Efficacy of Topical Tacrolimus for Erosive Oral Lichen Planus: A Meta-analysis
Chun-lan Guo, Ji-zhi Zhao*, Jie Zhang, and Hai-tao Dong
Department of Stomatology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
Efficacy of Topical Tacrolimus for Erosive Oral Lichen Planus: A Meta-analysis
Chun-lan Guo, Ji-zhi Zhao*, Jie Zhang, and Hai-tao Dong
Department of Stomatology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
tacrolimus; corticosteroids; oral lichen planus; Meta-analysis
Objective To assess the efficacy and safety of topical tacrolimus for erosive oral lichen planus (EOLP).
Methods Literatures published up to December 2013 were searched from PubMed, Embase,CENTRAL, Chinese BioMedical Literature Database (CBM), and System for Information on Grey Literature in Europe (SIGLE). All randomized controlled trials (RCTs) of topical tacrolimus for EOLP which compared with other interventions or a placebo were considered in this Meta-analysis. Two researchers collected data independently. The assessment of methodological quality was based on Cochrane Handbook and the materials were analyzed with the software Revman 5.2.5. The primary outcome measures were the symptoms (e.g. pain,discomfort) complained by patients. The secondary outcome measures included the improvement rate of clinical signs assessed by the investigators and the incidence of adverse effects (e.g. clinical candidiasis).
Results A total of 9 RCTs involving 476 patients were finally included. The pooled odds ratio (OR) of clinical improvement for topical tacrolimus vs. topical corticosteroids was 1.19 [95% confidence interval (CI): 0.64-2.22, I2: 44%]. Regarding to 0.1% tacrolimus and 0.03% tacrolimus, the pooled OR were 1.87 (95 % CI: 0.60-5.82) and 1.47 (95 % CI: 0.14-16.04) respectively in subgroup analysis. No serious adverse events were reported in topical tacrolimus group.
Conclusions There was no evidence to support that topical tacrolimus for EOLP was more effective and safer than topical corticosteroids in this Meta-analysis. Clinical assessment criteria should be established and accepted by clinicians and researchers before further RCTs are undertaken.
Chin Med Sci J 2015; 30(4):210-217
O RAL lichen planus (OLP) is a common chronic inflammatory disease of oral mucosa, with a worldwide distribution and an overall prevalence of 1%-2.2%.1The clinical appearances of OLP present in various manifestations such as reticular, plaque,and erosive patterns. Erosive OLP (EOLP) is symptomatic and hardly undergoes spontaneous remission, which leads to persistent pain, difficulty to eat, and suboptimal dental hygiene. The quality of life in the patients with EOLP is impaired significantly. Long-standing intractable EOLP is a condition with potential malignancy. It is important that affected individuals are given the most efficacious and safe treatments. However, the exact etiology of OLP is still unclear and the treatment of EOLP remains a challenging problem.
The histopathological researches at the molecular level have found that massive activated CD8+and CD4+T cells infiltrated in the basement membrane of OLP lesions and the release of cytokines such as tumor necrosis factoralpha (TNF-alpha) and interferon-gamma (IFN-gamma) increased significantly.2These phenomena suggested that T-cell-mediated autoimmune dysfunction would be one of the essential pathogenesis of OLP. Therefore topical corticosteroids (TCSs), including betamethasone, clobetasol, dexamethasone, and triamcinolone, have been the first-line medication for OLP for years.3However, TCSs only alleviate pain and severity of lesion temporally. And the prolonged use of TCSs may result in drug tolerance, secondary candidiasis infection, and auto-adrenal insufficiency, etc. Various other treatment regimens, such as topical and systemic retinoids, topical cyclosporine, thalidomide, photo-chemotherapy, and traditional Chinese medicine, have been attempted to improve the symptom. There is still poor consensus in clinical practices.
In the past decade, it has been proved that tacrolimus, a new kind of calcineurin inhibitors produced by Streptomyces tsukubaensis, can inhibit the dephosphorylation of nuclear factor in activated T lymphocytes, reduce the release of cytokines, and suppress the T-cell-mediated pathophysiological reaction in OLP.4More and more clinical studies reported that topical tacrolimus had very significant effect for EOLP and caused few side effects. But complete cure is still difficult to achieve. At present, there are no systematic reviews and Meta-analysis published to provide sufficient evidence to support the effectiveness of any specific treatment for EOLP as being superior. So we conducted a Meta-analysis of randomized controlled trials (RCTs) available comparing topical tacrolimus with other interventions or placebo for EOLP in order to assess the efficacy and safety of topical tacrolimus.
MATERIALS AND METHODS
Inclusion criteria
All RCTs which compared topical tacrolimus with another intervention or placebo in the treatment of EOLP were considered in this Meta-analysis. The patients in the included studies satisfied the following criteria: (1) having a clinical and/or histological diagnosis of EOLP; (2) not taking any other treatment for EOLP (e.g. systemic steroids, anti-fungals or immunosuppressants) concurrently. The primary outcome measures were the symptoms (e.g. pain, discomfort) complained by patients. The secondary outcome measures included the improvement rate of clinical signs assessed by the investigators and the incidence of adverse effects (e.g. clinical candidiasis).
Search strategy
The published trials were searched from PubMed (from 1990), Embase (from 1990), the Cochrance Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 4, 2013), and Chinese BioMedical Literature Database (CBM) (from 1990) up to Dec 2013. For the identification of studies included or considered in this Meta-analysis, detailed search strategies were developed appropriately for each database. For example, the search strategy developed by using a combination of controlled vocabulary and free text terms for MEDLINE was as follow:“tacrolimus"(MeSH Terms) or "tacrolimus"(All Fields) AND "lichen planus, oral"(MeSH Terms) or "lichen"(All Fields) AND "planus"(All Fields) AND "oral" (All Fields) or "oral lichen planus"(All Fields) or "oral"(All Fields) AND "lichen"(All Fields) AND "planus"(All Fields) AND “Clinical Trial” (ptyp). Moreover, System for Information on Grey Literature in Europe (SIGLE) was searched for grey literature and bibliographies of included studies, unpublished reviews. Abstracts of international conference proceedings were also scanned. No language restrictions were imposed.
Data collection and analysis
The titles and abstracts of all retrieved articles were scanned independently by two independent researchers (CL Guo and JZ Zhao). Full texts of the included studies were obtained and assessed independently by both of the two researchers. Differences in opinion were resolved by discussion with a third researcher (J Zhang) until the consensus was met.
All data collection and extraction were performed independently by two researchers (CL Guo and JZ Zhao) using specially designed form. The data on study characteristics including study design details, participants’characteristics, interventions and outcomes were extracted (Table 1). For dichotomous outcomes, the estimates of effects of an intervention were expressed as risk ratios (RR) together with 95% confidence interval (CI). For continuous outcomes, mean differences (MD) and 95% CI were used to summarize the data for each group.
The risk of bias of the included trials was assessed by all the researchers on the basis of Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0.5The following six specific domains were evaluated: (i) random sequence generation (selection bias); (ii) allocation concealment (selection bias); (iii) blinding (performancebias and detection bias); (iv) incomplete outcome data (attrition bias); (v) selective reporting (reporting bias); and (vi) other bias. Studies were graded into the following categories: low risk of bias–low risk of bias for all key domains; unclear risk of bias–unclear risk of bias for one or more key domains; high risk of bias–high risk of bias for one or more key domains. Any disagreement was resolved through discussion.
Statistical analysis
Statistical analyses were conducted using the software Revman 5.2.5 (the Cochrane Collaboration). When studies comparing similar interventions reported the same outcome measures, data were combined for Meta-analysis, such as the comparison in terms of clinical improvement of different interventions. The presence of heterogeneity among the included studies was detected by Q-test and the statistical heterogeneity was quantified by I2statistic. Heterogeneity was considered statistically significant if the P value was less than 0.1. The guide to the interpretation of I2given in the Cochrane Handbook is: 0 to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% may represent considerable heterogeneity. If there was considerable heterogeneity, the data of the studies could not be pooled into the Meta-analysis, the descriptive quality analysis should be conducted.
RESULTS
Description of studies
A total of 45 articles were initially retrieved, of which 32 were excluded based on abstracts and full-texts. The remaining 13 papers6-18met the inclusion criteria, of which 4 RCTs,15-18compared topical tacrolimus with four different kinds of topical medication respectively, such as pemicrolimus ointment, amlexanox paste, Kangfuxin solution, and topical traditional Chinese medicine, were excluded. The remaining 9 RCTs6-14which compared topical tacrolimus for EOLP with topical corticosteroids (TCSs) were included in the Meta-analysis finally (Fig. 1). The total number of participants included was 476 cases.
All the included 9 RCTs were conducted as a parallel design. The diagnosis of EOLP was confirmed clinically in all studies and histologically in four studies.8,11,12,14Topical tacrolimus were the active intervention in these studies, compared with clobetasol in three trials,7,11,12triamcinolone in two trials,8,10fluocinonide in one trial,13and dexamethasone and in one trial.14TCSs in different delivery vehicles (lipid-loaded microspheres versus ointment) and with different concentration (0.1%, 0.015%, 0.03%) were used in these studies. The characteristics of the included studies are listed in Table 1.
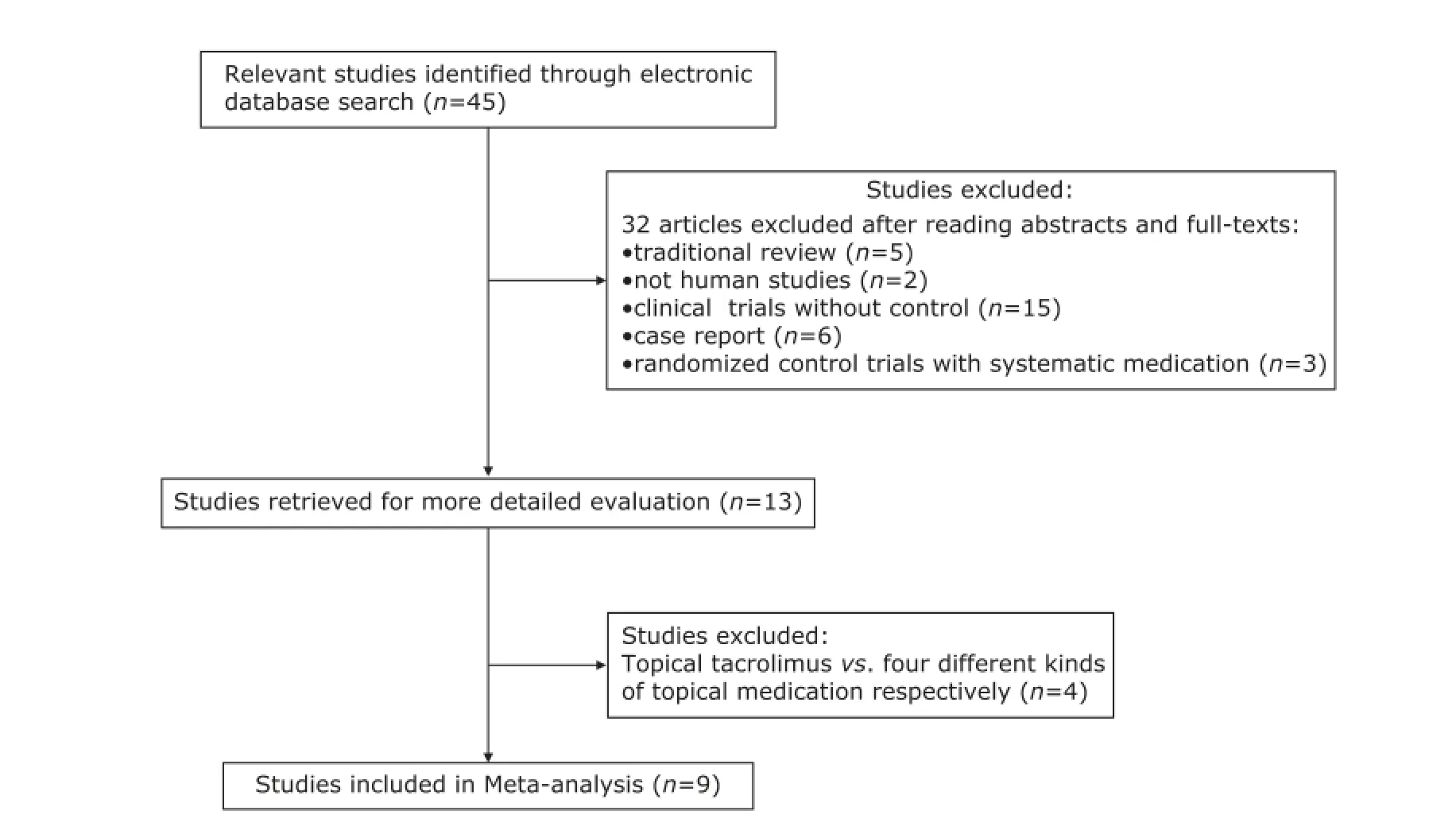
Figure 1. Flow chart for the study selection and exclusion process.

Table 1. Characteristics of the included studies
Risk of bias of the included studies
The risk of bias of the included studies was summarized in Figure 2. None of the studies met all the risk of bias criteria and therefore be assessed as low risk of bias. All the studies had one or more domains assessed as unclear and were considered to be at unclear risk of bias. Three studies7,11,12had reported the detailed information of randomized sequence generation, blinding of participants and personnel, and blinding of outcome assessment. They were considered as high quality according to the rules of CONSORT guidelines for reporting RCTs. The funnel plot showed no evidence of publication bias in this Meta-analysis in terms of clinical improvement (Fig. 3).
Effects of interventions
Pain, as one of the primary outcomes, was considered by all the studies. In these studies, there were different kinds of patterns for the evaluation of pain releasing, such as visual analogue scale (VAS) of pain,11,14Reticulation/keratosis, erythema, and ulceration (REU) scoring system for the lesions,6,14four-point scale score of symptoms,7,9,13and net clinical score,12etc. These outcome data could not been pooled quantitively. Neither study showed any statistical significant difference between topical tacrolimus and topical corticosteroids for the relief of pain in patients.
The clinical improvement rate of patients, the predefined secondary outcome, was reported in every included study and the data were synthesized in this Meta-analysis. Heterogeneity was found in those studies (P=0.001, I2=44%), so random effects models were used. The pooled odds ratio (OR) of clinical improvement was 1.19 [95% CI: 0.64-2.22] (Fig. 4).
Because the value of I2(44%) was close to 50%, subgroup analysis was conducted. The detected heterogeneity across studies may come from different concentration of topical tacrolimus. So, subgroup analyses regarding 0.1% and 0.03% tacrolimus were performed (Fig. 5 and Fig. 6). The pooled ORs were 1.87 (95 % CI: 0.60-5.82) and 1.47 (95 % CI: 0.14-16.04) respectively.
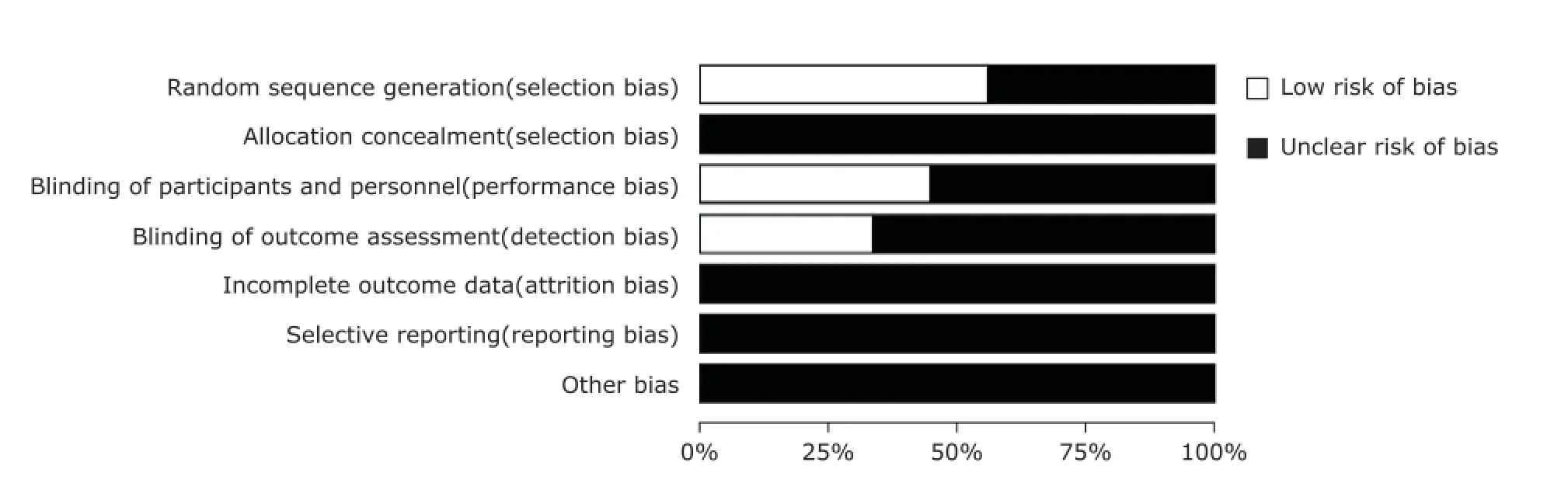
Figure 2. Risk of bias’ graph: review authors’ judgements about each risk of bias item presented as percentages across all the included studies.
Figure 3. Funnel plot for publication bias. OR: odds ratio; SE: standard error.
Adverse effects
Data on adverse effects were available from all the 9 included studies. The most frequently reported adverse effects were transient burning, stinging, or local swelling due to tacrolimus or corticosteroids application. No serious adverse effects were reported, such as secondary candidiasis, induced kidney/liver function impairment. One study reported that one patient treated by corticosteroid injection dropped out for the intolerable injection pain. The Meta-analysis of adverse effects showed no statistically difference between the tacrolimus group and corticosteroid group (Fig. 7).
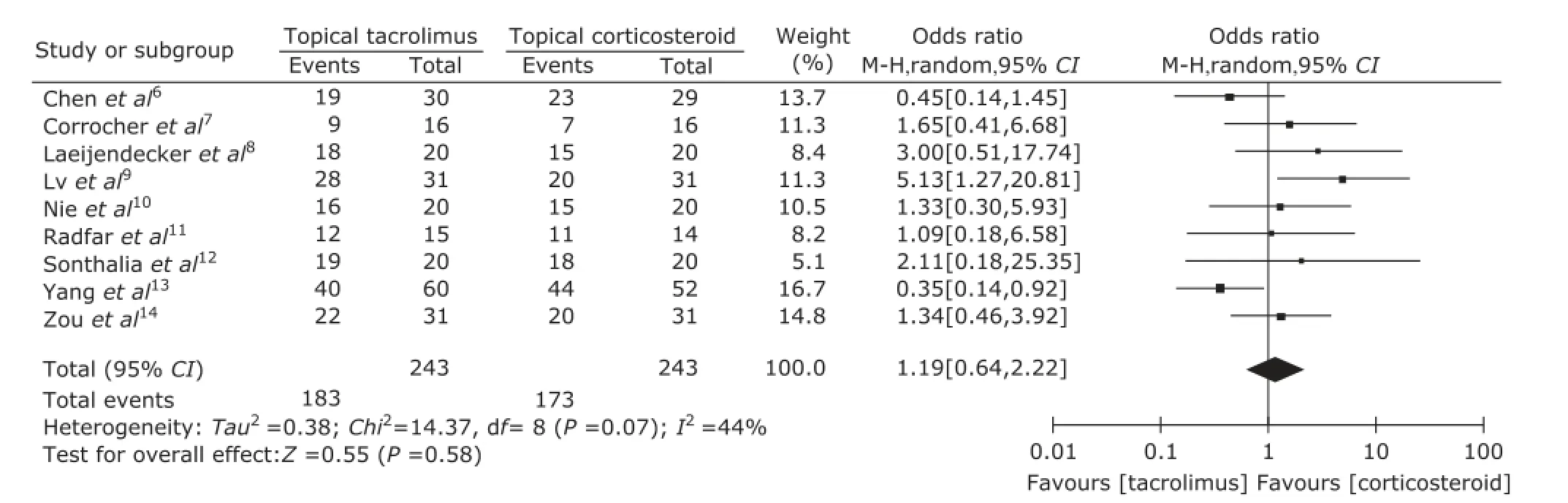
Figure 4. Forest plot of clinical improvement for topical tacrolimus vs. topical corticosteroid.
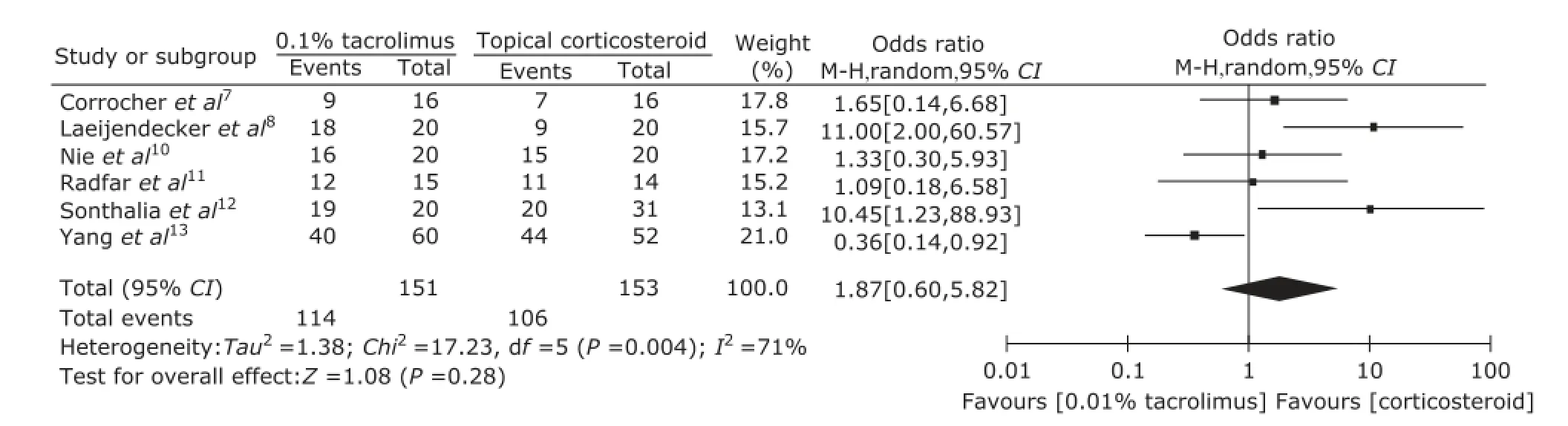
Figure 5. Forest plot of subgroup analysis of clinical improvement regarding 0.1% tacroliums vs. topical corticosteroid.
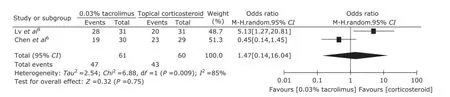
Figure 6. Forest plot of subgroup analysis of clinical improvement regarding 0.03% tarcolimus vs. topical corticosteroid.
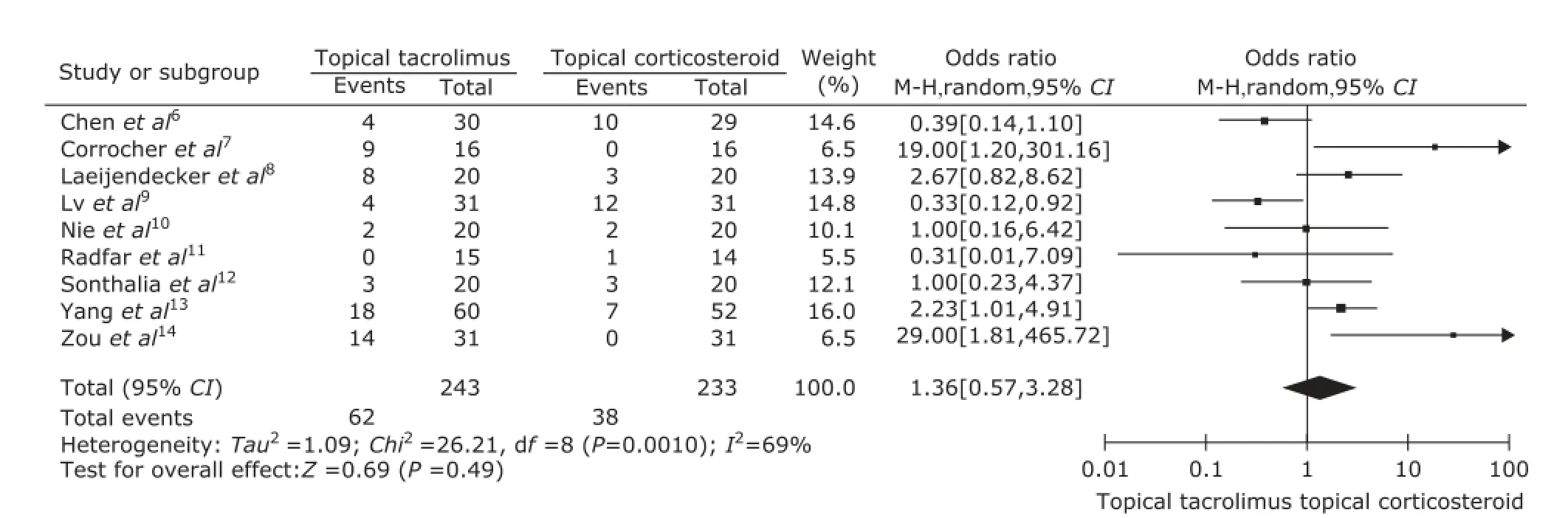
Figure 7. Forest plot of adverse effects of topical tacrolimus vs. topical corticosteroids.
DISCUSSION
There are not so many RCTs in the literature about the treatment of EOLP. In this Meta-analysis, 9 RCTs were included, involving 476 patients with EOLP. These studies came from different countries and regions and 5 studies7,8,11,12,14described the detailed information of random sequence generation method and blinding, complying with the rules of the CONSORT guidelines for reporting studies.
The included studies were inconformity in primary outcome variables and disease severity scoring tools. This is one of important obstacle in comparing and pooling outcome data of different studies together. Only the secondary outcome (clinical improvement rate of patients) could be pooled finally. The analysis results did not show that topical tacrolimus could be more effective and safer in the treatment of EOLP than topical corticosteroids. The different concentrations, dosages, vehicles of the medications, and the times of application used in the trials led to the potential heterogeneities across these studies. Most included studies were small sample-sized (range 32 to 112). It is likely that there was lack of power to detect significant differences between treatments if indeed such a difference existed.
There is a stringent need to standardize the methodology of clinical trials for EOLP, especially in the assessment of therapeutic response. The symptoms and clinical features should be measured by objective indexes in future RCTs. The indexes should be reliable (i.e. giving the same value each time it is used), sensible (i.e. able to catch even minimal clinically important changes), accurate and feasible (relatively easy to use).19For example, VAS scales of pain and the size of erosive area should be included, so that the accurate and quantitative treatment effects can be compared between patients and between interventions. And, follow-up periods should be sufficiently long to determine whether treatment confers an important long-term benefit. EOLP affects the patients’ daily life in many ways which including a psycho-social effect as well as a functional impact. Therefore, oral health-related quality of life should also be considered in future trials. The questionnaire, such as oral health impact profile (OHIP)-14, chronic oral mucosa diseases questionnaire (COMDQ), could be used to measure quality of life.
In conclusion, there is no evidence to support that topical tacrolimus could be more effective and safer for EOLP than topical corticosteroids. Tacrolimus could not replace corticosteroids to be the first-line medication at present. Clinical assessment criteria should be established and accepted by clinicians and researchers before further RCTs are undertaken.
REFERENCES
1. Silverman S, Gorsky M, Lozada-Nur F. A prospective follow-up study of 570 patients with oral lichen planus: persistence, remission and malignant association. Oral Surg Oral Med Oral Pathol 1985; 60:30-4.
2. Lozada-Nur F, Miranda C. Oral lichen planus: epidemiology, clinical characteristics, and associated diseases. Semin Cutan Med Surg 1997; 16:273-7.
3. Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell
clones in lichen planus. Br J Dematol 2000; 142:449-56.
4. Kelly PA, Burckart GJ, Venkataramanan R. Tacrolimus: a new immunosuppressive agent. Am J Health Syst Pharm 1995; 52:1521-35.
5. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. 2011 [cited 2011 March 1]. Available from: http://www.cochrane-handbook. org
6. Chen C, Cheng SH, Di ZH, et al. Comparative study of 0.03% tacrolimus ointment and diprospan in the treatment of oral lichen planus (OLP). Prac Phar Clin Rem 2013; 10:924-5.
7. Corrocher G, Di Lorenzo G, Martinelli N, et al. Comparative effect of tacrolimus 0.1% ointment and clobetasol 0.05% ointment in patients with oral lichen planus. J Clin Periodontol 2008; 35:244-9.
8. Laeijendecker R, Tank B, Dekker SK, et al. A comparison of treatment of oral lichen planus with topical tacrolimus and triamcinolone acetonide ointment. Acta Derm Venereol 2006; 86:227-9.
9. Lv XJ. Clinical observation of therapeutic effect of tacrolimus ointment in the treatment of patients with erosive oral lichen planus. Chin Med Herald 2012; 9:141-2.
10. Nie Z, Cai Z, Li ZH. Efficacy evaluation of tacrolimus on oral lichen planus. Chin J Leprosy Skin Dis 2010; 26:491-2.
11. Redfar L, Wild RC, Suresh L. A comparative treatment study of topical tacrolimus and clobetasol in oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 105:187-93.
12. Sonthalia S, Singal A. Comparative efficacy of tacrolimus 0.1% ointment and clobetasol propionate 0.05% ointment in oral lichen planus: a randomized double-blind trial. Int J Dermatol 2012; 51:1371-8.
13. Yang FH, Bai JW, Ren YZ, et al. The efficacy of tacrolimus 0.1% ointment in elderly patients with oral lichen planus. Chin J Geriatr Dent 2010; 8:145-7.
14. Zuo WX, Li XY, Cai JY, et al. A randomized single-blind controlled clinical trial of tacrolimus mouth rinse on erosive oral lichen planus. Shanghai J Stomatol 2013; 22:708-10.
15. Fu H, Zhang Q, Zhou XW. Treatment of chronic erosive oral lichen planus with low concentrations of tacrolimus. J Oral Sci Res 2013; 29:1135-40.
16. Arduino PG, Carbone M, Della Ferrera F, et al. Pimecrolimus vs. tacrolimus for the topical treatment of unresponsive oral erosive lichen planus: a 8-week randomized double-blind controlled study. J Eur Acad Dermatol Venereol 2013; 10:1-8.
17. Jiang LY, Luo G, Yin C. The efficacy of 0.03% tacrolimus in the treatment of erosive oral lichen planus. J Dent Prev Treatment 2009; 17:476-8.
18. Su SW, Cheng Z, Yan JJ. Efficacy evaluation of tacrolimus on erosive oral lichen planus. Strait Phar J 2010; 22:153-4.
19. Thongprasom K, Carrozzo M, Furness S, et al. Interventions for treating oral lichen planus. Cochrane Database Syst Rev 2011; (7):CD001168.
Notice of Correction
In the Notice of Retraction in Vol. 30 Issue 3 page, the article involved should be:
Liu K, Hao J, Xu Y, et al. Breast milk lead and cadmium levels in suburban areas of Nanjing, China. 2013; 28(1):7-15.
Dr Liu takes full responsibility for the retraction of the article.
for publication March 9, 2015.
Tel: 86-10-69157140, E-mail: zhaojizhi@ 126.com
 Chinese Medical Sciences Journal2015年4期
Chinese Medical Sciences Journal2015年4期
- Chinese Medical Sciences Journal的其它文章
- Effect of Ursolic Acid on Breast Cancer Resistance Protein-mediated Transport of Rosuvastatin In Vivo and Vitro△
- Management of Cesarean Scar Pregnancy: A Case Series
- Gender Differences in Ventricular-vascular Coupling Following Exercise△
- Retroperitoneal Versus Transperitoneal Laparoscopic Partial Nephrectomy: A Systematic Review and Meta-analysis△
- Effect of Atorvastatin on Expression of Peroxisome Proliferator-activated Receptor Beta/delta in AngiotensinⅡ-induced Hypertrophic Myocardial Cells In Vitro△
- Influence of Photodynamic Therapy on Apoptosis and Invasion of Human Cholangiocarcinoma QBC939 Cell Line
