Gender Differences in Ventricular-vascular Coupling Following Exercise△
Zhao-jun Li, Lian-fang Du, and Xiang-hong Luo*
Department of Ultrasound, Shanghai General Hospital, Shanghai Jiaotong University, Shanghai 200080, China
Gender Differences in Ventricular-vascular Coupling Following Exercise△
Zhao-jun Li, Lian-fang Du, and Xiang-hong Luo*
Department of Ultrasound, Shanghai General Hospital, Shanghai Jiaotong University, Shanghai 200080, China
ventricular-vascular coupling; exercise stress; gender; exercise echocardiography
Objective To study the differences of cardiovascular system between men and women in response to exercise stress.
Methods Forty healthy youth were tested according to Bruce protocol of exercise stress. They were detected by ultrasonography during the rest, peak exercise, and recovery stages, respectively. The left ventricular diastolic elastance (Ed), effective arterial elastance (Ea), left ventricular end-systolic elatance (Ees),ventricular-vascular coupling index (VVI), and total stiffness index (TSI) were measured and calculated according to the formulas. The results of all stages were compared according to genders.
Results All stages, the Ed, TSI, and VVI of women were higher than those of men, but the Ees was lower than that of men (all p<0.05); there was no significant difference in Ea between men and women. The Ed, Ees, Ea, and TSI were closely related with left ventricular oxygen consumption and heart function, and women showed more closely. Before and after exercise, the changes were different in Ed, Ees, Ea, TSI, and VVI (all p<0.05), and VVI changed least.
Conclusions Before and after exercise, the ventricular stiffness matched well with arterial stiffness and maintained within a narrow range. For women, the tolerance of exercise was lower than that of men. Chin Med Sci J 2015; 30(4):231-238
T HE ventricular function and structure are affected by the elastic properties of the entire arterial tree. The interaction between the heart and the systemic vasculature, termed ventricularvascular coupling, is essential for the heart to achievemaximal cardiac work. Furthermore, the clinical studies have reported that women tend to develop greater hypertrophy in response to hypertension1,2or aortic stenosis3compared to men, and shown a higher prevalence of diastolic heart failure in women.4The intrinsic differences in gender may contribute to cardiovascular structure and function.5The purpose of the present study was to determine that ventricular and arterial stiffness might be changed synchronously and achieve a new match during exercise in healthy individuals; and some of the gender differences in cardiovascular function might be an expression of differences in ventricular and arterial stiffness during exercise stress.
SUBJECTS AND METHODS
Study population
From October 2O13 to March 2O14, a total of 4O healthy volunteers were recruited including 22 men and 18 women,aged from 2O to 35 years with a mean age of (25.5±2.8)years. The men and women were matched for age. They were all from the Shanghai General Hospital, Shanghai Jiaotong University. The subjects reported initially to the study room and underwent the examinations including physical examination, chest X-ray, electrocardiogram, and echocardiography. All subjects were healthy, had no history of cardiovascular diseases, and were not taking any medication. All subjects gave informed consents to participate, and the study was approved by the institutional Ethics Committee of Shanghai General Hospital, Shanghai Jiaotong University.
Exercise test
All subjects underwent a multistage symptom-limited supine graded exercise protocol6on a variable load bicycle ergometer (ergoselect 1OOO LP, Ergoline, Germany). The subjects began at a workload of 25 W, and then the workload was increased by 25 W every 3 minutes until exhaustion. Pedal speed was maintained constant at 6O-7O rpm. Maximal workload was defined as the maximal wattage attained during the exercise test. Symptoms-limited exercise testing was done on a treadmill according to the standard or modified Bruce protocol. The test was ended in the following cases: ① exhaustion or if the heart rate reached the maximum age-predicted heart rate (22O beats/min-age);② significant decrease in systolic blood pressure (SBP) with progressive exercise, particularly in presence of other signs of ischemia, or a hypertensive response (SBP>23O mm Hg or diastolic blood pressure>13O mm Hg); ③ dizziness, intense dyspnea, severe claudication, or evidence of peripheral hypoperfusion; ④ ventricular arrhythmias (frequent ventricular extrasystoles, multiform complexes, or bursts of 3 or more beats); ⑤ technical problems with monitoring the echocardiogram or blood pressure (BP); ⑥ severe angina pectoris;⑦ ST-segment depression of 3 mm or more; and ⑧ at the request of subject. BP was measured on the right arm during rest, peak exercise, and recovery stages using an oscillometric monitoring device. End-systolic pressure (ESP)was calculated as (2×systolic pressure+diastolic pressure). Echocardiography also was performed during the rest,peak exercise, and recovery stages, respectively. Prior to postexercise echocardiography measures, participants rested and rehydrated in a controlled laboratory environment for 3O minutes and were allowed to consume fluids ad libitum.
Echocardiography
During the rest, peak exercise, and recovery stages, the conventional echocardiography and stress echocardiography were performed using a Mylab 7O ultrasound system (Esaote,Italy) with a 1-4 MHz transducer. Left ventricular (LV) chamber volumes and stroke volume were determined from 2-dimensional echocardiography. The LV ejection fraction (LVEF)was measured by the biplane Simpson's rule. In the fivechamber view, Doppler sample volume was placed about 1 cm below the aortic valve, the LV outflow (LVOT) velocities were traced, and the following variables were obtained including peak velocity, velocity time integral, and acceleration time. Through the apical four-chamber view, a 2-mm pulsed Doppler sample volume was placed at the mitral valve tip, and mitral flow parameters were obtained:peak velocity of early filling (E) and late filling (A), and ratio of E to A. Furthermore, Doppler tissue imaging was carried out in the four-chamber view at the septal mitral annular level. The peak velocity of myocardial systolic wave (s),early diastolic wave (e), and late diastolic wave (a) were recorded; the e/a and E/e ratios were calculated. The isovolumic contraction time (ICT), ejection time (ET), and isovolumic relaxation time (IRT) were measured, and anatomic M-mode and Tei index [(ICT+IRT)/ET] were calculated. All measurements were reported as an average of 3 consecutive beats.
Echo-derived parameters
During the rest, peak exercise, and recovery stages, the echo-derived parameters about ventricular and vascular stiffness were calculated. E/e was used as a surrogate of LV end-diastolic pressure and E/e divided by stroke volume(SV) as an index of LV diastolic elastance (Ed).7LV systolic elastance (Ees) was calculated using a LVOT peak velocity/ accelerating time as reported in a previous study.8The effective arterial elastance (Ea) was estimated as the ESP/SV.9The interaction between peripheral resistance and LV systolic stiffness, the ventricular-vascular coupling index (VVI, 1O×Ea/Ees) was calculated. Moreover, total stiffness index (TSI, Ed×VVI) was calculated.9To assess the inter-observer variability, 2 investigators measured independently values of the rest, peak exercise, and recovery stages. To assess the intraobserver variability, an investigator measured each echo-Doppler value twice.
Statistical analysis
Statistical analyses were performed using SPSS 13.O software package (SPSS Inc., IL, USA). Continuous variables were expressed as mean±standard deviation. Comparison between male and female groups was donewith independent sample t-test. Data among rest, peak exercise, and recovery stages were compared using one-way analysis of variance (ANOVA) followed by least significant difference (LDS) test. A receiver operating characteristic (ROC) curve was performed to compare stiffness parameters between rest and peak exercise. To assess the inter- and intra-observer variability of echoindices, Pearson's correlation and Bland-Altman analysis were performed with each measured value. The degree of correlation between LV function and cardiovascular stiffness was analyzed by Pearson's correlation method. P<O.O5 was considered statistically significant.
RESULTS
In the exercise stress test, all the volunteers reached the target load and successfully completed the entire test. None of them showed adverse reaction.
BP and heart rate
BP and heart rate (HR) are shown in Table 1. At peak exercise, the SBP, mean BP (MBP), ESP, HR, and rate pressure product (HR×MBP) significantly increased in both men and women (man: F=55.695, 29.886, 2O.44O, 32O.2O6,265.3O9; woman: F=26.37O, 11.733, 21.332, 244.843,147.573; all P<O.O5). The DBP insignificantly decreased between rest and exercise in both men and women. Both men and women had a higher HR at reserve compare with rest (man: F=9.316; woman: F=4.939; P<O.O5).
Inter- and intra-observer variability
Ten subjects were randomly selected from the forty volunteers for coincidence test. The inter-observer correlation coefficient was O.961 for resting Ed, O.998 for resting VVI, O.871 for Ed after exercise, and O.987 for VVI after exercise. The correlation coefficient of the TSI measured by 2 independent investigators was O.979 in the resting stage and O.96O after peak exercise. The intra-observer correlation coefficient was O.895 for resting Ed, O.994 for resting VVI, O.875 for Ed after exercise, and O.983 for VVI after exercise. The intra-observer correlation coefficient of TSI was O.965 in the resting stage and O.954 after peak exercise (Fig. 1).
LV geometry and function
LV volumes were not significantly modified by exercise in men and women (man: F=1.628, 1.OO1, 1.223; woman:F=1.1OO, O.271, 1.152; all P>O.O5). Both men and women,the cardiac output and "s" wave increased at the peak exercise than that at rest (man: F=7.197 and 16.251;woman: F=16.244 and 2O.975; all P<O.O5), but the ejection fraction was not significantly different between the two stages (man: F=3.788; woman: F=3.411; P>O.O5). At the peak exercise, the E/A significantly decreased, and the E/e notably increased than that at rest (man: F=14.348,23.475; woman: F=1O.5O9, 14.O29; all P<O.O1). Tei index was not significantly changed after exercise (man: F=2.53O;woman: F=3.664; P>O.O5). (Table 2)
Changs of ventricular-arterial stiffness and coupling at different states of exercise
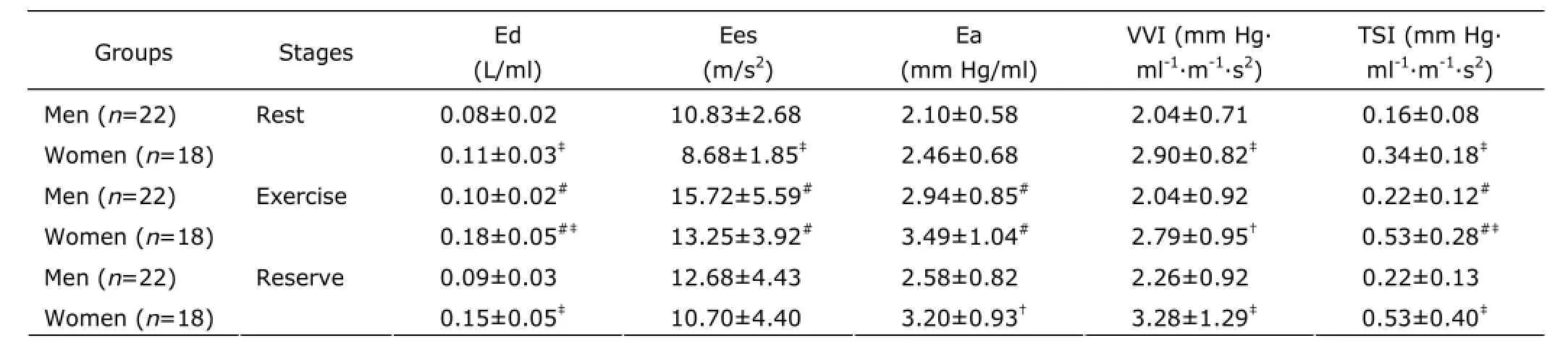
In both genders, Ed, Ees, Ea, and TSI were all significantly increased during exercise (man: F=9.5O7, 6.O15, 5.321,4.566; woman: F=8.774, 5.472, 6.5O8, 4.344; all P<O.O5). There were no significant difference of VVI which presented ventricular-arterial coupling in either men or women during exercise (man: F=1.184; woman: F=O.423; P>O.O5).
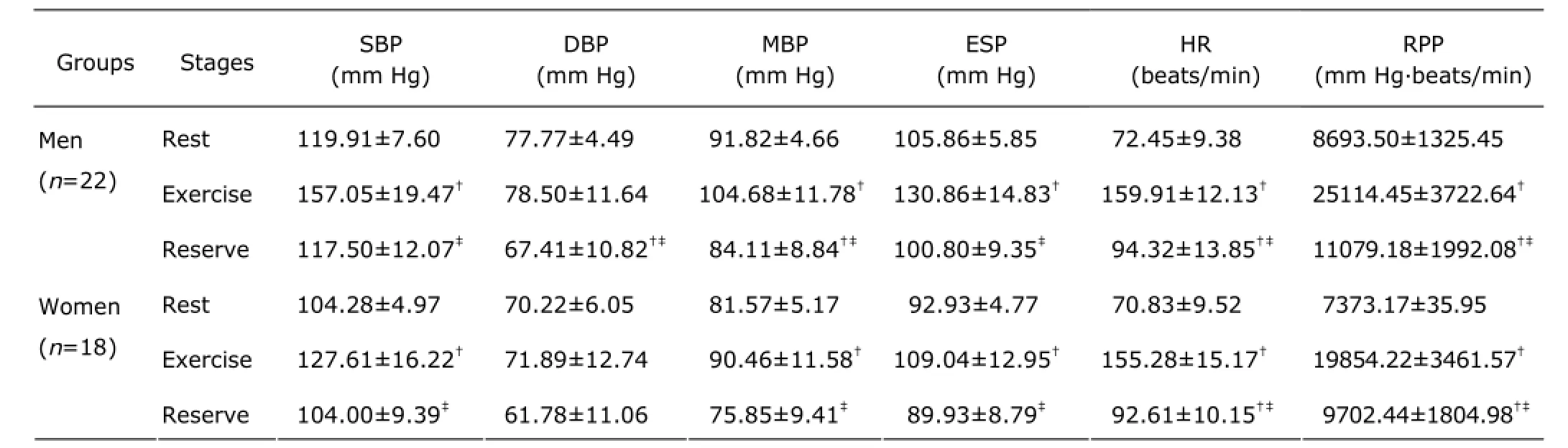
Table 1. Exercise test characteristics of the study groups§
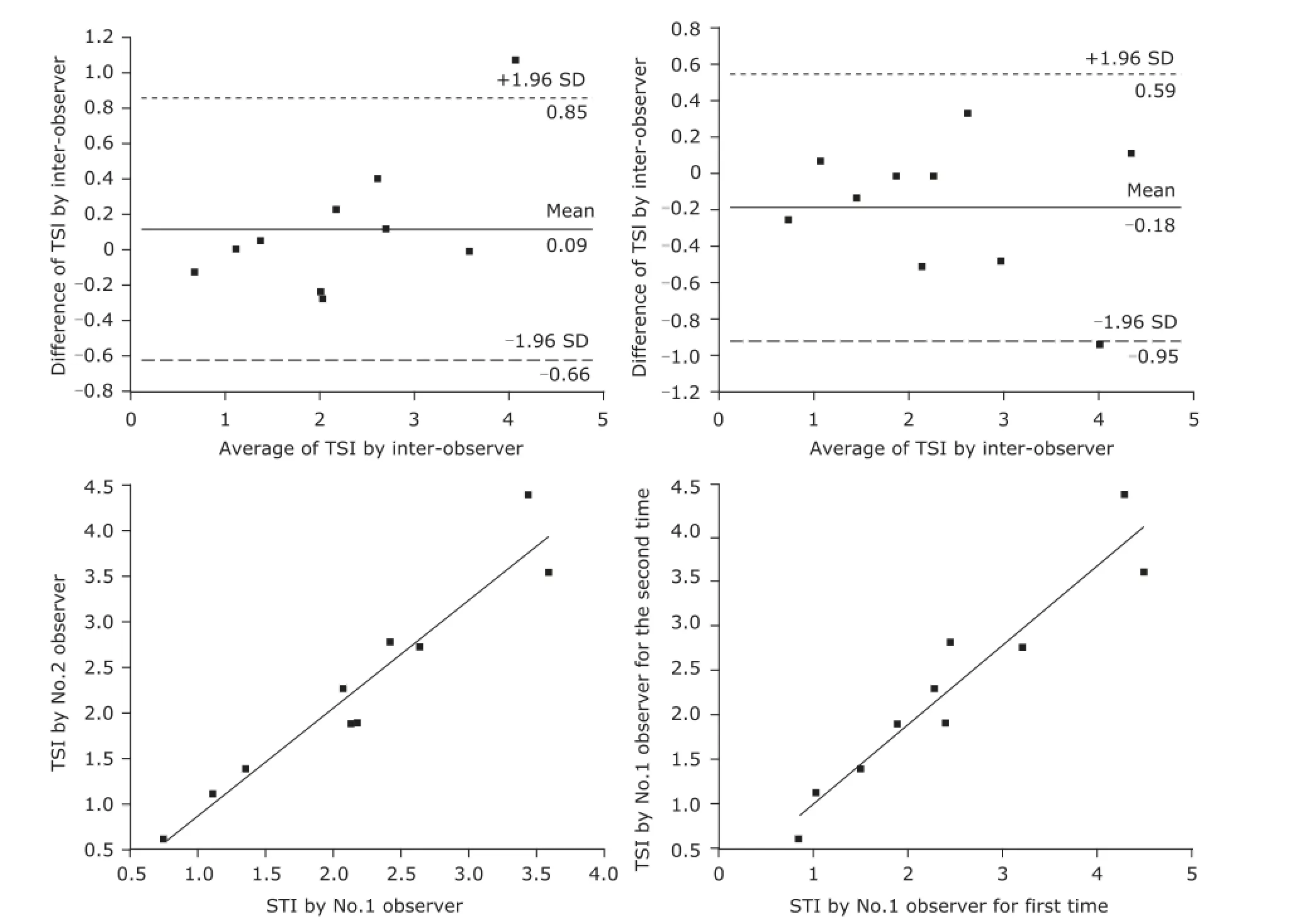
Figure 1. Linear correlation analysis and Bland-Altman plots were adopted to analyze the repeatability of echo-derived indices at rest and peak exercise. Correlation and agreement of total stiffness index (TSI) were measured by inter-observers and intra-observers. SD: standard deviation.
At rest, exercise, and reserve, Ed increased to a greater extent in women than in men (P=O.OO7, P=O.OO7,and P=O.OO6). At rest, the Ees was lower in women than that in men (P=O.OO5), but at exercise and reserve, there were no significant differences between women and men(P=O.31O and P=O.29O). Ea was not gender-related at either rest or exercise (P=O.22O and P=O.19O). There was a significant gender difference in VVI and TSI at the resting,exercising, and reserving states, with women having higher values (P<O.O1)(Table 3).
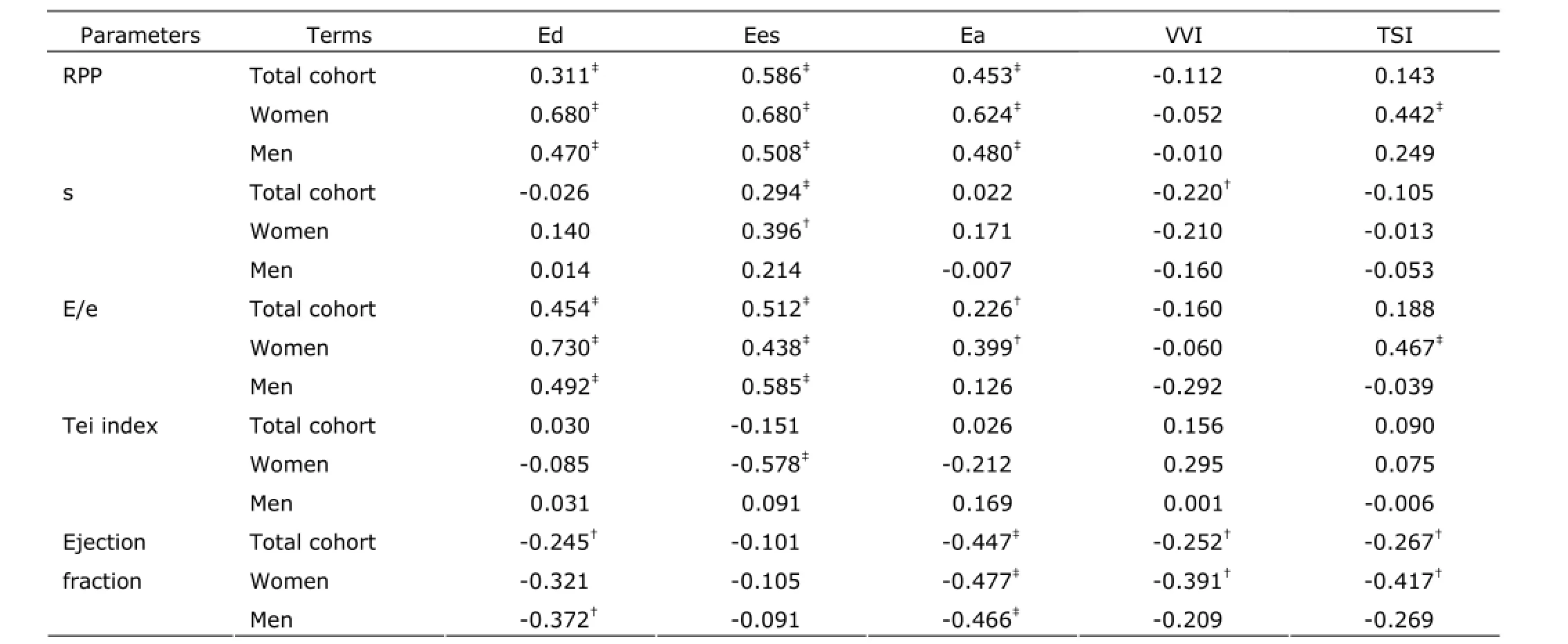
The ROC curve was performed to investigate the changes of Ed, Ees, Ea, TSI, and VVI during exercise. The areas of Ed, Ees, Ea, TSI, and VVI under the ROC curve were O.778 (95% CI: O.676, O.879), O.797 (95% CI: O.698,O.896), O.783 (95% CI: O.783, O.882), O.641 (95% CI:O.519, O.762), and O.48O (95% CI: O.351, O.6O9),respectively. Moreover, the areas of Ed, Ees, Ea, and TSI were all larger than VVI's. There were significant differences in Ed, Ees, Ea, and TSI between rest and exercise (all P Correlation of cardiovascular stiffness with heart function To men, women, and total cohort, Ed and Ees were all positively correlated with RPP. To women and total cohort,Ea was positively correlated with E/e. The results showed Ed, Ees, and Ea were closely related with LV oxygen consumption and cardiac diastolic function. VVI was negatively related to ejection fraction, though the correlation between VVI and RPP, s, E/e, Tei was statistically insignificant. This suggested the myocardial function and oxygen consumption had little effect on VVI. Under the condition of exercise, the ventricular stiffness matched well with arterial stiffness. For women, TSI was positively related to RPP and E/e. For men and total cohort, the correlation between TSI and RPP, s, E/e, Tei was no statistically significant. (Table 4) Table 2. Two-dimensional and harmonic Doppler echocardiography findings§ Table 3. Ventricular-arterial stiffness and coupling§ Figure 2. Exercise-induced changes of Ed, Ees, Ea, VVI, and TSI were analyzed by receiver operating characteristic curve. Therewas no significant difference in VVI after exercise compared with rest.RUC: area under receiver operating characteristic curve. Table 4. Correlation of cardiovascular stiffness with heart function In this study, we performed a comprehensive evaluation of resting and exercising cardiovascular function in healthy subjects using traditional and echo-derived ventricular elastance index, TSI, and ventricular-arterial coupling index. We found that ① Both at rest and exercise, the women had the higher Ed, TSI, and VVI, but the lower ventricular Ees than men's; ② Both men and women, at resting, the Ed, Ees, Ea, and TSI were lower than those at exercise. There was no significant difference of VVI between resting and exercising; ③ Ed, Ees, Ea, and TSI were closely correlated with LV oxygen consumption and function; ④ At rest and exercise, the changes of Ed, Ees, Ea,TSI, and VVI were detected, and the smallest change was VVI. Ed, Ees, and Ea could be considered as independent determinants of exercise capacity in healthy person. The ventricular and vascular elastance was matching and coupling with healthy person at various stages. Ed is the slope of end-diastolic pressure-volume relationship and is used to evaluate the ventricular relaxation and stiffness.1OEd can be obtained by noninvasive echocardiography.11Echo-Doppler-derived E/e, a reliable index of ventricular filling, is significantly correlated with the LV end-diastolic pressure.12In the absence of significant aortic regurgitation, SV can be used as an indicator of ventricular filling volume. So, the combination of these 2 parameters as E/e/SV represents the LV end-diastolic pressure/LV filling volume and can be used as the Ed index.7,13The community-based study reported that the gender-related differences of Ed were independent of cardiovascular disease. In subjects without cardiovascular disease, LV diastolic stiffness is higher in women than that in men,14and the results of this study supported the view. Shapiro et al15showed that the Ed described with acute preload reduction to release external forces was shifted upward with increase in Ea. In our study, E/e was similar between men and women, while the SV was smaller in women, so E/e/SV was higher in women. It suggested gender-related differences in systolic chamber function should be considered when ventricular stiffness was analyzed. In this study, the Ed was significantly related to LV oxygen consumption and function, Ed is a better determinant of exercise capacity than E/e. Understanding the performance of left ventricle requires not only examining the properties of the left ventricle itself,but also investigating the modulating effects of the arterial system, termed ventricular-arterial coupling. The interaction of the heart with the arterial system (ventricular-arterial coupling) is a key determinant of cardiovascular performance and cardiac energetic. Ventricular-arterial coupling is expressed by the Ea/Ees ratio,16so Ea and Ees were its components. Ea is a lumped parameter reflecting total arterial afterload,incorporating mean and pulsatile components. Ea will be greater during exercise because of a complex interplay among the changes in BP, arterial stiffness, and HR. In this study, we found that both women and men had higher Ea and Ees at peak exercise than at resting. Decreases in aortic distensibility have been reported to correlate with exercise intolerance in elderly hypertension for heart fail with a normal ejection fraction.15The LV systolic stiffness was increased with increased arterial stiffness.17Ees is determined invasively from the slope and intercept of the end-systolic pressure-volume relationship, but may also be measured noninvasively by echocardiography. Our study showed that Ees was increased and appeared higher in men than that in women. Ees was higher at exercise than that at rest. This indicated that myocardial contractility was higher in men compared to women and Ees may represent myocardial contractility. At the resting state, previous studies have shown that Ea/Ees is tightly controlled within a narrow range to optimize energetic efficiency.18During exercise, the subjects showed to have both arterial and ventricular stiffening, so an acute mismatch between the arterial and ventricular systems occurred, due to a disproportionate increase in Ees vs. Ea,to ensure that sufficient cardiac performance, and then the Ea vs. Ees progressively achieved new balance. The area of VVI under the ROC curve had no significant difference between rest and exercise (P=O.758), and indicated VVI was remain relatively stable. In addition, for both women and men, a higher Ees and Ea were associated with higher RPP,which suggested that higher Ees and Ea required a greater energetic requirement. Moreover, the associations were stronger for women than for men. It is currently not known whether long-term consumption of higher energy could lead to energetic depletion in the heart.19Chantler et al2Oreported that systolic hypertension women demonstrated a disproportionate increase in Ees compared with Ea, suggesting an adaptation by these in women to limit the impact of systolic hypertension on the ventricular vs. arterial elastance. The indexes of arterial and ventricular elastance were all assessed noninvasively. At rest, the single-beat elastance approach is regarded as the preferred noninvasive method to measure Ees. However, the single-beat elastance approach may be technically challenging during exercise, because of the difficulties in measuring cardiac volumes and systolictime intervals with echocardiography during exercise. With the fast maturity of cardiac three-dimensional technology,the measuring accuracy of SV should be further improved,and then, there would be more clinical value in the ultrasonic evaluation of ventricular-vascular coupling. The recovery stage which was only 3O minutes after peak exercise was a shorter time to recover and the capillaries in skin, skeletal muscle and the other peripheral organs were expensed after exercise. These factors attributes to faster HR and lower DBP of subjects than rest. In the following experiments,we would allow more time to recover and standardize on date by surface area. 1. Saba PS, Ganau A, Devereux RB, et al. Impact of arterial elastance as a measure of vascular load on left ventricular geometry in hypertension. J Hypertens 1999; 17:1OO7-15. 2. Asanoi H, Kameyama T, Ishizaka S, et al. Ventriculoarterial coupling during exercise in normal human subjects. Int J Cardiol 1992; 36:177-86. 3. Tanoue Y, Maeda T, Oda S, et al. Left ventricular performance in aortic valve replacement. Interact Cardiovasc Thorac Surg 2OO9; 9:255-9. 4. Tartière-Kesri L, Tartière JM, Logeart D, et al. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2O12; 59:455-61. 5. Coutinho T, Borlaug BA, Pellikka PA, et al. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol 2O13; 61:96-1O3. 6. Her AY, Kim JY, Choi EY, et al. Value of ventricular stiffness index and ventricular arterial interaction in patients with no ischemic dilated cardiomyopathy. Circ J 2OO9;73:1683-9O. 7. Redfield MM, Jacobsen SJ, Borlaug BA, et al. Age- and gender-related ventricular-vascular stiffening: a communitybased study. Circulation 2OO5; 112:2254-62. 8. Bauer F, Jones M, Shiota T, et al. Left ventricular outflow tract mean systolic accelerations a surrogate for the slope of the left ventricular end-systolic pressure-volume relationship. J Am Coll Cardiol 2OO2; 4O:132O-7. 9. Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastances index of arterial vascular load in humans. Circulation 1992; 86:513-21. 1O. Li ZJ, Du LF, Luo XH. Evaluation of ventricular-vascular coupling in patients with type 2 diabetes mellitus using 2-dimensional speckle tracking imaging. J Huazhong Univ Sci Technolog Med Sci 2O14; 34:929-34. 11. Ha JW, Choi D, Park S, et al. Left ventricular diastolic functional reserve during exercise in patients with impaired myocardial relaxation at rest. Heart 2OO9; 95:399-4O4. 12. Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997; 3O:1527-33. 13. Goto T, Ohte N, Fukuta H, et al. Relationship between effective arterial elastance, total vascular resistance, and augmentation index at the ascending aorta and left ventricular diastolic function in older women. Circ J 2O12;77:123-9. 14. Ha JW, Lee HC, Park S, et al. Gender-related difference in left ventricular diastolic elastance during exercise in patients with diabetes mellitus. Circ J 2OO8; 72:1443-8. 15. Shapiro BP, Lam CS, Patel JB, et al. Acute and chronic ventricular-arterial coupling in systole and diastole:insights from an elderly hypertensive model. Hypertension 2OO7; 5O:5O3-11. 16. Kass DA. Age-related changes in ventricular-arterial coupling: pathophysiologic implications. Heart Fail Rev 2OO2; 7:51-62. 17. Hay I, Rich J, Ferber P, et al. Role of impaired myocardial relaxation in the production of elevated left ventricular filling pressure. Am J Physiol Heart Circ Physiol 2OO5;288:H12O3-8. 18. Suga H. Global cardiac function: mechano-energetic-informatics. J Biomech 36:713-2O. 19. Ingwall JS, Weiss RG. Is the failing heart energy starved?On using chemical energy to support cardiac function. Circ Res 2OO4; 95:135-45. 2O. Chantler PD, Melenovsky V, Schulman SP, et al. The sex-specific impact of systolic hypertension and systolic blood pressure on arterial-ventricular coupling at rest and during exercise. Am J Physiol Heart Circ Physiol 2OO8;295:H145-53. for publication March 25, 2014. Tel: 86-21-36126236, E-mail: lxh_20050703@ sina.com △Supported by the Shanghai Health and Family Planning Commission Fund (201440290), Shanghai Science and Technology Committee Fund (15411969100), and Shanghai Jiaotong University Interdisciplinary Projects of Medicine and Engineering (YG2015MS28).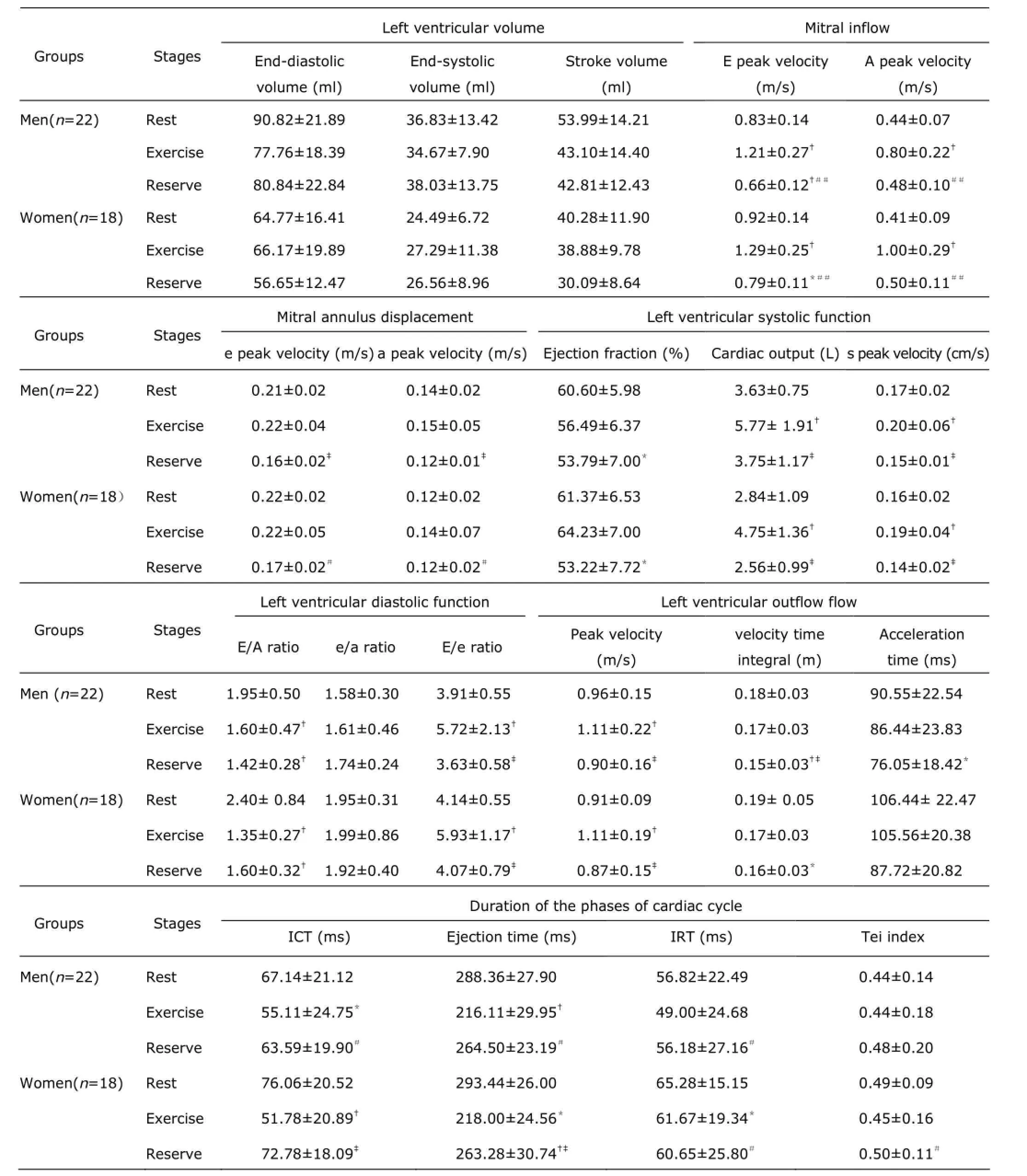

DISCUSSION
REFERENCES
 Chinese Medical Sciences Journal2015年4期
Chinese Medical Sciences Journal2015年4期
- Chinese Medical Sciences Journal的其它文章
- Efficacy of Topical Tacrolimus for Erosive Oral Lichen Planus: A Meta-analysis
- Effect of Ursolic Acid on Breast Cancer Resistance Protein-mediated Transport of Rosuvastatin In Vivo and Vitro△
- Management of Cesarean Scar Pregnancy: A Case Series
- Retroperitoneal Versus Transperitoneal Laparoscopic Partial Nephrectomy: A Systematic Review and Meta-analysis△
- Effect of Atorvastatin on Expression of Peroxisome Proliferator-activated Receptor Beta/delta in AngiotensinⅡ-induced Hypertrophic Myocardial Cells In Vitro△
- Influence of Photodynamic Therapy on Apoptosis and Invasion of Human Cholangiocarcinoma QBC939 Cell Line
