In Vitro Propagation of Ardisia mamillata Hance
Bihua CHEN,Juan ZHANG,Zhuoxi WU,Huihua FAN,Qianzhen Ll
1.Fujian Academy of Forestry Sciences,Fuzhou 350012,China;
2.Key Laboratory of Timber Forest Breeding and Cultivation for Mountainous Areas in Southern China,China Forestry Bureau,Fuzhou 350012,China
In Vitro Propagation of Ardisia mamillata Hance
Bihua CHEN1,2*,Juan ZHANG1,2,Zhuoxi WU1,2,Huihua FAN1,2,Qianzhen Ll1,2
1.Fujian Academy of Forestry Sciences,Fuzhou 350012,China;
2.Key Laboratory of Timber Forest Breeding and Cultivation for Mountainous Areas in Southern China,China Forestry Bureau,Fuzhou 350012,China
Ardisia mamillata Hance is a rare plant with highly ornamental and medic-inal value.The traditional propagation methods for A.mamillata by seeds or cutting provided low proliferation rate.This study is to optimize the propagation technique of A.mamillata by tissue culture and set up an industrial production system to provide plenty of A.mamillata seedlings for the human demand.The optimal initiation medi-um for A.mamillata is MS+2.0 mg/L BA+0.1 mg/L NAA+30 g/L sugar,providing 76.4%initiation rate.The optimal shoot proliferation medium for A.mamillata is MS+ 1.0 mg/L BA+0.1 mg/L NAA+30 g/L sugar,providing 4.56 fold proliferation rate and 3.10 cm shoot in height.The optimal shoot elongation medium for A.mamillata is MS+0.5 mg/L BA+0.1 mg/L NAA+30 g/L sugar,providing 2.77 fold proliferation rate and 4.27 cm shoot in height.The optimal rooting medium for A.mamillata is 1/2MS+ 0.1 mg/L IBA+15 g/L sugar,providing 99.7%rooting rate,4.0 roots per individual,7.53 cm root in length and 3.94 cm shoot in height.This provides a reliable mass propagation method for A.mamillata.
Ardisia mamillata;In vitro;Propagation;Medium;Red-core soil
A rdisia mamillata Hance belongs to genus Ardisia,family Myrsi-naceae.It is a rare plant with highly ornamentallandscape and medicinal value(Zhong et al.,2008).It has lovely white or pink flowers,and round red fruits lasting for more than 9 months.Previous studies on the ex-tracts of alkaloid,polysaccharide,triterpenoid saponins and bergenin from A.mamillata proved its medicinal value (Huang et al.,2000;Huang et al.,2003;Kobayashi& De Mejía,2005;Ling&Zeng,2007a;Ling& Zeng,2007b;Luo et al.,2008).
The traditional propagation meth-ods for A.mamillata by seeds or cut-ting provided low proliferation rate.Al-though few studies on tissue culture of A.mamillata have been reported,mass propagation of this species is not realized.This study is to optimize the propagation technique of A.mamillata by tissue culture and set up an indus-trialproduction system to provide plenty of A.mamillata seedlings for the human demand.The excellent individ-uals of A.mamillata were selected to study the tissue culture.
Materials and Methods
New buds from the elite individual A.mamillata were used as the plant materials.The apical buds and hemi-lignified stems were adopted as the explants.
Surface sterilization
The stems were initially washed and immersed in the washing power liquid for 15 minutes,then cleaned un-der running tap water for 10 min,and cut into pieces with 2-3 nodes each,immersed in 70%ethanol for 60 sec-onds under aseptic condition in a lami-nar flow machine,rinsed in sterilized water once,transferred to 0.1%HgCl2supplemented with dishwashing deter-gent for 15 min,and then rinsed in sterilized water four to five times.The explants were cut into two ends with one node each (1.0-1.5 cm length)and then transferred onto shoot initia-tion medium.
Culture conditions
The explants were incubated in dark for the initiation.The illumination intensity was 1 000-1 500 lux for shoot proliferation and root induction,and 3 000-6 000 lux for plantlet hardening provided with 12 h light photoperiod. All the cultures were maintained in room temperature of(24±2)℃.
Experimental design
Explant initiation The explant initi-ation medium comprised:(1)MS+2.0 mg/L BA+0.1 mg/L NAA;(2)1/2MS+ 2.0 mg/L BA+0.1 mg/L NAA.All me-dia contained 30 g/L sugar and 6.0 g/L carrageenan (produced in Quanzhou,Fujian,China),pH 6.0.There were 20 jars for each medium and 3 replica-tions for the same experiment.One bud or shoot occupied one jar respec-tively.The data of initiation frequency were recorded after 30-day incubation.
For surface sterilization,100 ml 0.1%HgCl2was supplemented with 2-3 drops of dishwashing detergent.
Shoot multiplication The shoot proliferation medium comprised:(3)MS+1.0 mg/L BA+0.1 mg/L NAA;(4)1/2MS+1.0 mg/L BA+0.1 mg/L NAA;(5)MS+0.5 mg/L BA+0.1 mg/L NAA;(6)1/2MS+0.5 mg/L BA+0.1 mg/L NAA.All media contained 30 g/L sugar and 6.0 g/L carrageenan,pH 6.0. There were 20 jars(3 shoots per jar)for each medium and 3 replications for the same experiment.The data of pro-liferation rate and shoot height were recorded after 30-day incubation.
Root induction The rooting medium comprised:(7)1/2MS+0.1 mg/L IBA;(8)1/2MS+0.25 mg/L IBA;(9)1/2MS+ 0.5 mg/L IBA;(10)1/2MS+1.0 mg/L IBA.All media contained 15 g/L sugar and 7.0 g/L carrageenan,pH 6.0. There were 20 jars (3 shoots per jar)for each medium and 3 replications for the same experiment.
Plantlet hardening After 45-day cul-ture in rooting medium in culture room,the plantlets with the bottles weretransferred into the glass greenhouse for 15-day hardening.Rooting percent-age,root number per individual,root length and plantlet height were rec-orded at the end of 15-day hardening.
Plantlet transplant The red-core soil(natural local soil)was adopted as the transplant medium.The soil or media was put into the quadrate plas-tic containers with drain holes,placed in the greenhouse,and sterilized by 0.03%-0.05%KMnO4.The hard-ened plantlets were rinsed by tap waterto eliminate the remains of carrageenan.The plantlets were im-mersedin1/1000dilutionof 70%Thio-phanate methyl or 1/1 000 dilution of 80%Mancozeb solution for 10 min,then transplanted into the soil or media and covered with a transparent plastic film to maintain high humidity.The plantlets were sprayed with water once everyday.The film was disclosed after 4 weeks and the spray frequency in-creased to 2-3 times per day.
Statistical analysis Data were pro-cessed statistically with SPSS 17.0 software.Data were performed by analysis of variance(ANOVA)(for 3-6 means)or t-test(for 2 means),with a post-hoc Tukey's test if the ANOVA was significant.Means were provided with standard errors,and means were considered significantly different at P<0.05.
Results
Explant initiation medium
The results of explant initiation were shown on Table 1.The results showed no significant difference be-tween medium No.1 and No.2.New shoots in medium No.1 appeared vig-orous shoots and rapid growth,but new shoots in medium No.2 grew slowly. Therefore,medium No.1:MS+2.0 mg/L BA+0.1 mg/L NAA was optimal for explant initiation of A.mamillata.
Proliferation medium
On a laminar flow machine,the elongating shoots were excised and placed on the different multiplication media for three successive subcul-tures in same medium.The results(Table 2)showed that there were sig-nificant differences between the con-centrations of BA or basic media on the aspects of proliferation rate and shoot height.The proliferation rates on medium No.3 and No.4 were the high-est of all,namely 4.56(a)and 3.47(b),respectively,which indicated that 1 mg/L was the best for proliferation. The shoot heights on medium No.3 and No.5 were the highest of all,namely,3.10 cm (b)and 4.27 cm(a),respectively,which indicated that MS medium was the best for proliferation. The shoot heights on medium No.4 and No.6 were not enough for prepar-ing rooting material;the proliferation rate on medium No.6 was not enough for mass propagation. Therefore,medium No.3:MS+1.0 mg/L BA+0.1 mg/L NAA was optimal for shoot pro-liferation of A.mamillata at the begin-ning;and medium No.5:MS+0.5 mg/L BA+0.1 mg/L NAA was opti-mal for shoot elongation of A.mamillata(Fig.1).
Rooting medium
The elongated shoots were ex-cised and placed on rooting media. After45-day incubation in culture room,the plantlets with bottles were transferred into the glass greenhouse for 15-day hardening.The roots were recorded and analyzed in Table 3.The results showed that the average root-ing rate,average root number per indi-vidual and average shoot height were highest on medium No.7 which provid-ed 99.7%(a)rooting rate,4.00(a)roots per individual and 3.94 (a)cm shoots in length,respectively.Although the average root length was only 7.53(b)cm,it was eligible for transplant.The rooting rate was the most important factor on rooting test.The average rooting rates on medium No.8,9 and 10 were among 72.2%-80.0%,which were less than that of medium No.7. Therefore,only medium No.7:0.1 mg/L IBA (with three “a”level)was optimal to be the rooting medium for A. mamillata(Fig.2 and 3).
Plantlet transplant
After hardening,the plantlets were rinsed by tap water to eliminate the remains of carrageenan,and trans-ferred to the soil in a greenhouse.The soil was sterilized by KMnO4three days prior to use.After transferring to soil or light media,the plantlets were immediately covered with a transpar-ent plastic film upon arched racks. The plantlets were sprayed 1/1 000 dilution of 10% Difenoconazole or 1/800 dilution of 70%Thiophanate methyl to control the diseases every week.The data of survival rate were recorded after 60 days.The plantlets grew well in red core soil,providing 97%survival rate (data not shown). Therefore,the red core soil was opti-mal for A.mamillata plantlets conver-sion.

Table 1 The effect of different basal media on explant initiation of A.mamillata
Conclusions and Discus-sion
The optimal initiation medium for A.mamillata is MS+2.0 mg/L BA+0.1 mg/L NAA+30 g/L sugar,providing 76.4%initiation rate.Luo et al.(2004),and Jiang&Deng (2001)applied MS as the basal medium whereas Lu(2002)used 1/2MS or B5 for explant initiation.Luo et al.(2004)also adopt-ed BA and NAA,but the concentration was higher than that of this experi-ment.The full-strength inorganic MS benefited to the new shoot growth of A.mamillata,but 1/2MS caused new shoot growing slowly.
The optimalshootproliferation medium for A.mamillata is MS+1.0 mg/L BA+0.1 mg/L NAA+30 g/L sugar,providing 4.56 fold proliferation rate and 3.10 cm shoot in height.The optimal shoot elongation medium for A.mamillata is MS+0.5 mg/L BA+0.1 mg/L NAA+30 g/L sugar,providing 2.77 fold proliferation rate and 4.27 cm shoot in height.Luo et al.(2004)and Jiang&Deng (2001)applied MS as the basal medium for shoot prolifera-tion,but the types and concentrationsof plant growth regulators were differ-ent.The duration for one generation was at least 90 days(Luo et al.,2004),which was much more than that of this experiment,30 days.Du et al.(2007)used MS supplemented with 0.4 mg/L CPPU (forchlorfenuron)and 0.2 mg/L NAA as multiplication medium,but the CPPU was more expensive and not easy to obtain.Lu (2002)adopted 1/ 2MS as the basal medium which was inferior to MS in this experiment.
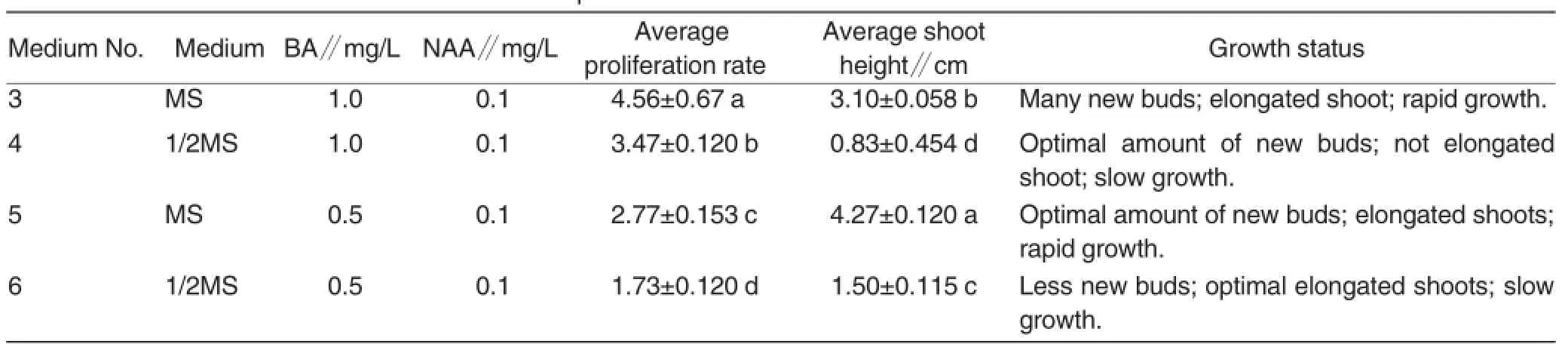
Table 2 The effect of different media on shoot multiplication of A.mamillata
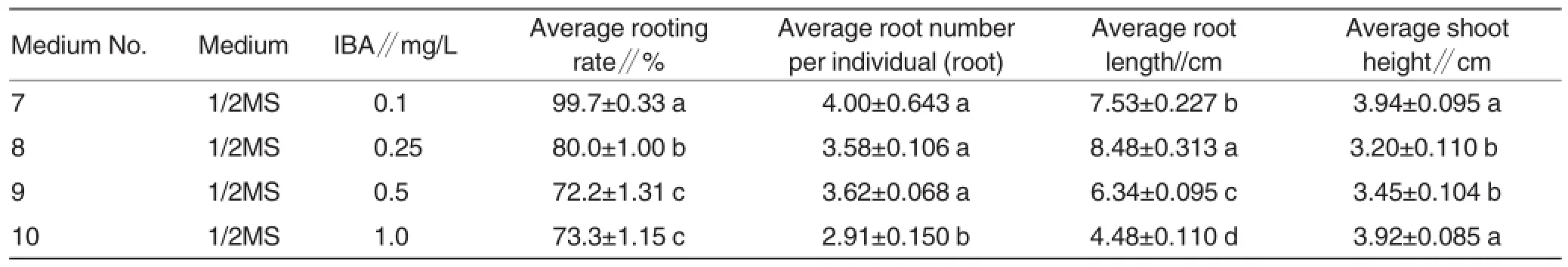
Table 3 The effect of different media on root induction of A.mamillata
The optimal rooting medium for A.mamillata is 1/2MS+0.1 mg/L IBA+ 15 g/L sugar,providing 99.7%rooting rate,4.0 roots per individual,7.53 cm root in length and 3.94 cm shoot in height.Luo et al.(2004)applied MS as the basal medium for rooting,provid-ing 80%rooting rate,which is lower than that of this experiment,indicating MS is not optimal for rooting medium.References
[1]DU M,LI Y,TIAN L,et al.Establishment of high-frequency regeneration system for stem apexes of Ardisia mamillata Hance[J].Journal of Northeast Forestry University,2007,35(3):21-22,30.
[2]HUANG J,ZHANG H,SHIMIZU N,et al. Ardisima millosides G and H,two new triterpenoid saponins from Ardisia mamillata[J].Chemical and Pharma-ceutical Bulletin,2003,51(7):875-877.
[3]HUANG J,OGIHARAY,ZHANG H,et al.Triterpenoid saponins from Ardisia mamillata[J].Phytochemistry,2000,54(8):817-822.
[4]JIANG X,DENG X.Tissue culture and plantlet regeneration of Ardisia mamillata[J].Jiangxi Forestry Science and Technology,2001(6):3,11.
[5]KOBAYASHI H,DE MEJIA E.The genus Ardisia:a novel source of healthpromoting compounds and phytophar-maceuticals[J].Journal of Ethnophar-macology,2005,96(3):347-354.
[6]LING YZ,ZENG MZ.Extraction,frac-tionation and structure identification of the alkaloids from Ardisia mamillata[J]. Fine Chemicals,2007,24(7):667-670.
[7]LING YZ,ZENG MZ.Isolation,purifica-tion and property study of polysaccha-ride from Ardisia mamillata[J].Chinese Journal of Analysis Laboratory,2007,26(4):93-96.
[8]LU Q.Study on biological characteristics and tissue culture of Ardisia mamillata[J].Jiangxi Forestry Science and Tech-nology,2002(1):5-6.
[9]LUO J,CHENG Z,LONG C.Tissue cul-tureof Ardisia mamillata[J].Plant Physi-ology Communications,2004,40(4):465.
[10]LUO M,LUO Y,MIN B.Comparison of bergenin content in cultivated and wild Ardisia mamillata Hance[J].Journal of Anhui Agricultural Sciences,2008,36(10):4136-4137.
[11]ZENG Y.Advances in study of re-search on Ardisia mamillata Hance tis-sue culture[J].Northern Horticulture,2009(8):155-157.
[12]ZHONG J,YE M,ZHUANG P,et al.A review of Ardisia mamillata Hance[J]. Northern Horticulture,2008(5):65-69.
Responsible editor:Nanling WANG
Responsible proofreader:Xiaoyan WU
Supported by Fujian Modern Agriculture Project:The Innovation and Industrialization Techniques of Dominant Woody Flowering Plants(No.:Min Lin Ji Cai[2012]137).
*Corresponding author.E-mail:chenbihua@hotmail.com
Received:May 29,2015 Accepted:September 13,2015
 Agricultural Science & Technology2015年10期
Agricultural Science & Technology2015年10期
- Agricultural Science & Technology的其它文章
- Effects of Different Light Emitting Diodes on Growth and Quality of Lettuce
- Analysis on Carotenoids Content and Other Quality Traits of 185 Wheat Varieties
- Extraction and Structure Elucidation of Phenols from Dendrobium thyrsiflorum
- lnfluences of Different Habitats on Asexual Propagation of Wild Gastrodia elata f.glauca being Domesticated in Ganzi
- Breeding and Evaluation of New lron Yam(Dioscorea opposita Thunb)Varieties
- Evaluation on ldeal Test Sites and Regional Characteristics of Cotton Fiber Quality in Jiangsu Province
