexperiment,a ratmodelofAlexposurewasbuiltthroughdrinking waterinordertostudythedamageofAltotherenal structure.
Materials and Methods
Animals and treatments
Atotalof60healthymaleWistarrats(5weeksold) weighed110-120gwereusedafter1weekofthe feedingwithastandarddiet.Theywererandomly allocatedintofourgroups(n=15):controlgroup(GC), low-dosegroup(GL),medium-dosegroup(GM),and high-dosegroup(GH).Theratswereorallyadministratedwith0,64.18,128.36,and256.72mg•kg-1BW AlCl3indrinkingwater(distilledwater)for120days. Ratswerefreelyaccessedtofoodandwater.Thehealthstatusoftheratswasmonitoreddaily,andthe bodyweightoftheratswasrecordedmonthly.
Tissue sampling and processing
After120days,ratsweresubsequentlyeuthanizedby cervicaldislocationandwereweighed.Thekidneys werecollected,about2.0×1.5×0.3cm3sizewasput into10%formaldehydefluidformassonstaining, about1.0×1.0×1.0mm3sizewasputinto2.5% glutaraldehydesolutiontoobservetheultrastructure.
Observation of kidney pathologic structure
Thekidneypathologicstructurewasobservedby massonstainingaccordingtothemethodbyZhang et al(2005).
Observation of kidney ultrastructure
Theultrastructurewasobservedaccordingtothe methodbyYamadaet al(2014).
Statistical analysis
Datawerepresentedasmean±standarddeviation (SD)andanalyzedbyusingone-wayanalysisofthe variance(ANOVA)followedbyStudent'sttest(SPSS 17.0software,SPSSIncorporated,Chicago,IL,USA) consideringtheinteractiveeffectsanddifferences betweenAl-treatedgroupsandGC.P<0.05was consideredstatisticallysignificantandP<0.01was markedlysignificant.
Results
Clinical symptoms and body weight
ClinicalsymptomsofthemostAl-treatedratswere anorexia,dreariment,shaggyhair,stumble,astasia, andpaletail.Theserioussymptomswereprincipally shortnessofbreath,dyspnea,andgeneralizedconvulsion.However,thecontrolgroupratsgrewwell.
Thebodyweightofalltheratsincreasedthroughout theexperimentperiod,whilethebodyweightofAltreatedratswaslowerthanthatinGC.Thebody weightoftheratswaslowerinGL(P<0.05),GMand GH(P<0.01)thanthatinGC(Table1).
Result of masson staining
ThecollagensinGCwerelocatedmainlyinrenal tubularbasementmembrane,renalcapsule,glomerular mesangialarea,andaroundthesmallbloodvessels (Fig.1).InAl-treatedrats,thecollagenwasdeposited intherenalinterstitiumandaggravatedwithAldose increasesinAl-treatedrats(Figs.2-4).Thecollagen fiberwasgreen,redbloodcellwasorange,and cytoplasmwasred.

Table 1 Body weight changes of rats (g)
Kidney ultrastructure
Thepodocyte,microvillus,nuclearenvelope,rough endoplasmicreticulum,microfilamentandthe mitochondriastructureofthekidneyareshown inFigs.5-8forGCandFigs.9-12forGH.Thedifferentsizes,anditscristaewasfused(Fig.10), andmicrovilluswasslightfused(Fig.11).Microvillus wasswollen,fusedandthestructurewasunclear (Fig.12). damagesofplasmamembrane,mitochondrion, andmicrovilluswereobviousinGH.Theinfoldingofplasmamembranewasslightswollen (Fig.9).Themitochondrionwasabundantwith

Fig. 1 Kidney in rats of GC (masson staining ×400)

Fig. 2 Kidney in rats of GL (masson staining ×400)

Fig. 3 Kidney in rats of GM (masson staining ×400)

Fig. 4 Kidney in rats of GH (masson staining ×400)

Fig. 5 Podocyte of kidney in rats of GC (×4 200)

Fig. 6 Microvillus of kidney in rats of GC (×9 900)

Fig. 7 Mitochondria and nuclear membrane of kidney in rats of GC (×16 500)
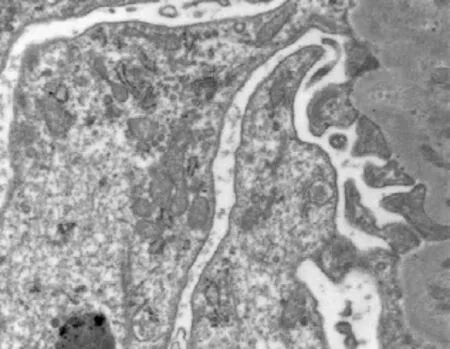
Fig. 8 Mitochondria, rough endoplasmic reticulum and microfilament of kidney in rats of GC (×16 500)

Fig. 9 Plasma membrane infolding and nuclear membrane of kidney in rats of GH (×4 200)

Fig. 10 Mitochondria and and microvillus of kidney in rats of GH (×9 900)

Fig. 11 Mitochondria and microvillus of kidney in rats of GH (×9 900)

Fig. 12 Microvillus of kidney in rats of GH (×16 500)
Discussion
Nodeathwasdiscoveredinalltheratsduringthe experiment.However,Al-treatedgroupswereless activethanthatinGC.Theslownessofthebody weightgrowthinAl-treatedgroupsindicatedthatAl couldinhibitthegrowthoftherats.Thesimilarresults werediscoveredinourpreviousresearches(Wang et al.,2012;Zhuet al.,2014).Theresultssuggested thatAlCl3inducedthechangeofclinicalsymptoms andinhibitedthegrowthofbodyweight.
Kidneyisoneoftheprimarytargetorgansofmany chemicalsanddrugs.Thekidneylesionswerecaused bymanyfactors(Mahieuet al.,2009).Jiet al.(2011) foundthatglomerulusshrankinratstreatedwith 100mg•kg-1AlCl3throughnasaldrip.Zhuo(2008) discoveredthatovermuchAlaccumulatedinrats inducedtheswelloftherenaltubules,capillariesand theepithelialcellsoftheproximalconvolutedtubule. Thisstudyshowedthatglomerulus,renaltubular basementmembrane,andrenalinterstitiumweredyed green,redbloodcellwasdyedorange,andcytoplasm wasdyedredbymassonstaining.Theresultsshowed thatsub-chronicAlexposurecouldinducerenal interstitialfibrosis,andtherenalinterstitialfibrosis wasaggravatedwithAldoseincreases.
Inthisstudy,thepathologicaldamageofthekidney focusedonrenaltubularepithelialcells,especially thechangesofmitochondrionandmicrovillus underelectronmicroscope.Themitochondrionwas abundantwithdifferentsizes,themitochondrion cristaewasfused,andthemicrovilliwereslight fused,swollenandfusedinGH.Ourprevious researchesreportedthatAlCl3exposureledto Alaccumulationinkidney,inhabitedthekidney growth,andcausedthepathologicalchangesof glomerulusandrenaltubuleinratsafterkidney hematoxylinandeosinwerestained(Xiaet al., 2013).Theresultsshowedthatsub-chronicAl exposurecouldinducethedamageofthekidney structure.
References
BohrerD,DessuyMB,KaizerR,et al.2008.Tissuedigestionfor aluminumdeterminationinexperimentalanimalstudies. Analytical Biochemistry,377(2):120-127.
BolignanoD,Mattace-RasoF,SijbrandsEJ,et al.2014.Theagingkidneyrevisited:asystematicreview. Ageing Research Reviews,14:65-80.
LinardakiZI,OrkoulaMG,KokkosisAG,et al.2013.Investigationof theneuroprotectiveactionofsaffron(Crocus sativus L.)inaluminumexposedadultmicethroughbehavioralandneurobiochemical assessment. Food and Chemical Toxicology,52:163-170.
JiJW,ZhangQL,GaoFP, et al.2011.Thebasicpathologicalchanges inmiceinducedbynano-alumina. Journal of Toxicology,25(6): 442-446.
KumarV,GillKD.2009.Aluminiumneurotoxicity:neurobehavioural andoxidativeaspects. Archives of Toxicology,83(11):965-978.
WangN,SheY,ZhuYZ, et al. 2012.Effectsofsubchronicaluminum exposureonthereproductivefunctioninfemalerats. Biological Trace Element Research, 145(3):382-387.MahieuS,ContiniM C,GonzálezM, et al. 2009.Melatoninreducesoxidativedamage inducedbyaluminiuminratkidney. Toxicology Letters,190(1):9-15.
XiaSL,LiM,ShaoB,et al.2013.Effectsofsub-chronicaluminum exposureonrenalpathologicstructureinrats.Journal of Northeast Agricultural University, 20(1):49-52.
YamadaH,ChikamatsuK,AonoA,et al.2014.Pre-fixationof virulentMycobacterium tuberculosiswithglutaraldehydepreserves exquisiteultrastructureontransmissionelectronmicroscopythrough cryofixationandfreeze-substitutionwithosmium-acetoneatultralow temperature. Journal of Microbiological Methods,96:50-55.
ZhangW,ZhuXL,WangCJ,et al.2005.Applicationofimproved massonstainingmethodtothestainingofrenalbiopsytissue.Chinese Journal of Pathology,34(6):375-376.
ZhuYZ,HanYF,ZhaoHS, et al.2013.Suppressiveeffectof accumulatedaluminumtrichlorideonthehepaticmicrosomal cytochromeP450enzymesysteminrats. Food and Chemical Toxicology,51:210-214.
ZhuYZ,SunH,FuY,et al. 2014.Effectsofsub-chronicaluminum chlorideonspermatogenesisandtesticularenzymaticactivityinmale rats.Life Sciences,102(1):36-40.
ZhuoJH.2008. The mechanism of DFP protecting against nervous system, liver and kidney in aluminum-exposed rats.Shandong University,Jinan.
S852.2Document code: A Article ID: 1006-8104(2015)-02-0047-05
10January2015
SupportedbytheScienceandTechnologyProgramofHeilongjiangEducationalBureau(12541025);theNaturalScienceFoundationofHeilongjiang Province(C201425)
LiYan-fei(1967-),female,professor,supervisorofPh.Dstudent,engagedintheresearchofveterinarymedicine.E-mail:yanfeili_200@126.com
 Journal of Northeast Agricultural University(English Edition)2015年2期
Journal of Northeast Agricultural University(English Edition)2015年2期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Effects of Rice Yield and Quality Across Accumulated Temperature Zone Planting in Cold Area
- Separation and Purification of Total Phloroglucinols in Dryopteris crassirhizoma with DM-130 Macroporous Adsorption Resin
- Characterization and Expression of Outer Membrane Protein A I Gene of Aeromonas veronii
- Construction and Expression of Methionine-rich and Lysine-rich Fusion Gene in Bacillus natto
- Isolation and Pathogenicity Analyses on Yersinia enterocolitica from Pelteobagrus vachelli
- Effects of Three Different Diluents on Quality of Boar Semen Stored at 17℃
