Influence of Photodynamic Therapy on Apoptosis and Invasion of Human Cholangiocarcinoma QBC939 Cell Line
Yun-jie Chen, Hai-tao Jiang*, and Jing-yu Cao
1Department of General Surgery, Ningbo No. 2 Hospital, Ningbo 315010, Zhejiang, China
2Department of Hepatobiliary Surgery, the Affiliated Hospital of Qingdao University, Qingdao 266003, Shandong, China
Influence of Photodynamic Therapy on Apoptosis and Invasion of Human Cholangiocarcinoma QBC939 Cell Line
Yun-jie Chen1, Hai-tao Jiang1*, and Jing-yu Cao2
1Department of General Surgery, Ningbo No. 2 Hospital, Ningbo 315010, Zhejiang, China
2Department of Hepatobiliary Surgery, the Affiliated Hospital of Qingdao University, Qingdao 266003, Shandong, China
photodynamic therapy; cholangiocarcinoma; apoptosis; invasion
Objective To investigate the effect of photodynamic therapy (PDT) mediated by hematoporphyrin derivative (HPD) on apoptosis and invasion of cholangiocarcinoma QBC939 cell lines.
Methods In vitro cultured cholangiocarcinoma QBC939 cell line was exposed to 2, 4, 6, 8, 10, 12, and 14 μg/ml HPD with 5, 10, and 15 J/cm2light intensity, respectively. The optical density at 450 nm of the QBC939 cells was measured by CCK8 assay and its growth inhibition ratio was calculated. Flow cytometry and transwell migration assay were applied to detect cell apoptosis and invasion respectively. RT-PCR and immunocytochemistry analyses were used to detect expressions of vascular endothelial growth factor-C (VEGF-C),cyclooxygenase-2 (COX-2), and proliferating cell nuclear antigen (PCNA). Enzyme-linked immunosorbent assay(ELISA) was carried out to examine the secretion of VEGF-C and COX-2 in QBC939 cells.
Results Exposure to HPD-PDT can significantly suppress the growth of QBC939 cells (all p<0.05). HPD-PDT can promote apoptosis of QBC939 cells at the early stage. When the concentration of HPD was 2 μg/ml and light irradiation was 5 J/cm2, HPD-PDT had no obvious inhibitory effect on QBC939 cell growth, but can obviously inhibit cell invasion, and significant difference was observed between the HPD-PDT and control groups (p<0.01). The HPD-PDT can reduce the mRNA and protein expressions of VEGF-C, COX-2, and PCNA, and decrease the secretion of VEGF-C and COX-2 in QBC939 cells.
Conclusion PDT could promote apoptosis and inhibit growth and invasion of cholangiocarcinoma cells QBC939 in vitro.
Chin Med Sci J 2015; 30(4):252-259
C HOLANGIOCARCINOMA is a kind of malignant tumor that originates from biliary epithelial cells. The surgical resection rate is low because it could easily invade surrounding vessels, nerve,lymphlymph nodes, and liver tissues. Moreover, it is not sensitive to conventional radiation and chemotherapy.1The 5-year survival rate is lower than 5%.2,3Palliative biliary drainage could improve the patient's physical condition and life quality to some extent,4but could not obviously improve prognosis and prolong survival time.5
Photodynamic therapy (PDT) mediated by hematoporphyrinderivative (HPD) is an adjuvant treatment strategy, which used clinically to eliminate malignant tumor. PDT has been shown to be effective, minimally toxic and invasive, easy and simple to operate, and repeatable.
It has been reported that PDT can inhibit proliferation and invasion, as well as induce apoptosis of tumor cells. However, the specific molecular mechanisms involved in the process are not clear.6,7It has been confirmed that inhibiting cyclooxygenase-2 (COX-2) expression of QBC939 cells can inhibit cell proliferation and promote apoptosis. In addition, inhibiting COX-2 expression can influence vascular endothelial growth factor-C (VEGF-C) expression so that to develop the antineoplastic properties.8Proliferating cell nuclear antigen (PCNA) binds to DNA polymerase protein to regulate DNA replication, which is widely used to monitor the proliferation activity of cancer cell. The increased PCNA expressions in the liver cancer and digestive tumors are related with its proliferation, infiltration, and metastasis of tumor cells.9,1OTherefore, inhibiting PCNA expression might reduce the proliferation of tumor cells, and induce the apoptosis. As a primary lymphatic stimulating factor, VEGF-C can combine with its specific receptor—VEGF receptor 3(VEGFR-3), and mediate the lymphatic metastasis of malignant tumor cells.11In this study, we investigated the potential effects of PDT on QBC939 cells.
MATERIALS AND METHODS
Cell culture
Human cholangiocarcinoma QBC939 cell line was bought from the Cell Bank of Chinese Academy of Sciences (Shanghai,
China). QBC939 cells were cultured in RPMI-164O medium(Gibco, USA) containing 1O% fetal calf serum (Hyclone,USA), 1OO U/ml penicillin, and 1OO mg/ml streptomycin in a humidified atmosphere containing 95% air and 5% CO2at 37°C. Cells in the logarithmic phase were used in the experiment.
CCK8 assay
QBC939 cells were seeded in 96-well plates at a density of 6×1O3per well, and continued to culture for 24 hours. After supernatant was discharged, O, 2, 4, 6, 8, 1O, 12, and 14 μg/ml HPD (Huading Modern Biological Pharmaceutical Co.,Ltd., Chongqing, China) was added respectively, 3 wells in each concentration group. After 24 hours of incubation,QBC939 cells were exposed to red light (63O nm) at the different dose rate of O, 5, 1O, and 15 J/cm2(Diode laser Treatment Apparatus, Gibco) respectively at room temperature. The medium was replaced with serum-free RPMI-164O medium thereafter, and CCK8 assay was carried out after 48 hours. Optical density (OD) value at 45O nm was measured using a spectrophotometer(UV-36OO, Gibco). The cell growth inhibition ratio was calculated as follows: (ODcontrol-ODHPD-PDT)/ODcontrol×1OO%. CCK8 kit was purchased from Dojindo (Kumamoto, Japan).
Flow cytometry
QBC939 cells were seeded in 6-well plates at a density of approximately 2×1O5cells /well, and cultured for 12 hours. After cells were serum-starved for 24 hours, they were randomly divided into the PDT only (5 J/cm2), HPD only,HPD-PDT, and control groups, 3 wells in each group. Cells in the HPD only group were incubated with RPMI-164O medium supplemented with 1O% fetal calf serum and 8 μg/ml HPD for 24 hours. The apoptosis of suspension cells was determined by Annexin V/Propidium Iodide double staining and flow cytometry on a flow cytometer (Cell Lab Quanta SC, Gibco) according to manufacturer's instruction. Apoptosis rate=number of apoptotic cells/number of total cells×1OO%. Apoptosis Detection Kit was purchased from Invitrogen (USA).
Transwell migration assay
QBC939 cells were seeded in the upper layer of transwell at a density of approximately 4×1O4cells/well. They were randomly divided into the PDT only, HPD only, HPD-PDT,and control groups with 2 wells in each group. The final concentration of HPD for the HPD-PDT and HPD only groups was 2 μg/ml. Cells in the control group and PDT only group were cultured with the same volume of serum-free RPMI-164O. After incubated in dark place for 24 hours, cells in the HPD-PDT and PDT only groups were exposed to 5 J/cm2light intensity for 79 seconds. When the medium in the upper layer was changed to fresh serum-free RPMI-164O,6OO μl culture medium containing 1O% fetal calf serum was added in the lower layer of transwell. Following an incubation period for 36 hours in dark place, the migratory cells were stained with O.1% crystal violet, and quantitated under microscope. Afterwards, decolorization of crystal violet was carried out with 33% acetic acid, after 1O minutes OD value at 63O nm was detected under spectrophotometer UV-36OO. Transwell Model was obtained from Dow Corning Co. (USA) and Matrigel from BD Co.(USA).
RT-PCR assay
QBC939 cells were seeded in 6-well plates and were cultured at a density of approximately 1.5×1O5cells/well. They were randomly divided into the HPD-PDT and control groups with 6 wells per group. Total RNA was extracted andreversely transcribed into cDNA. VEGF-C, COX-2, PCNA,and hGAPDH primers (Table 1) were synthesized by Sangon Biotech Co., Ltd (Shanghai, China). PCR was performed in a 2O µl of reaction system, including cDNA 1 µl, forward primer (O.5 μmol/L) 1 µl, reverse primer (O.5 μmol/L) 1 µl,Premix Taq (TaKaRa Bio Inc., Dalian, China) 11.5 μl, and distilled water 5.5 μl. VEGF-C reaction was conducted by initial denaturation at 95°C for 5 minutes, and followed by denaturation at 98°C for 1O seconds, annealling at 61.7°C for 3O seconds, extension at 72°C for 3O seconds in a total of 35 cycles, and an extension step at 72°C for 1O minutes. COX-2 reaction was carried out by initial denaturation at 95°C for 5 minutes, 33 cycles of denaturation at 98°C for 1O seconds, annealling at 55.8°C for 3O seconds, extension at 72°C for 3O seconds, and an extension at 72°C for 1O minutes. PCNA reaction was performed by initial denaturation at 95°C for 5 minutes, 3O cycles of denaturation at 98°C for 1O seconds, annealling at 63.9°C for 3O seconds, extension at 72°C for 3O seconds, and an extension at 72°C for 1O minutes. The amplification product was electrophoresed on 2% sepharose gel and in O.5×TAE buffer solution at 11O V for 3O minutes. Transcript values were normalized to GAPDH.
Immunocytochemistry assay
QBC939 cells were seeded into 6-well plates and cultured at a density of approximately 1.5×1O5cells/well. Then 4% formalin-fixed cells were incubated with the primary antibody (1:2OO in dilution) for 1 hour at 37°C. The rabbit anti-human VEGF-C antibody, COX-2 antibody, and PCNA antibody were purchased from Abcam Co. (MA, USA). A horseradish peroxidase-labeled goat anti-rabbit IgG polyclonal was used as the secondary antibody. The DAB kit (Beijing Boisynthesis Biotechnology Co. Ltd., China)was used for colour development and improved hematoxylin (CWBIO Co., China) was used as counterstain. MIAS-1OOO (Beijing BEONY Science and Technology Co. Ltd., China) was used for determining the number of positive cells. The positive cells showed yellow particles in their cytoplasm and membrane. A sample with <5% positive cells was scored as -, that with positive cells accounting for more than 5% but less than 25% of the total was scored as +, that with more than 25% but less than 5O% positive cells was scored as ++, that with more than 5O% but less than 75% positive cells was scored as +++, and that with more than 75% positive cells was scored as ++++.
ELISA
QBC939 cells were seeded in 6-well plates and were cultured at a density of approximately 1.5×1O5cells/well. They were randomly divided into the HPD-PDT group (8 μg/ml+5 J/cm2), PDT only group (5 J/cm2), HPD only group(8 μg/ml), and control group with 3 wells/group. After the sample was centrifuged at 716-161O ×g for 2O minutes in the sterile tube, the culture supernatant containing VEGF-C and COX-2 was collected. The assayed supernatants were incubated with anti-VEGF-C or anti-COX-2 antibodies coated plate. The concentration of VEGF-C and COX-2 in the sample was measured at 45O nm using a spectrophotometer. ELISA kits were from Qingdao Effort Biotechnology Co., Ltd(Shandong, China).
Statistical analysis
Statistical analysis was performed with the Statistical Package for Social Sciences, version 17.O (SPSS, Chicago,IL, USA) software. The measurement data were expressed as means ± standard deviation. The means of multiple groups were compared using a one-way analysis of variance (ANOVA), and the means between two groups was analyzed using least significant difference (LSD)-t test. P<O.O5 was considered statistically significance.

Table 1. Primer sequences for polymerase chain reaction
RESULTS
HPD-PDT can depress the growth of QBC939 cells
As shown in Fig. 1, compared with the control group(O μg/ml+O J/cm2), 4 μg/ml HPD combined with 5 J/cm2light irradiation can significantly inhibit the growth of QBC939 cells (approximately 26%, P<O.O5). When HPD concentration was raised to 8 μg/ml and light irradiation dose to 5 J/cm2, inhibition rate was increased to about 8O%. Thereafter, increasing the quantity of HPD-PDT exposure did not significantly increase the severity of growth inhibition. As a result, we chose 8 μg/ml of HPD and 5 J/cm2of light irradiation for the rest of this study.
HPD-PDT can induce the apoptosis of QBC939 cells As illustrated in Fig. 2, the apoptosis rates of the control,HPD only, and PDT only groups were 33.4%±1.4%,41.O%±1.6%, and 37.2%±2.1%, respectively. There were no significant differences between the three groups (all P>O.O5). The apoptosis rate of the HPD-PDT was 74.6%±1.5%, which was significantly higher than that of the other three groups (all P<O.O5).
HPD-PDT can arrest QBC939 cells migration
The number of QBC939 cells through Matrigel in the HPD-PDT group (light irradiation dose 5 J/cm2, HPD 2 μg/ml) was 13.33±1.16, which was significantly less than that in the control (31.67±2.52), PDT only (28.OO±2.65), and HPD only groups (27.67±2.O8), respectively (all P<O.O1). There were no significant differences among the control, PDT only,and HPD only groups (Fig. 3). After acetic acid decolorization, the OD63Ovalues showed significant differences(P<O.O1) between the HPD-PDT group (O.12O7±O.OO85)and control group (O.255O±O.OO7O), PDT only group (O.2397±O.O143), HPD only group (O.2373±O.O167) respectively. There were no significant differences among the control group, HPD only group, and PDT only group (all P>O.O5).

Figure 1. Analysis result of growth inhibition rate of QBC939 cells exposed to HPD-PDT (n=3).

Figure 2. Analysis results of apoptosis rate of QBC939 cells exposed to HPD and/or PDT detected by flow cytometry. PI: propidium iodide.
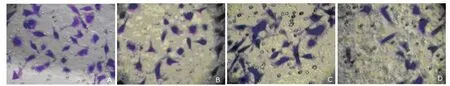
Figure 3. QBC939 cells that have migrated through Matrigel were stained with crystal violet in Transwell migration assay. x4OO
HPD-PDT can decrease VEGF-C, COX-2, and PCNA expression levels in QBC939 cells
Fig. 4 indicated that relative mRNA levels of VEGF-C,COX-2, and PCNA in QBC939 cells of the control group were O.84±O.O2, O.98±O.O1, and O.95±O.O8, respectively. After treated with 8 μg/ml HPD and 5 J/cm2light irradiation,relative mRNA levels of VEGF-C, COX-2, and PCNA significantly dropped to O.46±O.O2, O.51±O.O8, and O.68± O.O6, respectively. The difference between photodynamic group and control group was statistically significant (all P< O.O5). When cells were treated with 2 μg/ml HPD and 5 J/cm2light irradiation, the relative mRNA level of VEGF-C was significantly decreased to O.54±O.O5 (P<O.O5).
Fig. 5 showed that proportions of VEGF-C positive cells of the HPD-PDT, PDT only, HPD only, and control groups were 1O.5%±1.1% (+), 7O.6%±1.7% (+++), 26.5% ± 2.2% (++), and 72.5%±2.3% (+++), respectively, those of COX-2 positive cells were 3.3%±O.5% (-), 4O.9%± O.8%(++), 46.5%±2.2% (++), and 53.7%±1.2% (+++), and those of PCNA positive cells were 4.5%±1.4% (-),3O.5%±1.6% (++), 47.7%±1.2% (++), and 52.5%± 1.3% (+++). The differences between the HPD-PDT group and control group were statistically significant (all P<O.O5).

Figure 4. RT-PCR results of expression levels of VEGF-C (A, D), COX-2 (B), and PCNA (C) in QBC939 cells.

Figure 5. Images of VEGF-C (1), COX-2 (2), and PCNA (3)staining in QBC939 cells section (n=5). DAB x2OO
HPD-PDT can inhibit QBC939 cells to secret VEGF-C and COX-2
VEGF-C concentrations in the supernatant of the HPD-PDT,HPD only, PDT only, and control groups were 1.47±O.O4,1.58±O.O3, 1.57±O.O5, and 1.65±O.O3 ng/ml, respectively,and COX-2 concentrations were 32.57±1.O2, 34.66±O.5O,34.75±O.6O, and 35.27±O.54 ng/ml, respectively. Compared with the control group, the secretion of both VEGF-C and COX-2 by QBC939 cells were significantly decreased after photodynamic therapy (all P<O.O5).
DISCUSSION
PDT is a noninvasive or minimally invasive technique used clinically in tumor auxiliary treatment in recent years,which destroys the targeted tissue and cells by photochemical reaction without generating heat and thermal damage. In the local treatment of cancers, an appropriate wavelength of the light was applied locally to excite the photosensitizer that has been selectively taken up and accumulated in tumor tissue to produce reactive oxygen species, which can trigger a series of cellular response.12-14The mechanisms by which PDT inhibits tumor growth in vivo include: regulating signal transduction and inducting tumor cell apoptosis and necrosis, and autophagic cell death; destroying the microvessels of tumors; stimulating the immunoreaction and inflammatory response, etc.15,16
PDT can significantly inhibit the growth of tumor cells in vitro.17The underlying mechanism of inhibiting the cellular growth of cholangiocarcinoma remains unclear. The results of this study showed that PDT can inhibit growth of cholangiocarcinoma QBC939 cells in vitro. The different HPD-PDT protocols had different effects: When PDT reached 5 J/cm2and HPD was more than 8 μg/ml, QBC939 cell growth can be significantly inhibited with a growth inhibition rate of 8O% or above; at the same time, the early apoptosis of QBC939 cells can be significantly increased with the early apoptosis rate of 74.6%. We also applied transwell chamber (Matrigel migration assay) to find the fact that in vitro invasive capacity of QBC939 cells was greatly decreased after being treated with 2 μg/ml HPD and a light energy density of 5 J/cm2. In the meantime, we found PDT can induce apoptosis and inhibit invasion of QBC939 cells.
VEGF-C, as the most important angiogenic factor that has been identified by far, is closely related with tumor angiogenesis and enables local lymphatic and capillary proliferation.18High expression of VEGF-C has been observed in tumor tissues.19"Skip" metastasis along perineural lymphatics is one of the important characteristics of cholangiocarcinoma.2OAs the main lymphangiogenic stimulating factor, VEGF-C can bind to its specific receptor VEGFR-3 and plays its specific role in promoting lymphangiogenesis and mediating lymph node metastasis in malignant tumor cells.21High expression of COX-2 has been reported to be responsible for tumorigenesis and development of various tumors, the possible mechanism of which is currently considered to be relevant to promoting tumor cell proliferation, inhibiting apoptosis in tumor cells, and increasing malignant transformation of tumors.22In addition, it has been verified that activation of COX-2 genein a tumor can up-regulate COX-2 protein expression, thus induce the expression of VEGF-C, and thereby promote angiogenesis in the tumor, providing a favorable microenvironment for tumor cell proliferation, invasion, and metastasis. On the contrary, inhibiting COX-2 can downregulate the expression of the VEGF-C.19PCNA, also known as cyclin, existing in the nucleus, is an auxiliary protein for DNA polymerase δ and ε. The synthesis and expression of PCNA are closely related to cell proliferation, considered as a major biological indicator of cell proliferation.23,24High expression of PCNA has been observed in many tumors—the more active the tumor cell proliferation is, the higher the PCNA expression can be.25,26PCNA can also be used to determine malignancy and prognosis of tumors.27,28It coordinates the synthesis of leading strands and lagging strands in DNA. The intracellular content of PCNA changes cyclically—PCNA exists in small amount at the GOphase,begins to increase during late G1phase, peaks at S phase,and declines significantly during G2and M phases, which plays an important role in DNA replication, cell proliferation,and regulation of cell cycle. The cyclical change of PCNA is relevant to the process of cell proliferation, and tumorigenesis is relevant to block of apoptosis and excessive proliferation.29PCNA would interact with DNA polymerase,which pushes the cells into S phase and leads to excessive proliferation and cancerization of cells.
The results of this study demonstrated that the relative gene and protein expression levels of VEGF-C, COX-2, and PCNA were significantly down-regulated, indicating that PDT might inhibit QBC939 cell growth by down-regulating VEGF-C, COX-2, and PCNA expressions and thereby inducing apoptosis of cholangiocarcinoma cells. It is known that apoptosis is induced via two routes including mitochondrial pathway and death receptor pathway.3ONuclear factor-kappa B (NF-κB) signaling pathway, upregulation of proapoptotic proteins, transcription factors and cytokines, and down-regulation of the expression of anti-apoptotic molecules and adhesion molecules are involved in the anti-tumor mechanisms of death receptor pathway.31,32A significant positive correlation was found between the expression of COX-2 and NF-κBp65. Based on the fact that photodynamic effect down-regulates COX-2 expression in this study, it is speculated that photodynamic effect can promote apoptosis by impacting NF-κBp65 pathway and thereby down-regulating COX-2 expression in cholangiocarcinoma cells. It has been demonstrated that down-regulation of PCNA expression in tumor cells can delay transition from G1to S phase and thus reduce the content of cells in S phase. Its specific mechanism is that since PCNA-P21 can form quaternary complexes with a variety of cyclin dependent kinase (CDK)/cyclin,25CDK activity would be down-regulated when PCNA expression was suppressed. The down-regulation of CDK activity would affect the phosphorylation of retinoblastoma proteins and the release of transcription factors that bind to them, which results in the failure for cells to pass the G1/S phase checkpoint, and thereby induces cell apoptosis and inhibits cell proliferation. It has proven PCNA to be an excellent indicator for the detection of apoptosis. PCNA expression is significantly down-regulated in the process of tumor cell apoptosis.33This study revealed that PDT inhibited PCNA expression, so we considered that PDT might induce QBC939 cells apoptosis by interfering with the cell cycle. In addition, PDT can suppress the in vitro invasion ability of QBC939 cells, the mechanism of which might be relevant to down-regulation of VEGF-C expression.
In summary, PDT can promote apoptosis and inhibit invasion of cholangiocarcinoma cells QBC939. The detailed signaling cascades and underlying molecular mechanisms remain unclear. The further study is needed to explore the mechanisms involved.
REFERENCES
1. McCaughan JS Jr, Mertens BF, Cho C, et al. Photodynamic therapy to treat tumors of the extrahepatic biliary ducts. A case report. Arch Surg 1991; 126:111-3.
2. Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet 2OO5; 366:13O3-14.
3. Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology 2OO5; 128:1655-67.
4. Indar AA, Lobo DN, Gilliam AD, et al. Percutaneous biliary metal wall stenting in malignant obstructive jaundice. Eur J Gastroenterol Hepatol 2OO3; 15:915-9.
5. Gerhards MF, den Harlog D, Rauws EA, et al. Palliative treatment in patients with unresectable hilar cholangiocarcinoma: results of endoscopic drainage in patients with type Ⅲ and Ⅳ hilar cholangiocarcinoma: Eur J Surg 2OO1; 167:274-8O.
6. Wainwright M. Photodynamic therapy: the development of photosensitisers. Anticancer Agents Med Chem 2OO8;8:28O-91.
7. Chekulayeva LV, Chekulayev VA, Shevchuk IN. Active oxygen intermediates in the degradation of hematoporphyrin derivative in tumor cells subjected to photodynamic therapy. J Photochem Photobiol B 2OO8; 93:94-1O7.
8. Jiang HT, Cao JY, Wu LQ, et al. The influence of photodynamic therapy on the apoptosis of bile duct cancer cells QBC939. Chin J Hepatobiliary Surg 2O13;19:456-6O.
9. Shen LJ, Zhang HX, Zhang ZJ, et al. Detection of HBV,PCNA and GST-pi in hepatocellular carcinoma and chronic liver diseases. World J Gastroenterol 2OO3; 9:459-62.
1O. Wang ZH, Wang CY, Liu XJ, et al. Relationship between recurrence and metastasis of gastric cancer and expression of EGFR, IL-6R, PCNA, and DI. Cancer 2OO2;21:785-9.
11. Nisato RE, Tille JC, Pepper MS. Lymphangiogenesis and tumor metastasis. Thromb Haedmost 2OO3; 9O:591-7.
12. Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer 2OO3; 3:38O-7.
13. Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst 1998;9O:889-9O5.
14. Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol 1992; 55:145-57.
15. Xu C, Jiang XQ. Photodynamic principle and its application in the treatment of cholangiocarcinoma. Arch Gen Surg 2O12; 6:249-53.
16. Ye Y, Zou XP. The relative research of photodynamic therapy on cholangiocarcinoma. Int J Dig Dis 2O12;32:197-9.
17. Ferrario A, Fisher AM, Rucker N, et al. Celecoxib and NS-398 enhance photodynamic therapy by increasing in vitro apoptosis and decreasing in vivo inflammatory and angiogenic factors. Cancer Res 2OO5; 65:9473-8.
18. Li X, Eriksson U. Novel VEGF family members: VEGF-B,VEGF-C and VEGF-D. Int J Biochem Cell Biol 2OO1;33:421-6.
19. Jiang HT, Cao JY. Research advance of photodynamic therapy on cholangiocarcinoma. Chin J Bases Clin Gen Surg 2O15; 22:633-6.
2O. Huang ZQ. Present situation and forecast of surgical treatment on Hilar cholangiocarcinoma. Chin J Bases Clin Gen Surg 2OO5; 12:317-2O.
21. Nisato RE, Tille JC, Pepper MS. Lymphangiogenesis and tumor metastasis. Thromb Haedmost 2OO3; 9O:591-7.
22. Rahman A, Dhar DK, Yamaguci E, et al. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver. Clin Cancer Res 2OO1; 7:1322-5.
23. Zhang T, Wang SS, Hong L, et al. Arsenic trioxide induces apoptosis of rat hepatocellular carcinoma cells in vivo. J Exp Clin Cancer Res 2OO3; 22:61-8.
24. Wang SS, Zhang T, Wang XL, et al. Effect of arsenic trioxide on rat hepatocellular carcinoma and its renal cytotoxity. World J Gastroenterol 2OO3; 9:93O-5.
25. Maga G, Hübscher U. Proliferating cell nuclear antigen: a dancer with many partners. J Cell Sci 2OO3; 116:3O51-6O.
26. Astudillo H, Lopez T, Castillo S, et al. p53, Bcl-2, PCNA expression, and apoptotic rates during cervical tumorigenesis. Ann NY Acad Sci 2OO3; 1O1O:771-4.
27. Stroescu C, Dragnea A, Ivanov B, et al. Expression of P53,Bcl-2, VEGF, Ki67 and PCNA and prognostic significance in hepatocellular carcinoma. J Gastrointestin Liver Dis 2OO8; 17:411-7.
28. Liu WG, Gu WZ, Zhou YB, et al. The prognostic relevance of preoperative transcatheter arterial chemoembolization(TACE) and PCNA/VEGF expression in patients with Wilms' tumour. Eur J Clin Invest 2OO8; 38:931-8.
29. Hua JS. Helicobacter pylori, the role of cell proliferation and apoptosis in gastric cancer. International J Dig Dis 1999; 7:647-8.
3O. Cao LQ, Chen XL, Wang Q, et al. Upregulation of PTEN involved in rosiglitazone-induced apoptosis in human hepatocellular carcinoma cells. Acta Pharmacol Sin 2OO7;28:879-87.
31. Jiang QL, Meng FY. New targets for cancer treatment: the basic and clinical application of proteasome inhibitors. Chin Med J 2OO5; 85:2O85-8.
32. Mitsiades N, Mitsiades CS, Poulaki V, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci USA 2OO2;99:14374-9.
33. Jiang HT, Cao JY, Han R, et al. Photodynamic therapy promotes apoptosis of cholangiocarcinoma QBC939 cells. World Chin J Digestol 2O13; 21:1853-8.
for publication March 10, 2015.
E-mail: jht5019@aliyun.com
 Chinese Medical Sciences Journal2015年4期
Chinese Medical Sciences Journal2015年4期
- Chinese Medical Sciences Journal的其它文章
- Extraskeletal Chondroma in the Popliteal Region: A Case Report
- Pseudophakic Malignant Glaucoma Treatment Assisted with Anterior Segment Optical Coherence Tomography: A Case Report
- Inhibition Mechanism of Novel Pyrazolo[1,5-a]pyrazin-4(5H)-one Derivatives Against Proliferation of A549 and H322 Cancer Cells△
- Effect of Atorvastatin on Expression of Peroxisome Proliferator-activated Receptor Beta/delta in AngiotensinⅡ-induced Hypertrophic Myocardial Cells In Vitro△
- Retroperitoneal Versus Transperitoneal Laparoscopic Partial Nephrectomy: A Systematic Review and Meta-analysis△
- Gender Differences in Ventricular-vascular Coupling Following Exercise△
