Enhanced performance of g-C3N4/TiO2 photocatalysts for degradation of organic pollutants under visible light☆
Gaixue Song ,Zhenyu Chu ,Wanqin Jin ,*,Hongqi Sun *
1 State Key Laboratory of Materials-Oriented Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
2 Department of Chemical Engineering,Curtin University,Perth WA6845,Australia
Keywords:Carbon nitride Titanium dioxide Composite Photodegradation Sonication
ABSTRACT Photocatalytic degradation is one of the most promising remediation technologies in terms of advanced oxidation processes(AOPs)for water treatment.In this study,novel graphitic carbon nitride/titanium dioxide(g-C3N4/TiO2)composites were synthesized by a facile sonication method.The physicochemical properties of the photocatalyst with different mass ratios ofg-C3N4 to TiO2 were investigated by X-ray diffraction(XRD),scanning electron microscope(SEM),transmission electron microscopy(TEM),N2 sorption,Fourier transform infrared spectroscopy(FT-IR),X-ray photoelectron spectroscopy(XPS),and UV–vis DRS.The photocatalytic performances were evaluated by degradation of methylene blue.It was found thatg-C3N4/TiO2 with a mass ratio of1.5:1 exhibited the best degradation performance.Under UV,the degradation rate of g-C3N4/TiO2 was 6.92 and 2.65 times higher than g-C3N4 and TiO2,respectively.While under visible light,the enhancement factors became 9.27(to g-C3N4)and 7.03(to TiO2).The improved photocatalytic activity was ascribed to the interfacial charge transfer between g-C3N4 and TiO2.This work suggests that hybridization can produce promising solar materials for environmental remediation.
1.Introduction
Over the last few decades,the semiconductor-based photocatalysis has emerged as an environmentally benign technology for wastewater treatment due to its destructive ability towards a wide range of inorganic and organic pollutants[1].The efficiency of photocatalysis is dramatically dependent on the generation and separation of the photoinduced electron/hole pairs,which are mainly the intrinsic nature of the photocatalyst materials[2].Many investigations have been implemented on the development of photocatalyst materials,such as metal oxides,sulfides and nitrides,among which titanium dioxide(TiO2)has demonstrated to be the most popular photocatalyst for potential commercialization[2–5].TiO2,an n-type semiconductor,has been then widely used owing to the high efficiency,low cost,non-toxicity and long-term stability[6–9].However,the large band gap energy of3.2 eV of TiO2(anatase)and the high recombination rate of electron–hole pairs have hindered its wide applications.Many studies have been carried out to improve the visible light absorption and/or separation rate of photoinduced carriers by means of metal or non-metal doping,dye-sensitizing,coupling,surface modification,and semiconductor-coupling[10].
Recently,a novel metal-free photocatalyst,graphite-like carbon nitride(g-C3N4)with high thermal and chemical stability together with a moderate band gap energy of2.7–2.8 eV has demonstrated to be effective for solar energy conversion[11–15].The delocalized conjugated structure of g-C3N4offers a relatively slow charge recombination rate and a rapid photoinduced charge separation in the electron transfer process,making this materiala sensitizer candidate for the design of efficient visible-light-driven photocatalyst[16].However,due to the low quantum efficiency and high recombination rate of photogenerated electron–hole pairs[17],photocatalytic efficiency of pure g-C3N4is rather low.Moreover,photoreduction capability of g-C3N4is strongly facilitated by the high redox potential of−1.3 V[vs.normal hydrogen electrode(NHE)at pH=7,compared to−0.5 V of TiO2].On the other hand,the top energy level of valence band(VB)of g-C3N4is 1.4 V(vs.NHE at pH=7,compared to 2.7 V of TiO2),resulting in a weak thermodynamical force(oxidative capability)for water oxidation and not enough for producing hydroxyl radicals[18].As a result,pristine g-C3N4has been rarely used for photodegradation of organic pollutants in water[18–20].
It is expected that coupling TiO2with g-C3N4would be able to significantly improve the visible light absorption,carriers'separation,and capability for photooxidation by the created interfaces.Liu et al.[21]prepared g-C3N4/ZnO hybrid photocatalyst with a higher activity in Rhodamine B(RhB)degradation than either a single phase of ZnO or g-C3N4.Ge et al.[22]synthesized PANI:g-C3N4by “in situ”deposition of oxidative polymerization of aniline monomer in the presence of g-C3N4powder.The PANI-g-C3N4composites showed significantly enhanced photocatalytic activities in the MB degradation.Ge et al.[23]also prepared MoS2-g-C3N4via a facile impregnation method,and MoS2-g-C3N4samples demonstrated improved photocatalytic H2evolution under visible light irradiations.The 0.5%(by mass)MoS2-g-C3N4exhibited the highest H2evolution rate of 23.10 μmol·h−1,which was about 11.3 times higher than that of pure g-C3N4.Tian et al.[24]reported the synthesis of g-C3N4-Bi2WO6heterojunctions,and found that the photocatalytic activity in degradation of methyl orange(MO)was almost 3 and 155 times higher than individual C3N4and Bi2WO6.Composites of g-C3N4/Ag3VO4were prepared by anchoring Ag3VO4to g-C3N4,and showed higher activity for basic fuchsin degradation than either Ag3VO4or g-C3N4[25].He et al.[26]reported a novel Z-scheme type MoO3-g-C3N4,which showed an activity in MO degradation 10.4 times higher than g-C3N4.WO3and Co3O4were also applied to couple with g-C3N4for enhanced photocatalytic activities in degradation of organic pollutants[19,20,27].For employing the promising properties of TiO2,the heterojunctions of g-C3N4/TiO2have attracted particular interests.Chai et al.[28]observed an enhanced hydrogen production owing to the synergistic effects by coupling g-C3N4to Pt-TiO2,such as enhanced light harvesting,improved photostability and efficient photo excited charge separation.Miranda et al.[29]reported that coupling of TiO2and g-C3N4can increase the photocatalytic degradation of phenol due to the improved electron–hole separation which results from the appropriate band structures of the composite photocatalyst.Kondo et al.[30]developed a highly efficient sulfur-doped TiO2hybridized with g-C3N4,on which both visible light response and photocatalytic activity in decomposition of acetaldehyde were improved.The catalytic activity of the hybrid was 4 times higher than sulfurdoped TiO2.
In this study,hydrothermal-induced TiO2was hybridized with g-C3N4by a simple sonication procedure with different g-C3N4to TiO2mass ratios.The photocatalysts were investigated for photodegradation of MB under UV and visible light,respectively.When g-C3N4:TiO2was 1.5,the photocatalytic activity in MB degradation was the highest among all the photocatalysts under both UV and visible light irradiations.The synergistic effects of the g-C3N4/TiO2composites and the enhancements of photocatalytic capacity in the MB degradation process were discussed.
2.Experimental Methods
2.1.Preparation of g-C3N4,TiO2 and g-C3N4/TiO2 hybrid photocatalyst
The polymeric carbon nitride was prepared by a simple thermal condensation method.In a typical synthesis,2 g melamine was put into an alumina crucible with a cover in order to prevent sublimation of melamine[31],then heated to 520°C for 4 h in a muffle furnace in air.The synthetic orange-yellow g-C3N4was ground into fine powders and collected for use without further treatment.
TiO2nanoparticles were prepared by hydrolysis of titanium tetrachloride(TiCl4)via a hydrothermal route.3.5 ml TiCl4was firstly added into 40 ml absolute ethyl alcohol.Afterwards,the obtained mixture was placed in a Te flon-lined autoclave.The hydrothermal treatment was performed at 120°C for 20 h.The produced precipitate was then filtered,washed repeatedly and dried at80°C for 6 h.The obtained TiO2was ground and collected for further use.
The fabrication process of g-C3N4/TiO2photocatalysts with various mass ratios(mg-C3N4:mTiO2=1.1,1.3,1.5,1.7 and 1.9,resulting composites were denoted as g-C3N4/TiO2-1.1,−1.3,−1.5,−1.7 and −1.9,respectively.)was described as follows:certain amount of g-C3N4and TiO2were dispersed in absolute ethyl alcohol by vigorously stir for 4 h,followed by a sonication for 60 min at room temperature.After that,the product was collected and dried in an oven at 70°C for 24 h,and then cooled down to room temperature.
2.2.Characterizations
X-ray diffraction(XRD)was performed on a Rigaku,Mini flex600 X-ray diffractometer,using a Cu Kαradiation(0.15406 nm).The diffraction patterns were recorded from 2θ 10 to 80°.Fourier transform infrared spectra(FT-IR)were recorded on a Nicolet 8700 FT-IR spectrometer using a KBr method.BET surface area and porosity measurements were carried out by N2sorption using an ASAP2020 instrument after degassing at 350 °C in vacuum.The UV–visible diffuse reflectance spectra(UV–vis DRS)were obtained on a Perkin Elmer Lambda 950 UV–vis spectrometer equipped with an integrating sphere assembly using BaSO4as the reference sample.The morphologies of samples were investigated by field emission scanning electron microscopy(FESEM S-4800).The samples were dispersed in ethanol using an ultrasonicator and dropped on a copper grid.X-ray photoelectron spectra(XPS)were acquired on Thermo ESCALAB 250 with hemispherical energy analyzer(SPECS)equipped with Al,Ka radiation in the fixed analyzer transmission mode.All peaks in XPS spectra were calibrated by C1s peak at 284.8 eV.
2.3.Photodegradation of methylene blue(MB)
Methylene blue(MB)aqueous solution was used as a modelpollutant to evaluate the photocatalytic efficiencies of the samples.The photocatalytic degradation of MB was carried out in a 250 ml reactor at room temperature.The solution volume was 100 ml,the initial concentration of MB solution was 20 mg·L−1,and the photocatalyst amount was 1 g·L−1.The irradiations were provided by two light sources,UV lamp(2 mW·cm−2)and a high pressure Xenon short arc lamp(22.5 mW·cm−2)with a filter(λ≥400 nm).The catalyst was magnetically stirred in the dark for reaching the adsorption equilibrium before commencing photocatalytic reaction.After the photocatalytic reaction was initiated by switching on the lamp,MB samples were withdrawn at certain intervals and were filtered for analysis.The solution concentration of MB was determined by a UV–vis spectrophotometer(Perkin Elmer Lambda 950).
3.Results and Discussion
3.1.XRD studies

Fig.1.XRD patterns of pure TiO2,g-C3N4 and g-C3N4/TiO2 composites.
The XRD patterns of TiO2,g-C3N4and g-C3N4/TiO2composites are shown in Fig.1.Two peaks were found from the diffraction pattern of the pristine g-C3N4,indicating a typical graphitic structure without any impurity phase[11].The strong peak at 27.5°represents stacking of conjugated aromatic system,which is indexed to graphitic materials as the(002)crystal plan[32].The weak peak at12.9°is indexed as(100)associated with interlayer.The peaks of pure TiO2at 25.3,37.8,48.0,53.9,55.1,and 62.7°correspond to the(101),(004),(200),(105),(211)and(204)crystal planes of anatase TiO2(JCPDS 21–1272)[33].The crystal size of TiO2particles was estimated to be 14.2 nm according to the Scherrer equation[8].The g-C3N4characteristic peaks were found in allg-C3N4/TiO2photocatalysts.The positions and shapes of characteristic TiO2peaks of g-C3N4/TiO2did not significantly change compared with pure TiO2,indicating that coupling with g-C3N4did not influence lattice structure of TiO2,which might be beneficial for the photocatalytic activity of hybrid photocatalyst.
3.2.SEM and TEM imaging
The morphologies of pure TiO2,pristine g-C3N4and g-C3N4/TiO2composites were investigated by FESEM,and the images of TiO2,g-C3N4and g-C3N4/TiO2-1.5 were shown in Fig.2.Pristine g-C3N4presents a bulk structure with sheet-like sub-blocks.Pure TiO2presents spherical-like particles that clearly agglomerate and show a particle size around 50 nm.Fig.2(c)and(d)show the images of g-C3N4/TiO2-1.5.It can be seen that TiO2particles disperse on the surface of g-C3N4.Before SEM imaging,all samples underwent the ultrasonic treatment for 1 h.The still firm connection between TiO2and g-C3N4displayed in the g-C3N4/TiO2composites indicated that there would be an intense interaction between g-C3N4and TiO2rather than a simply physical adsorption.Compared with Fig.2(a)and(b),it also deduced that g-C3N4could inhibit the aggregation of TiO2nanoparticles.The good dispersion can close interfacial connections between g-C3N4and TiO2nanoparticles and improve the electron–hole separation efficiency.Similar results were observed on other g-C3N4/TiO2composites shown in Fig.3.

Fig.2.SEM images of(a)pure g-C3N4,(b)pure TiO2,(c)g-C3N4/TiO2-1.5 and(d)the larger magnification of g-C3N4/TiO2-1.5.
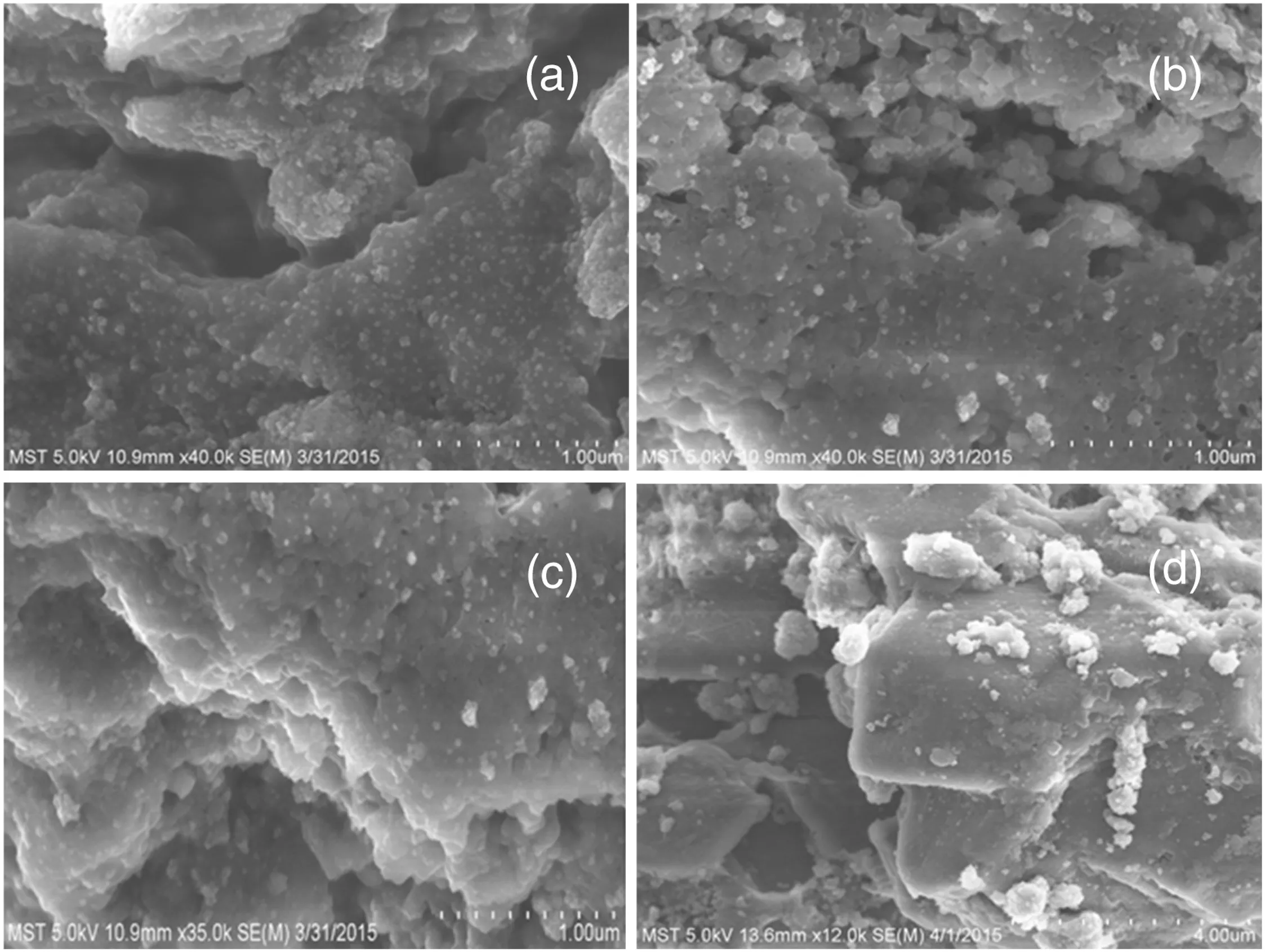
Fig.3.SEM images of(a)g-C3N4/TiO2-1.1,(b)g-C3N4/TiO2-1.3,(c)g-C3N4/TiO2-1.7 and(d)g-C3N4/TiO2-1.9.
TEM and HRTEM images of g-C3N4/TiO2-1.5 were shown in Fig.4(c)and(d),suggesting TiO2nanoparticles embed in the lamellar structure.TEM images demonstrated that there are close interfacial connections between g-C3N4and TiO2,which are beneficial for carrier transfer between g-C3N4and TiO2.EDS spectrum of g-C3N4/TiO2-1.5 was provided together with TEM.The spectrum shows the element species and contents of g-C3N4/TiO2-1.5,confirming the formation of heterojunction between g-C3N4and TiO2.
3.3.N2 sorption isotherms
The textural properties of pure TiO2,pure g-C3N4and g-C3N4/TiO2composites were investigated by N2sorption.Fig.5(a)shows the nitrogen adsorption/desorption isotherms of pure g-C3N4,TiO2and the composite of g-C3N4/TiO2-1.5.It was found that the nitrogen adsorption on g-C3N4was minor,corresponding to a low specific surface area(SSA)of 6.3 m2·g−1.The TiO2via the hydrothermal synthesis showed a very high nitrogen sorption volume,giving rise to a high SSA of 198.1 m2·g−1.Upon hybridization,the sorption curves of g-C3N4/TiO2-1.5 were between pure TiO2and pristine g-C3N4,associated with a moderate SSA of49.8 m2·g−1.An IV-type N2isotherm with hysteresis loop between P/P00.4–0.9 was identified on both TiO2and the composite,indicating the mesoporous porosity characteristics.The pore volumes were calculated to be 0.029,0.364 and 0.094 cm3·g−1for g-C3N4,TiO2and g-C3N4/TiO2-1.5,respectively.
Fig.5(b)shows the comparison of textural properties affected by the different mass ratios of g-C3N4to TiO2.Due to the dramatic difference in SSA and porosity between g-C3N4and TiO2,the increase of g-C3N4resulted in the decrease of SSA and pore volume.The SSA and pore volumes of TiO2,g-C3N4and the composites were listed in Table 1.
3.4.FT-IR spectra

Fig.4.TEM images of(a)pure g-C3N4,(b)pure TiO2,(c)g-C3N4/TiO2-1.5,(d)HRTEM of g-C3N4/TiO2-1.5,and(e)EDS spectrum of g-C3N4/TiO2-1.5.

Fig.5.N2 sorption isotherms of(a)g-C3N4,TiO2 and g-C3N4/TiO2-1.5;(b)g-C3N4/TiO2 composites.

Table 1 Textural,optical and photocatalytic properties of TiO2,g-C3N4 and g-C3N4/TiO2 photocatalysts
Fig.6 shows the FT-IR spectra of g-C3N4,TiO2and g-C3N4/TiO2composites.FT-IR spectrum of pure g-C3N4shows several strong bands in 1200–1650 cm−1,corresponding to typical stretching modes of CN heterocycles[34].The peak at 1638 cm−1is assigned to C–N stretching vibration mode,while those at 1247,1332,1417 and 1461 cm−1are associated with C–N heterocycle stretching of g-C3N4.Among that,the peaks at 1332 and 1247 cm−1were assigned to stretching vibration of connected trigonal units of C–N(−C)–C or bridging C–NH–C(partial condensation)[35].The band at812 cm−1corresponds to the breathing mode of the heptazine arrangement[36].A broad band in the range of 3150–3300 cm−1attributes to the stretching vibration modes of terminal NH groups[37,38].The spectra display that the main characteristic peaks of both g-C3N4and TiO2appeared in all g-C3N4/TiO2samples,suggesting the formation of a composite between g-C3N4and TiO2[38].
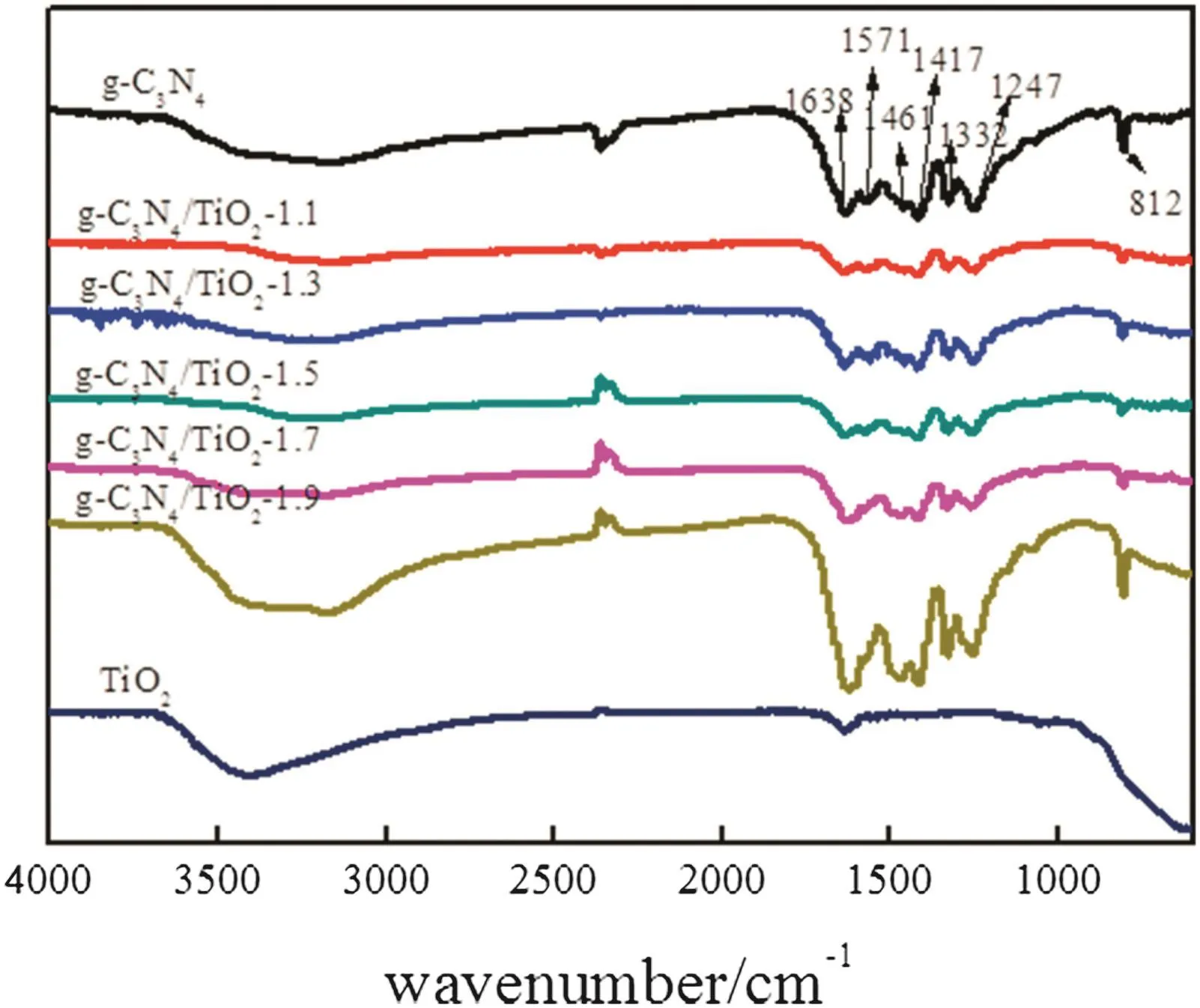
Fig.6.FTIR spectra of pure g-C3N4,TiO2 and g-C3N4/TiO2 composites.
3.5.XPS studies
XPS was used to analyze the chemical states and the surface chemical components of the prepared photocatalysts.Fig.7(a)shows the peaks of Ti,C and O in pure TiO2and the peaks of Ti,C,O,and N in g-C3N4/TiO2-1.5 photocatalyst.Fig.7(b)displays C1s spectrum of g-C3N4/TiO2-1.5.The peaks centering at 284.66,285.91,288.1 eV are corresponding to C–C,C–N–C and C–(N)3,respectively,and the peak at 293.12 eV is ascribed to carbon attached to terminal uncondensed H–N–H species in aromatic rings.The N1s spectrum of g-C3N4/TiO2-1.5 is shown in Fig.7(c).Peaks are found and ascribable to C–N=C at 398.63 eV,N–(C)3at 399.85 eV[39]and–NH2or=NH of uncondensed terminal amino groups at 401.02 eV[40].Fig.7(d)shows Ti 2p spectra of g-C3N4/TiO2-1.5 and pure TiO2.The binding energy values of Ti 2p3/2and Ti2p1/2are at 458.4 and 464.2 eV,represent Ti4+species in the form of TiO2clusters[41].However,a small red-shift to lower binding energy was found in the g-C3N4/TiO2-1.5,which was possibly due to the increased electron density around Ti.The O1s spectrum[Fig.7(e)]of g-C3N4/TiO2-1.5 can be fitted into three peaks,and the binding energies of 529.53 and 531.03 eV are attributed to O2−ions surrounded by Tiatoms and the O2−ions in the surface oxygen deficient regions respectively.From the O1s spectrum,a small blue-shift to higher binding energy is observed in g-C3N4/TiO2-1.5 compared with pure TiO2.
3.6.UV–vis DRS

Fig.7.XPS spectra of TiO2 and g-C3N4/TiO2-1.5:(a)survey spectrum;(b)C1s spectrum;(c)N1s spectrum;(d)Ti2p spectrum;and(e)O1s spectrum.
The optical properties of the photocatalyst materials are shown in Fig.8.A sharp fundamental absorption edge for pure TiO2was observed at395 nmand the band gap energy was estimated to be 3.14 eV.The absorption threshold of pure g-C3N4was at 463.4 nm and the band gap was estimated to be 2.68 eV.Upon loading TiO2with g-C3N4,the light absorption region of TiO2could be further extended to a longer wavelength and the highest red shift appearing at about 457 nm is found on g-C3N4/TiO2-1.5.The absorption thresholds and band gap energies of pure g-C3N4,TiO2and hybrid photocatalysts were displayed in Table 1.The results indicated that the improved visible light response of the photocatalysts is contributed by g-C3N4and suggested thatthe composites could provide an efficient utilization of visible light which would be beneficial to photocatalytic process.
3.7.Photodegradation of methylene blue
The photocatalytic properties of g-C3N4/TiO2were evaluated by the degradation of MB under UV and visible light,respectively.For comparison,MB photolysis was studied under the same experimental conditions.Without the irradiation,the MB adsorption tests were carried out for 1 h.MB adsorption rates on g-C3N4,TiO2,g-C3N4/TiO2-1.1,g-C3N4/TiO2-1.3,g-C3N4/TiO2-1.5,g-C3N4/TiO2-1.7,and g-C3N4/TiO2-1.9 were 1.02%,24.6%,3.26%,5.11%,11.89%,8.25%,and 4.78%,respectively.Fig.9(a)shows the photocatalytic activities of TiO2,g-C3N4and a series of g-C3N4/TiO2photocatalysts under UV.It was found that the photocatalytic activity increased gradually with the increase of g-C3N4.Once the mass ratio of g-C3N4to TiO2reached 1.5,the composite exhibited the highest activity,and the photocatalyst was able to degrade 95.32%MB within 120 min.while pure TiO2and g-C3N4only degraded MB by 68.56%and 35.75%,respectively.All hybrid photocatalysts possessed better photocatalytic activities than pure TiO2and g-C3N4.The results demonstrated that the loading level of g-C3N4played a crucial role in improving the MB photodegradation.Fig.9(c)shows photocatalytic activities of TiO2,g-C3N4and a series of g-C3N4/TiO2photocatalysts under visible light.Photodegradation under visible light demonstrated same order compared with that under UV.To further study the reaction kinetics,the obtained data were fitted by a first-order kinetic model,the equation is as follow:
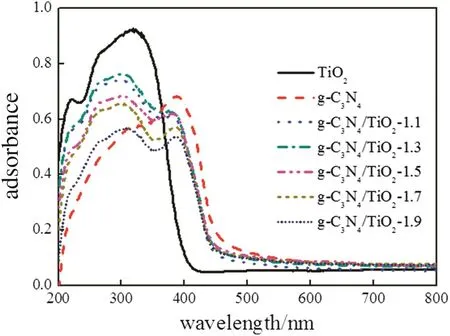
Fig.8.UV–vis DRS of TiO2,g-C3N4 and g-C3N4/TiO2 photocatalysts.

where C0is the initial dye concentration in solution,C is the concentration at time t,and k is the apparent first-order rate constant.Fig.9(b)and(d)shows the reaction rate constants of MB degradation on different materials under UV and visible light,respectively.The photocatalytic rate constant enhanced with increasing the g-C3N4contents.When the loading level of g-C3N4reached 1.5,the apparent rate constants were the highest under both UV and visible light.Table 1 lists the rate constants of the photodegradation of MB.Under UV,the reaction rate constant of g-C3N4/TiO2-1.5 was 0.02552 min−1,which is 6.92 and 2.65 times higher than g-C3N4and TiO2,respectively.Under visible light,a rate constant of 0.00816 min−1was estimated for g-C3N4/TiO2-1.5,which is 9.27 and 7.03 times higher than g-C3N4and TiO2,respectively.The enhancement factor,the ratio of the activity of composite to g-C3N4and/or the other semiconductor,can be used to evaluate the synergistic effects from the hybridization between g-C3N4and another semiconductor[10].Table 2 lists a number of g-C3N4composites,and their enhanced efficiencies compared to the single component.It was found that the enhancement by the hybridization in this study is superior to most similar studies.The enhancements of photodegradation under both UV and visible light also strongly suggested that synergistic effects were created by the hybridization of g-C3N4to TiO2.

Fig.9.Photodegradation of MB and apparent rate constants under UV(a,b)and under visible light(c,d).[Initial MB:20 mg·L−1;catalyst:1 g·L−1;volume:100 ml].

Table 2 Enhanced photocatalytic activity of g-C3N4 composites over pure g-C3N4

Fig.10.Photomineralization of MB using TiO2,g-C3N4 and g-C3N4/TiO2-1.5 under visible light.

Fig.11.Proposed mechanism for the separation and transport processes of photoexcited electron–holes at the g-C3N4/TiO2 interfaces.
The process of photocatalytic degradation of organic dye molecules involves many complicated intermediate products.The advantage of photocatalytic reaction is that the organic pollutants could be decomposed into H2O,CO2and other inorganic groups.Fig.10 shows MB photomineralization rates of g-C3N4,TiO2and g-C3N4/TiO2-1.5 using a TOC analyzer.The TOC removal rates of g-C3N4,TiO2and g-C3N4/TiO2-1.5 under visible light were determined to be 26.75%,57.56%and 87.32%,respectively.MB photomineralization rates on TiO2,g-C3N4and g-C3N4/TiO2-1.9 demonstrated the photocatalytic essence of MB degradation.
3.8.Mechanism of the enhanced activity of g-C3N4/TiO2
The processes of electron–hole separation and transport at the interfaces of g-C3N4/TiO2hybrid photocatalyst as well as the MB degradation mechanism are schematically shown in Fig.11.The conduction band(CB)and valence band(VB)potentials of g-C3N4are−1.3 and 1.4 eV,compared with−0.5 and 2.7 eV of TiO2,respectively.Only g-C3N4can be activated by the visible light,so the photogenerated electrons would be excited from VB to CB of g-C3N4.Since the CB edge potential of g-C3N4was negative than that of TiO2,the photoinduced electrons in CB of g-C3N4can be transferred to the CB of TiO2easily via intense interfacial connections.Hence,the electron–hole recombination rate can be reduced and the separation efficiency would be enhanced.The photoinduced electrons(from both g-C3N4and TiO2)at the CB of TiO2would be donated to an electron acceptor such as dissolved oxygen(leading to the formation of ·O2−),and then a sequence of reactions occurred include ·O2−combine with protons to yield HOO·and then H2O2to oxidize organic dyes[43,44].At the same time,the photoinduced holes(including g-C3N4and TiO2)at the VB of g-C3N4may contact with H2O adsorbed on the surface of hybrid catalyst to produce hydroxyl radicals to oxidize pollutants.
The process is described as follows[21]:

As for MB degradation,it mainly occurs on the external surface of the catalysts.The adsorption on g-C3N4/TiO2has promoted direct contact between MB molecules and photocatalyst surfaces.According to MB adsorption rates,TiO2has the highest adsorption capacity due to its largest SSA.However,the photocatalytic activity of TiO2is lower than g-C3N4/TiO2photocatalysts.It is noteworthy that higher g-C3N4loading will not only decrease SSA,which results in a lower adsorption of MB,but also decrease TiO2,which is used for fast transfer of excited electrons.When considering the highest activity was on g-C3N4/TiO2-1.5,which has a much lower SSA than TiO2,it was concluded that adsorption was not the limiting step of photodegradation of MB,but the interfacial transfer of carriers between g-C3N4and TiO2.
4.Conclusions
In summary,a highly active g-C3N4/TiO2hybrid photocatalyst was obtained by a simple sonication method.The improved photocatalytic activities under UV and visible light were observed over all g-C3N4/TiO2photocatalysts and g-C3N4/TiO2-1.5 exhibited the highest efficiency.The apparent rate constant k of g-C3N4/TiO2-1.5 is 7.03 times higher than bare TiO2,and 9.27 times higher than pristine g-C3N4under visible light.The enhancement of photocatalytic performance was mainly attributed to the improved electron–hole separation efficiency,extended visible light absorption and direct contact between organic pollutants and photocatalyst.This study explores the capability of g-C3N4/TiO2in photodegradation of organic pollutants under visible light,and may lay the foundation to the potential applications of such materials in environmental remediation.
 Chinese Journal of Chemical Engineering2015年8期
Chinese Journal of Chemical Engineering2015年8期
- Chinese Journal of Chemical Engineering的其它文章
- A Reynolds mass flux model for gas separation process simulation:II.Application to adsorption on activated carbon in a packed column☆
- Turbulent forced convection in a heat exchanger square channel with wavy-ribs vortex generator☆
- Optimization of natural convection heat transfer of Newtonian nanofluids in a cylindrical enclosure
- Gas adsorption in shaped zeolitic imidazolate framework-8☆
- Preparation of pH-responsive membranes with amphiphilic copolymers by surface segregation method☆
- Computational exploration of H2S/CH4 mixture separation using acid-functionalized UiO-66(Zr)membrane and composites☆
