Computational exploration of H2S/CH4 mixture separation using acid-functionalized UiO-66(Zr)membrane and composites☆
Shumeng Wang ,Dong Wu ,2,Hongliang Huang ,Qingyuan Yang ,*,Minman Tong ,Dahuan Liu ,Chongli Zhong
1 State Key Laboratory of Organic–inorganic Composites,Beijing University of Chemical Technology,Beijing 100029,China
2 China Institute of Atomic Energy,Beijing 102413,China
Keywords:Desulfurization Metal-organic framework Membranes Composites Molecular simulation
ABSTRACT A computational study was firstly performed in this work to examine the applicability of an acid-functionalized metal-organic framework(MOF),UiO-66(Zr)-(COOH)2,in membrane-based H2S/CH4 separation.The results showthatthis MOF could be potentially interesting when being used as the pure membrane materialfor the separation of the mixture with low H2S concentration.Further,the performance of 10 different mixed matrix membranes(MMMs)on the basis of the MOF was predicted by combing the molecular simulation data and the Maxwell permeation model.The results indicate that using this MOF as filler particles in MMMs can significantly enhance the permeation performance of pure polymers.The findings obtained in this work may be helpful in facilitating the application of this promising MOF for practical desulfurization process of fuel gas.
1.Introduction
Before being transported in pipelines or as vehicle fuel,CH4originating from natural gas,coal gas,and biogas must meet strict specifications with respect to CO2and H2S[1].CO2removal is essential to enhance the energy content of these fuel gases,satisfy the pipeline standards and reduce the greenhouse effect.H2S is a very toxic and corrosive gas and harmful sulfuric substances will be formed when its burning product encounters with water.In view of the rapid growth in the demand of cleaner energy sources,the separation of the two acid gas impurities is of economic and environmental importance for the efficient utility of the above fuel gases[2].Compared to the traditional methods such as chemical absorption,distillation,extraction and metal-oxide conversion[3],membrane-based separation processes show competitive prospect because of the low cost and easy operating.A fundamental challenge in this area is to select appropriate membrane materials from the large numbers of materials that are available so far.To date,great attention has been paid to assess the feasibilities of various materials,including polymers[4]and other conventional inorganic materials[5,6].Currently,increasing efforts are being paid to find new promising materials with even better performance.
Metal-organic frameworks(MOFs),as a novel class of crystal nanoporous materials,have attracted tremendous interest in the membrane-based gas separation and purification during the past decade[7–10].Considering the large number of existing materials,it would be very time consuming to experimentally examine the separation properties of new MOF-based membranes.In this respect,molecular modeling is a powerful tool that can provide a valuable complement to experimental studies,allowing subsequent experimental endeavors to be devoted to the candidates that exhibit promising performance.As a result,significant computational researches have been carried out to investigate the performance of various pure MOF membranes for the desired gas separation[11].For example,Keskin et al.have computationally evaluated the performance of a series of MOF membranes for CO2/CH4separation[8,12].The group of Krishna has also paid great efforts to examine the membrane properties of various MOFs for this gas mixture using atomically detailed calculations[9,13].Watanabe et al.[14]studied the separation of CO2/CH4mixture in a microporous MOF membrane,Cu(h fipbb)(H2h fipbb)0.5,by a composite computational method that involves molecular simulation and density functional theory(DFT)calculations.These theoretical studies not only provide important information at molecular level for the design of new high performance membranes,but also demonstrate that some existing MOFs can be regarded as the promising membrane materials.On the other hand,polymeric membranes currently play a dominant role in the membrane market for mixture separation due to their low fabrication cost and easy process ability.However,polymer membranes usually bear a trade-off between selectivity and permeability[15].Due to the good affinity of their structures with polymer chains,MOF particles are considered as good fillers for the fabrication of mixed matrix membranes(MMMs)toward gas separation[10].Although the related investigation on polymer/MOF composite membranes is still in an early stage,some computational studies have shown that such types of MMMs can exhibit great superiority over the corresponding pure polymeric membranes[16,17].For instance,Erucar and Keskin[18,19]showed that with the addition of ZIF-11 or Zn-Atz,the performance of several polymers such as 6FDA-based polyimide,PIM-1,and PTMSP can be significantly enhanced to exceed the Robeson's upper bound for CO2/CH4separation.
In contrast to the significant progress achieved on MOFs for adsorption-based[20,21]as well as the above membrane-based CO2/CH4separation,the related studies on the important H2S/CH4separation for fuel gas purification are still in an extremely early stage.Actually,up to now,there is no research that was paid on the separation of this gas mixture using pure MOF membranes as well as MOF-containing mixed matrix membranes(MMMs).To the best of our knowledge,there are only three investigations that were all performed in the scope of adsorption-based process.Harmon et al.[22]studied H2S/CH4separation in MIL-47(V)with a combination of vacancy solution theory(VST)and experimental adsorption data of pure components.Peng and Cao[23]computationally screened the performance of 13 different MOFs for the removal of H2S from biogas and natural gas.With a combination of experimental measurements and molecular modeling,Vaesen et al.[24]revealed the potential of titanium(IV)-based material MIL-125(Ti)-NH2for the concomitant elimination of H2S from biogas and natural gas.
The zirconium-based MOF UiO-66(Zr)has received an intensive research interest due to its high thermal and chemical stability[25].Both the experimental[26]and computational[27–29]investigations revealed that such types of materials have very good properties for the removal of CO2from natural gas and flue gas.Recently,we synthesized a new ZrIVwater-stable MOF of the UiO-66 type incorporating benzene-1,2,4,5-tetracarboxylate(H2BETC)ligands,using an approach that is environmentally friendly and readily scaled up[30].Due to the existence of polar free carboxylic groups in the structure,this porous solid UiO-66-(COOH)2exhibits an outstanding performance for CO2capture from flue gas(CO2/N2separation),both in the adsorption-based and membrane-based separation processes[30,31].In addition,it was experimentally evidenced that it is feasible to prepare MMMs using the particles of UiO-66(Zr)or its amino-substituted analog UiO-66(Zr)–NH2as fillers,which show better separation ability for CO2/CH4mixture compared to several pure polymer membranes[32].Therefore,motivated by the facts described above,an extended computational study was performed in this work to provide the first information on the separation of H2S/CH4mixture in the pure UiO-66(Zr)-(COOH)2membrane and the MMMs composed of polymers as continuous phase and the MOF as dispersed phase.
2.Models and Computational Methods
2.1.MOF structure
The crystal structure of UiO-66(Zr)-(COOH)2was taken from our previous work[31]which was constructed through the combined use of experimental information and DFT calculation,the unit cell representation of which is shown in Fig.1.This porous solid is built up from the secondary building units Zr6-octahedra[Zr6O4(OH)4]but bounded to H2BTEC ligand with the two carboxylic groups located on the two sides of the benzene rings.Similar to other UiO-66 type materials,UiO-66(Zr)-(COOH)2,this MOF has a three dimensional arrangement of micropores in which each centric octahedral cage is connected to eight corner tetrahedral cages through triangular windows.

Fig.1.Illustration of the UiO-66(Zr)-(COOH)2 crystal structure.The spheres represent the void regions inside the octahedral and tetrahedral cages.The hydrogen atoms are omitted for clarity.
2.2.Force fields
In this study,H2S was treated with a three-site model that was specifically developed for this species by Kamath et al.[33],where only S atom is a Lennard–Jones(LJ)interacting site while partial charges are centered on each atom.The H–S bond length is 0.134 nm and the H–S–H angle is 92.5°.A single LJ interacting site model was used to depict a CH4molecule with potential parameters taken from the TraPPE force field[34].All the corresponding atomic partial charges and interatomic potential parameters are listed in Table A1(see Appendix),which has been successfully used to reproduce the experimental vapor-liquid phase equilibrium data of each gas.The interactions between the adsorbates and UiO-66(Zr)-(COOH)2were described by a combination of site-site LJ and Coulombic potentials,except for CH4where only a site–site LJ potential was considered.The LJ potential parameters for the framework atoms were taken from the DREIDING force field[35]while those for Zr metal atom were taken from the Universal force field(UFF)[36]due to not available in the former one,which are also given in Table A1.The partial charges of the framework atoms listed in Table A1 were taken from our previous work[31],which are the electrostatic potential(ESP)charges obtained from DFT calculation with ChelpG method.All the LJ cross interaction parameters between adsorbate molecules as well as between the adsorbates and MOF were determined by the Lorentz–Berthelot mixing rules.The above set of force fields has been successfully used to investigate the adsorption behaviors of CH4,H2S and their mixture in UiO-66(Zr)series MOFs[28,37]as well as in MIL-125(Ti)and its amino-functionalized material[24].It has been shown that the framework flexibility of UiO-66(Zr)-(COOH)2has a significant impact on the diffusion of guest species within the MOF[31].Thus,the flexible force field developed in that work was applied to calculate the diffusion properties of H2S and CH4in the material.In this flexible force field,the LJ potential parameters and partial charges for the framework atoms are the same as those given in Table A1.As a result,we found that the framework flexibility does not have a significant influence on the adsorption of both gases in the material,for which similar observations have also been found by others in the studies of some typical MOFs[38,39].
2.3.Simulation details
Grand canonical Monte Carlo(GCMC)simulations were conducted to calculate the adsorption isotherms of H2S,CH4and their mixture in UiO-66(Zr)-(COOH)2at 303 K using our newly developed simulation code CADSS(Complex Adsorption and Diffusion Simulation Suite).For the simulations of pure components,molecules involve four types of trials:attempts(i)to displace a molecule(translation or rotation),(ii)to regrow a molecule at a random position,(iii)to create a new molecule,and(iv)to delete an existing molecule.For the simulations of mixture,at tempt to exchange molecular identity was introduced as an additional type of trial to speed up the equilibrium and reduce the statistical errors.Details of the method can be found elsewhere[40,41].The simulation box consisted of 8(2×2×2)unit cells.A cutoff radius of 1.4 nm was applied to the LJ interactions,while the long-range electrostatic interactions were handled by the Ewald summation technique.Periodic boundary conditions were applied in all three dimensions.For each state point,GCMC simulation consisted of 2×107steps to ensure the equilibration,followed by 2×107steps to sample the desired thermodynamic properties.For the calculation of the zero-coverage adsorption enthalpies of H2S and CH4in the MOF,configuration-bias Monte Carlo(CBMC)simulations in the canonical(NVT)ensemble were performed using the revised Widom's testing particle method[41].
Molecular dynamic(MD)simulations in the canonical(NVT)ensemble,accomplished by DL_POLY 2.20 simulation package[42],were used to study the self and transport diffusivities of H2S and CH4in UiO-66(Zr)-(COOH)2at 303 K.All the MD runs were performed considering a full flexibility ofthe materials on the basis of the flexible force field described above.All the MD simulations were performed as follows:guest molecules were randomly inserted into the simulation box,and then relaxed using 2×105NVT Monte Carlo cycles.Velocities from the Maxwell–Boltzmann distribution at the required temperature were assigned to all the adsorbate molecules and the framework atoms.Further,the production run of 2×107MD steps(i.e.,20 ns)was performed for each MD calculation after an equilibration of the system with 2×106MD steps.The positions of adsorbed molecules and framework atoms were stored every 5000 MD steps for subsequent analysis.The Nosé–Hoover thermostat was employed to maintain the constant temperature condition and the velocity Verlet algorithm was used to integrate the Newton equations together with QUATERNION algorithm for the rotational motion of H2S molecules.It was checked that MD simulations conducted in microcanonical(NVE)ensemble lead to equivalent results.The time step used in the MD simulations was taken as 1.0 fs,and other simulation techniques are the same as those for GCMC simulations.
In order to obtain the separation properties of the membranes,it requires the adsorptive separation properties and the dynamic separation properties of the materials.In this work,after the validation of the applicability of ideal adsorption solution theory(IAST),this method was used to obtain the adsorption isotherms of H2S/CH4mixture.To predict mixture permeation through pure MOF membrane,the mixing theory(SSK method)proposed by Skoulidas et al.[43]was used with the data available from the IAST calculations and MD simulations of pure gases.Details of the implementation of the SSK method can be found in the Appendix.This method has been successfully used to investigate the separation performance of various zeolites[43]and MOF[12,31]membranes.For modeling the separation performance ofMMMs,the permeability of H2S/CH4mixtures through polymeric membranes were taken from the experimental data available in the literature,while those through the MOF phase were taken from the simulation results obtained in this work.Then,the Maxwell model[44]was used to predict gas permeabilities through the MMMs,as given by

where,P is the permeability of the MOF/polymer MMM,λdmis the permeability ratio Pd/Pm(Pdand Pmare the gas permeabilities in dispersed and continuous phases),Pris the relative permeability and φ is the volume fraction of the dispersed MOF particles in the polymer matrix that was varied from 0.1 to 0.5 in this work.The membrane selectivity was calculated as the ratio of the permeabilities of the two gas components.The above method using the Maxwell model has been successfully used to explore the membrane properties of various MOF-based MMMs within the range of φ examined here[17,31].
3.Results and Discussion
3.1.Performance of pure MOF membrane
Prior to investigating the membrane separation properties,GCMC simulations were first performed to examine the adsorption behaviors of single-component gases in UiO-66(Zr)-(COOH)2at 303 K.The single-component adsorption isotherms were shown in Fig.2.It can be seen that the H2S is more strongly adsorbed than CH4before saturation of the pores in the structure.This is not unexpected since the polar nature of H2S molecule leads to stronger interactions with the pore wall of the MOF than that of nonpolar CH4molecule,which can also be reflected from the zero-coverage adsorption enthalpies of the two gases(CH4:−24.2 kJ·mol−1;H2S:−38.8 kJ·mol−1).To apply the SSK method to predict mixture permeation through pure UiO-66(Zr)-(COOH)2membrane,it requires a set of continuous functions that quantifies mixture adsorption at very wide ranges of bulk compositions and pressures of interest.In this respect,IAST approach has been successfully used to describe the adsorption equilibria between mixture components in various nanoporous materials including MOFs.To examine the applicability of IAST for the system examined here,the dual-site Langmuir model(see Appendix)was firstly used to fit the single-component adsorption isotherms.Fig.2 shows the fitted results(illustrated with continuous lines),together with the fitted values of the model parameters given in Table A2(see Appendix).On the basis of good fitting results,the IAST was used to predict the mixture adsorption behaviors,and the calculation results for H2S/CH4mixture with three different bulk compositions are shown in Fig.3 as examples.By surveying the literature,it was found that the compositions of H2S in different kinds of fuel gases are quite different,depending on the locations[45–47].For example,in East Sichuan Basin of China,the typical H2S concentration(molar composition)in natural gas from Tieshan gas fields is about 0.59%,while that from Doukouhe gas fields can arrive 17.06%[46].Therefore,considering the corresponding CH4concentrations in fuel gases from these locations,this figure shows the simulation results for H2S/CH4mixture with three different bulk molar compositions(H2S:CH4=0.6:99.4,1.6:98.4 and 18.8:81.2).It can be found from Fig.3 that the IAST predictions agree well with the direct mixture GCMC simulation data.This validation makes us confidently use the IAST method for obtaining the mixture adsorption isotherms over wide ranges of bulk compositions and pressures.In this work,these calculations were performed for H2S/CH4mixtures at nine different compositions from 0.1 to 0.9 and at 30 different pressures from 0.00001 to 10 MPa.The mixture adsorption data generated from such a large collection of state points were further fitted using an extended dual-site Langmuir model defined in the Appendix,and the fitted model parameters are given in Table A3(see Appendix).Fig.A2(see Appendix)shows a comparison of the data obtained from the fitted model with the IAST-derived dataset,demonstrating good agreement between them.Such developed continuous function allows the binary adsorption isotherms to be used efficiently within membrane calculations.In addition,Fig.A3(see Appendix)presents the thermodynamic H2S/CH4adsorption selectivities from their mixtures at three different bulk compositions in UiO-66(Zr)-(COOH)2at 303 K.It can be found that for each bulk composition,the selectivity exhibits a sharply increasing trend with increasing fugacity and then becomes almost independent of the fugacity.The selectivity increases with increasing H2S molar composition in the bulk mixture at low fugacities,while the difference becomes small with further increasing fugacity.

Fig.2.Single-component adsorption isotherms of H2S and CH4 in UiO-66(Zr)-(COOH)2 at 303 K as a function of fugacity.Filled symbols and continuous lines represent the GCMC simulation results and those fitted by dual-site Langmuir model,respectively.(n.o.—number,u.c.—unit cell).

Fig.3.Mixture adsorption isotherms of H2S/CH4 in UiO-66(Zr)-(COOH)2 at 303 K as a function of fugacity.Filled symbols and continuous lines represent the results of mixture GCMC simulations and IAST predictions,respectively.(n.o.—number,u.c.—unit cell).
Apart from the information of mixture adsorption isotherms,the SSK method also needs continuous functions that are used to describe the single-component self-(Dself)and corrected(D0)diffusivities of H2S and CH4.For a single-component system,D0is also known as the Maxwell–Stefan diffusivity(Ð).Fig.4 shows the two types of diffusivities obtained from flexible force field-based MD simulations for the two gases in UiO-66(Zr)-(COOH)2at 303 K,as a function of loading.It can be found that both the Dselfand D0of CH4decreases with increasing the loading,For H2S,though the Dselfalso generally shows a decreasing trend with the loading,there is a maximum for the D0.In addition,this figure also indicates that H2S molecules diffuse slower than CH4molecules,especially more evident at low loadings.This can be attributed to the weaker interaction strength of CH4with the material,allowing the molecules to move more freely.Fig.A4(see Appendix)shows that the fitted results(continuous lines)using polynomial models are in very good agreement with these MD-simulated diffusivities,where the absolute loading are replaced by the fractional loading as required by the SSK method.
With the above fitted models,the permeation properties of UiO-66(Zr)-(COOH)2membrane for the separation of H2S/CH4gas mixture were calculated according to the procedure described in the Appendix.Fig.5 shows the predicted H2S selectivity of the membrane at 303 K for the mixture with three different bulk compositions examined previously,as a function of feed pressure.It can be observed that the membrane selectivities are almost independent of the feed pressure in the range examined in this work.In addition,the composition of the bulk gas mixture has a large influence on the mixture permeation selectivity.The lower the H2S concentration in the bulk mixture is,the higher the permeation selectivity will be.

Fig.4.Self-(D self)and corrected(D0)diffusivities of H2S,and CH4 in UiO-66(Zr)-(COOH)2 at 303 K,as a function of fractional loading.Symbols show the data from the MD simulations.(n.o.—number,u.c.—unit cell).

Fig.5.Mixture permeation selectivities for H2S/CH4 separation through UiO-66(Zr)-(COOH)2 membrane at 303 K,as a function of feed pressure(permeate side is considered under vacuum conditions).
Studies have been conducted to examine the membrane properties of carbon nanotubes(CNTs)for H2S/CH4separation.Gilani et al.[48]observed high permeability of H2S through CNT membrane,but the permeation selectivity of H2S over CH4is only about 1.4.Fig.6 compares the selectivity and permeability of H2S for H2S/CH4mixtures with two different compositions through UiO-66(Zr)-(COOH)2and CNT membranes at 303 K and 0.1 MPa,together with the comparisons under other conditions given in Table 1.Compared to the CNT membrane,the UiO-66(Zr)-(COOH)2membrane exhibits similar H2S permeability but with significant higher permeation selectivity,especially for the separation of the mixture with 0.6%H2S molar composition.At the same time,it can be seen from Table 1 that both the selectivity and permeability almost remain unchanged under the pressure range studied for each composition.As a result,the UiO-66(Zr)-(COOH)2pure membrane shows much better performance than the CNT one.Considering that the H2S concentrations in fuel gases from many locations are usually very low,UiO-66(Zr)-(COOH)2can be regarded as a good membrane material for the removal of H2S from them.

Fig.6.Selectivity and permeability of H2S for H2S/CH4 mixture through UiO-66(Zr)-(COOH)2 and CNT membranes at 303 K and 0.1 MPa.
3.2.Gas separation through MOF-based MMMs
On the basis of the above results,we further computationally predicted the performance of new MMMs with UiO-66(Zr)-(COOH)2particles embedded as fillers.To this target,10 different polymers with the experimental gas permeation data available in the literature[49–53]were chosen as the polymeric matrices.Although the temperatures in these experimental measurements vary from 298 to 303 K,we found that there is only a marginal change in adsorption and diffusion behaviors of H2S/CH4gas mixtures in UiO-66(Zr)-(COOH)2in this temperature range.As demonstrated by others[17],the temperature difference between these data sets can be neglected as long as the MMM results areinterpreted at the temperature of the polymer data.Fig.7 shows a comparison of the H2S selectivity and permeability through pure polymer membranes,pure UiO-66(Zr)-(COOH)2membrane and their composite membranes.Note that the conditions used to predict the performance of the pure MOF membrane and the MMMs are dependent on those at which the experimental measurements were performed on the corresponding pure polymer systems.
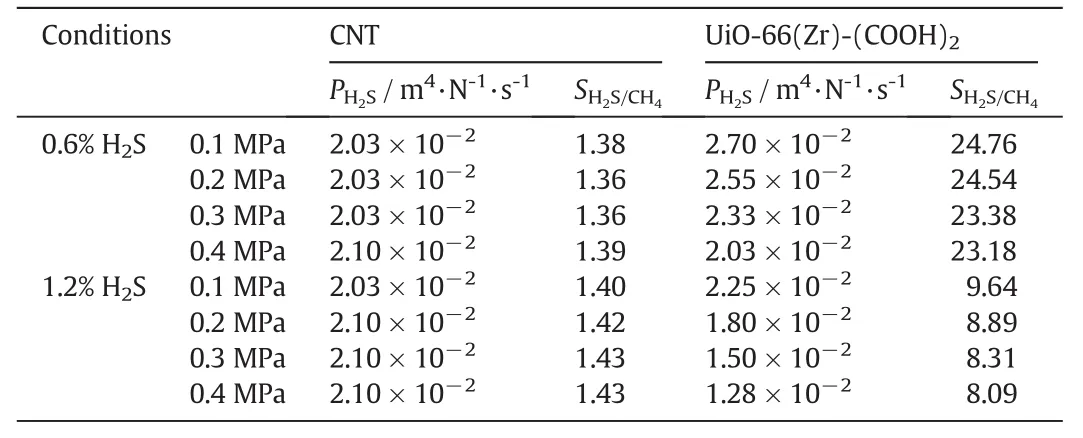
Table 1 Comparison of the permeability(P H2S)and selectivity(S H2S/CH4)ofH2S from H2S/CH4 mixtures at 303 K through UiO-66(Zr)-(COOH)2 membrane with those reported for CNT membrane[48]
All the selectivity and permeability data shown in Fig.7(a)were estimated at a feed pressure of 0.138 MPa with the permeate side at vacuum.The results for the membranes related to the dimethyl-silicone rubber present in Fig.7(b)were estimated at a feed pressure of 0.101 MPa,together with 0.456 MPa for those related to the other three polymer membranes.It can be found from the two figures that for these polymer membranes with low H2S permeabilities,the addition of the MOF into the polymer matrices can greatly increase the permeabilities and the selectivities almost remain unchanged with increasing the MOF volume fraction.For example,the permeability of H2S increases from 7.5 × 10−7to 6.3 × 10−6m4·N−1·s−1in the case of PPOP-COOH/UiO-66(Zr)-(COOH)2composite membrane when the volume fraction is changed from 0.0 to 0.5,while the permeation selectivity remains around 10 which is comparable to that of pure MOF membrane.In contrast,for the pure polymer membranes with high H2S permeability[see Fig.7(c)],both the permeability and permeation selectivity are enhanced in the presence of MOF particles as fillers.For example,the H2S permeability and the selectivity through the membranes related to the polymer PPO increase from 2.9×10−4to 1.1×10−3m4·N−1·s−1and from 7.70 to 18.19,respectively.In this figure,the membrane properties of the MMMs related to TFC polyamide was estimated at a feed pressure of 1.0 MPa,since the experimental data for pure polymer membranes were generally measured under these conditions.For the MMMs related to the polymers RITE-A and PPO,the membrane properties were estimated at a feed pressure of 0.69 MPa.
It can be concluded from Fig.7 that dispersing UiO-66(Zr)-(COOH)2particles into polymer matrices can enhance the permeability of H2S greatly,while the influence on the permeation selectivity can be divided into two cases.One is that using UiO-66(Zr)-(COOH)2as filler particles for polymers with low permeability at most increases the gas permeability of the membranes.The other one is that adding the MOF into the polymers with large permeability can enhance both the permeation selectivity and permeability.These observations underline the importance of the appropriate polymer/UiO-66(Zr)-(COOH)2combination in the fabrication of MMMs for practical applications.
4.Conclusions
With a combination of atomically detailed simulations and a theoretical permeation model,we performed a first study to explore the potential of one newly reported nanoporous material,UiO-66(Zr)-(COOH)2for the separation of H2S/CH4gas mixture.The results reveal that the MOF exhibits a good performance at low H2S concentrations when being used as pure membrane material.In addition,current work demonstrates that this MOF can be considered as promising nanoporous stuff for the fabrication of MMMs with a significant enhancement on the permeation performance of the polymer membranes.As a result,these observations make UiO-66(Zr)-(COOH)2with excellent thermal and chemical stabilities an alternative to the conventional adsorbents that are currently involved in fuel gas purification.

Fig.7.Performance of UiO-66(Zr)-(COOH)2 based MMMs predicted for H2S/CH4 separation.Filled triangular symbols represent the experiment data for polymer membranes taken from the literature,while the empty symbols represent the data of MMMs with different volume fractions of UiO-66(Zr)-(COOH)2.
Nomenclature
Dselfself-diffusivities,m2·s−1
D0corrected diffusivities,m2·s−1
P permeability of the MOF/polymer MMM,m4·N−1·s−1
Pdgas permeabilities in dispersed phases,m4·N−1·s−1
Pmgas permeabilities in continuous phases,m4·N−1·s−1
Prrelative permeability
λdmpermeability ratio
φ volume fraction of the dispersed MOF particles in the polymer matrix
Appendix
A1.Force field parameters

Fig.A1.Atom types for the framework of UiO-66(Zr)-(COOH)2.

Table A1 Potential parameters and partial charges for the framework atoms and the adsorbates
A2.Fitting of single-component adsorption isotherms
Single-component adsorption isotherms of H2S and CH4in UiO-66(Zr)-(COOH)2at 303 K were fitted using a dual-site Langmuir model

where Ciis the adsorbed amount(molecules/unit cell)of gas species i(i=H2S or CH4),f is the fugacity in units of MPa,and ai,bi,ci,and diare the model parameters to be fitted.Table A2 presents the values of the fitted parameters.

Table A2 Values and units of single component isotherm parameters
A3.Fitting of mixture adsorption isotherms
On this basis of the good agreement between the IAST predictions and direct mixture simulation data,an extended dual-site Langmuir model was used to fit the IAST-generated mixture adsorption data of H2S and CH4

where CH2Sand CCH4are the amounts(molecules/unit cell)of H2S and CH4in the adsorbed mixture,respectively;fH2Sand fCH4are the partial fugacities of the two components in the bulk phase,respectively;aiand biare parameters of the model which are given in Table A3.Fig.A2 shows the comparison of the fitted mixture adsorption data with those of IAST predictions.

Table A3 Values and units of binary isotherm parameters H2S/CH4 mixture
A4.Thermodynamic adsorption selectivities

Fig.A2.Comparison of the fitted binary adsorption data obtained from the fitted models with those of IAST predictions.(n.o.—number,u.c.—unit cell).

Fig.A3.Adsorption selectivities of H2S over CH4 in UiO-66-(COOH)2 from their mixture with three different bulk compositions.
A5.Fitting of single-component diffusivities
The single-component self-(Ds)and corrected(D0),also known as the Maxwell–Stefan diffusivity,diffusivities of H2S and CH4in UiO-66(Zr)-(COOH)2were extracted from MD simulations using the Einstein relations,which are further fitted using the continuous functions given in Eqs.(A4)–(A7)as a function of their fractional loadings
θi=,whereis saturation loading of species i derived from the corresponding fitted single-component adsorption isotherm.The fitted equations need to meet the conditions:at zero loading,the functions describing corrected diffusivity was constrained to reproduce the observed self-diffusivity;at the saturation loading,both diffusivities were constrained to be zero.The fitting results are shown in Fig.A4 with the resulting parameters given in Table A4.


Table A4 Values of the fitted parameters in units of 10−10 m2·s−1
A6.Calculation method for permeation properties
In the SSK method for predicting mixture diffusion behaviors,it requires two additional diffusion coefficients to define the correlation effects in a mixture;that is,the M–S self-exchange diffusivity()and the binary-exchange diffusivity().To calculate the two diffusivities,the SSK method replaces the fractional single component occupancy θ with the fractional total occupancy of mixture θ (θ = θ1+ θ2=c1/+c2/)and the M–S self exchange coefficients are estimated by
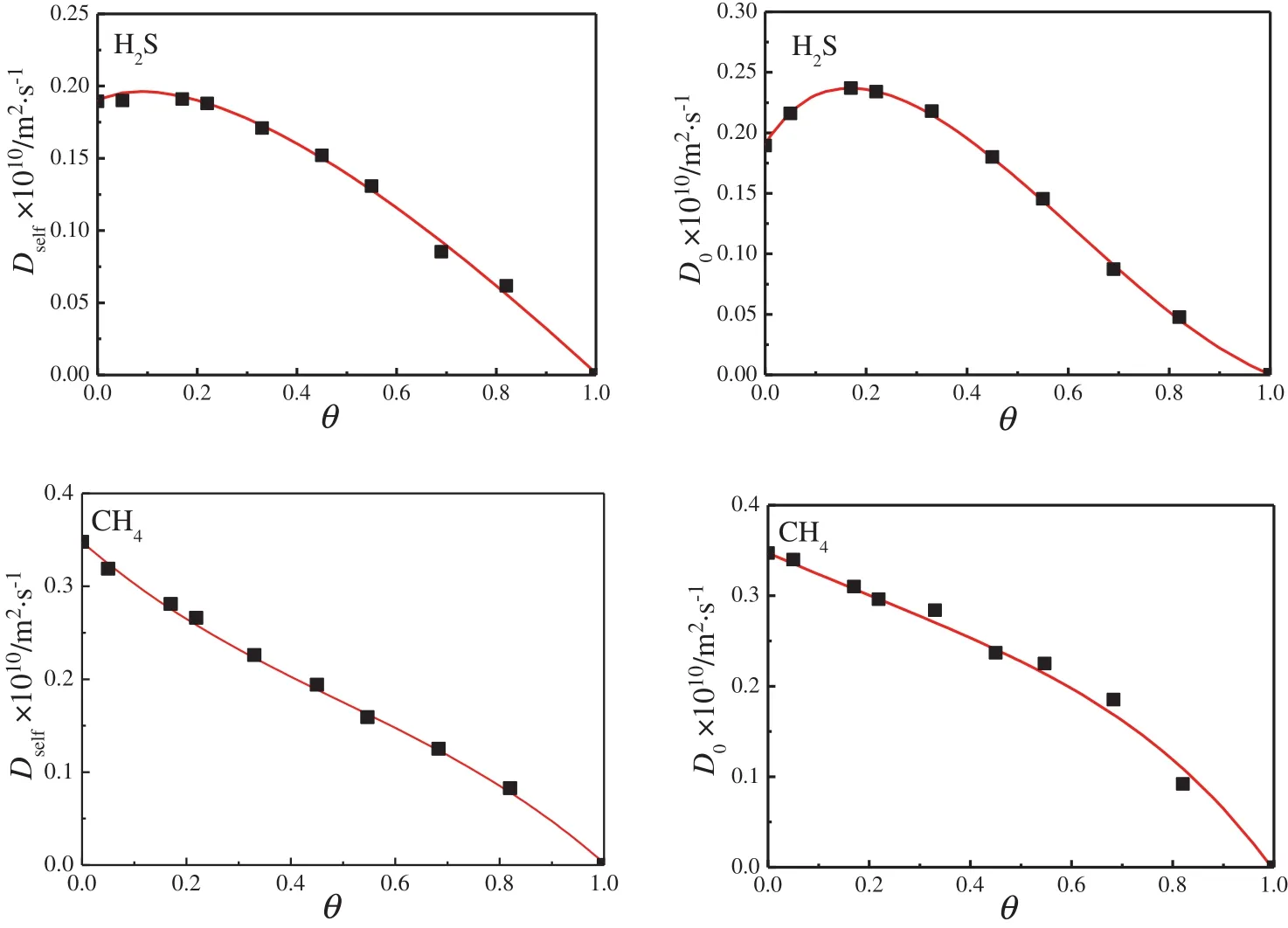
Fig.A4.Self and corrected diffusion coefficients of H2S,and CH4 in UiO-66(Zr)-(COOH)2 at303 K.Symbols show the data from the MD simulations,while curves show the fitting functions.

while the M–S binary-exchange coefficientsare estimated using a general interpolation formula

In the SSK method,the matrix of Fick diffusivities[D]for mixture diffusion is given as

where[B]and[Γ]are the correlation matrix and the matrix of thermodynamic correction factors respectively.The elements in the two matrices are defined by

where the values of thermodynamic correction factors can be obtained from the fitted mixture adsorption isotherms.Once the above quantities are obtained,steady-state permeance of mixture can be calculated by specifying the pressures on both the feed and permeate sides of the membrane,as well as the adsorption information of a mixture on feed side.For a binary mixture,the fluxes of the components are calculated using

where Jiis the net molecular flux of component i,ρ is the framework density,and∇ciis the gradient of the loading of component i.Then the permeation selectivity of component 1 over component 2 can be calculated by

and the permeability(Pperm,i)of component i is calculated by

where L is the membrane thickness and Δpiis the partial pressure drop across the membrane which is equal to the partial pressure difference of component i between the feed and permeate sites,Δpi=pfeed,i− pperm,i.In current study,the thickness of the pure MOF membrane was taken 10 μm,and the permeate side was considered under vacuum conditions.Steady-state fluxes were calculated using a shell description of the pure MOF membrane.When the performance of polymer/MOF composites,the permeabilities of H2S and CH4in the MOF phase were taken either from the mixture calculation or single-component calculation,depending on the experimental data for polymer phase are measured on pure gases or mixture
 Chinese Journal of Chemical Engineering2015年8期
Chinese Journal of Chemical Engineering2015年8期
- Chinese Journal of Chemical Engineering的其它文章
- A Reynolds mass flux model for gas separation process simulation:II.Application to adsorption on activated carbon in a packed column☆
- Turbulent forced convection in a heat exchanger square channel with wavy-ribs vortex generator☆
- Optimization of natural convection heat transfer of Newtonian nanofluids in a cylindrical enclosure
- Gas adsorption in shaped zeolitic imidazolate framework-8☆
- Preparation of pH-responsive membranes with amphiphilic copolymers by surface segregation method☆
- Gas separation using sol–gel derived microporous zirconia membranes with high hydrothermal stability☆
