High tap density of Ni3(PO4)2 coated LiNi1/3Co1/3Mn1/3O2 with enhanced cycling performance at high cut-off voltage☆
Yan Cui,Shengming Xu*
Institute of Nuclear and New Energy Technology,Tsinghua University,Beijing 100084,China
Keywords:LiNi1/3Co1/3Mn1/3O2 cathode material Electrochemistry Ni3(PO4)2 coating High tap density High rate discharge capacity
ABSTRACT The LiNi1/3Co1/3Mn1/3O2 is first obtained by the controlled crystallization method and then coated with Ni3(PO4)2 particles.The effects of the coating on rate capability and cycle life a thigh cut-off voltage are investigated by electrochemical impedance spectroscopy and galvanostatic measurements.The element ratio of Ni:Mn:Co is tested by inductively-coupled plasma spectrometer(ICP)analysis and it testified to be 1:1:1.It is indicated that Ni3(PO4)2-coated LiNi1/3Co1/3Mn1/3O2 has an outstanding capacity retention,where 99%capacity retention is maintained after 10 cycles at 5C discharge rate between 2.7 V and 4.6 V.The electrochemical impedance spectroscopy(EIS)results show that the current exchange density i0 of the coated sample is higher than that of LiNi1/3Co1/3Mn1/3O2,which is beneficial to its electrochemical performances.All the conclusions show that the Ni3(PO4)2 coating can prominently enhance the high rate performance of the LiNi1/3Co1/3Mn1/3O2,especially at high cut-off voltage.
1.Introduction
LiCoO2has been widely used as a cathode material in commercial lithium-ion batteries because of its good electrochemical properties and ease of preparation.But the high cost and toxicity of cobalt,and its safety when abused had led to the study of some new cathode materials,such as nickel-and manganese-based lithium oxides[1-5].Among these alternative materials,hexagonal α-NaFeO2-type LiNi1/3Co1/3Mn1/3O2has attracted significant attention on account of its many advantages over LiCoO2,namely,high capacity,structural and thermal stability,and excellent cycle ability.LiNi1/3Co1/3Mn1/3O2has a layered structure(space group:R-3m)with Li-ions located in-between the alternate layers of edge-shared MO6octahedra.Ni2+and Co3+involve in the electrochemical process while Mn4+with very high activation barrier for cation movement through tetrahedral site acts like a pillar to stabilize the structure during cycling.Also,LiNi1/3Co1/3Mn1/3O2has many disadvantages,such as poor cycle performance at high discharge rate and high cut-off voltage[6-11].It is reported that the electrochemical properties of LiNi1/3Co1/3Mn1/3O2are strongly affected by the synthesis condition[12-21].One way to enhance cycle performance of LiNi1/3Co1/3Mn1/3O2cathode materials is to coat their surfaces with nanoscale layers onto the cathode particle surface.These nanocoating layers protect the highly delithiated cathode active materials from HF attack in the electrolyte,which greatly suppresses undesirable increases in interfacial resistance[22-26].Coating the surface of LiNi1/3Co1/3Mn1/3O2with ZrO2,AlPO4,Al2O3and LiAlO2has been attempted.It is found that the cycle-life retention is improved to 93.9%of its initial capacity without decreasing its original specific capacity by coated with Al2O3.
In this paper,the LiNi1/3Co1/3Mn1/3O2is first obtained by the controlled crystallization method and then coated with Ni3(PO4)2particles.The principalaim of the present study is to clarify the effect of Ni3(PO4)2to the electrochemical characters.To the authors' knowledge this is the first time that Ni3(PO4)2particles are used as coating layer to improve the electrochemical properties of LiNi1/3Co1/3Mn1/3O2cathode.The effects of the coating on rate capability and cycle life a thigh cut-off voltage are investigated.
2.Experimental Procedure
2.1.Sample preparation
LiNi1/3Co1/3Mn1/3O2is first prepared by the controlled crystallization method.Stoichiometric amounts of MnSO4·H2O,NiSO4·6H2O and CoSO4·6H2O were pumped into a continuously stirred tank reactor(volume 2 L).At the same time,NH3·H2O and NaOH solution was also fed into the reactor.The reaction temperature and feed rate of the reactants to the reactor were controlled carefully.The pH value was maintained between 10.5 and 11 by controlling the amount of NaOH.After vigorous stirring at 60°C for a certain time,the homogenously Ni1/3Co1/3Mn1/3(OH)2was filtered and dried.The particle size was controlled by pH,reaction time and stirring speed.To prepared LiNi1/3Co1/3Mn1/3O2powders,stoichiometric amounts of the as-prepared Ni1/3Co1/3Mn1/3(OH)2and Li2CO3were mixed,and then sintered at 900°C for 15 h.
To prepare the Ni3(PO4)2-coated LiNi1/3Co1/3Mn1/3O2,1 g LiNi1/3Co1/3Mn1/3O2powder was dispersed in the solution of H3PO4and Ni(NO3)2·6H2O,and stirred at room temperature for 1 h.Then,the pH value was set to 5 by adding NH3·H2O,and stirred at room temperature for another 2 h.Finally,the precipitate was filtered,dried and heat treated at 500°C for 5 h.The mass percent of Ni3(PO4)2was 2%.
2.2.Powder X-ray diffraction and scanning electron microscope
The structure of LiNi1/3Co1/3Mn1/3O2material was determined by X-ray diffraction(XRD,Rigaku,RU-200).The scan data were collected in the 2θ range of 10°-80°in steps of 2°per minute.The particle size and morphological features of the obtained powders were observed via a scanning electron microscope equipped with energy dispersive spectroscopy(EDS).
The charge and discharge behaviors were investigated using CR2032-type coin cells.The cathode composite was prepared by mixing active material,acetylene black and polytetra fluoroethylene(PTFE)binder in the weight ratio of 80:10:10.The mixture was rolled into thin sheets.The sheets were cut into roundels of 6 mm in diameter.The strips were dried at 120°C overnight.The cells were assembled in an high purity argon- filled glove box(Mikrouna,Universal 2440/750)with lithium foil as the anode and 1 mol·L−1LiPF6/ethylene carbonate(EC)+dimethyl carbonate(DMC)(1:1,by volume)as the electrolyte.The assembled cells were then aged for 3 h before electrochemically testing between 2.7 and 4.6 V(versus Li/Li+)using CT2001A Land instrument(Wuhan Jinnuo Eleectronics Co.Ltd.,China).The amplitude used for impedance measurement was 5 mV and the frequency was from0.1 Hz to 100 kHz,and the tests were conducted by an M2273 electrochemistry analyzer(EG&G Princeton Applied Research,USA).ICP analysis is conducted by HK 2000 inductively coupled plasma spectrograph(Beijing Huakeyitong Co.Ltd.,China).All tests were performed at room temperature.
3.Results and Discussion
3.1.X-ray diffraction

Fig.1.XRD patterns of LiNi1/3Co1/3Mn1/3O2 and the coated sample.
The X-ray diffraction(XRD)patterns of LiNi1/3Co1/3Mn1/3O2and the coated sample are shown in Fig.1.All of the observed peaks can be indexed to a hexagonal α-NaFeO2structure(space group:R-3m).No peaks corresponding to Ni3(PO4)2were observed,indicating that they are amorphous or of low crystallinity in the sample.The clearly split(0 0 6,0 1 2)and(0 1 8,1 1 0)pairs in the two XRD patterns indicate that the layered structure is formed.On the other hand,c/a values of the both sample are more than 4.9,which is desirable for better hexagonal structure.The re fined cell parameters for the Ni3(PO4)2-coated sample are a=2.8639 and c=14.1958.The re fined cell parameters are approximately equal to those of the standard LiNi1/3Co1/3Mn1/3O2,implying that the Ni3(PO4)2coatings do not change the crystal structure of LiNi1/3Co1/3Mn1/3O2.The integrated intensity ratio of the(0 0 3)to(1 0 4)lines in the XRD patterns was shown to be a measure of the cation mixing.When I003/I104>1.2,the degree of cation mixing is smaller.The integrated intensity ratio of(003)to(104)peaks of these two samples is above 1.2,indicating lower amount of cation mixing.Also,the value of R[R=(I006+I102)/I101]is related to the hexagonal ordering.The lower the value of R,the more perfect the hexagonal structure.In order to identify the element ratios of Li:Ni:Mn:Co for the two samples,ICP analysis is conducted.The result indicates that the element ratios of Ni:Mn:Co are close to 1:1:1,and the Li/M is 1.03,while M represents the total amount of metal ions.The redundant Li,on the one hand,is to compensate the Livolatization in the high temperature sintering process,while,on the other hand,is to raise the valence of Mn to avoid the John-Teller effect accordingly.
3.2.Surface morphology
The SEM images of LiNi1/3Co1/3Mn1/3O2and the coated sample are shown in Fig.2.As shown in all SEM images,the secondary particles are composed of closely aggregated primary particles.From the images,it can be seen that the morphology of the particles is spherical and the particle size distribution is mainly located from 5 to 18 μm as indicated in Fig.3.The data in size distribution show that D10=5.683 μm,D50=11.508 and D90=23.295 μm.Thus,it has a relative wide size distribution.The relative small particles are filled in the interstices of the large particles,and therefore all particles are tightly packed.This structure is a benefit to the enhancement of the tap density.The tap density of prepared LiNi1/3Co1/3Mn1/3O2and the coated sample is 2.3 g·cm−3.In order to identify the distribution of Ni3(PO4)2coating on the surface of LiNi1/3Co1/3Mn1/3O2,EDS analysis is conducted.Fig.2(c)is performed by a surface scanning.The EDS spectrum and the element mappings of Ni,Co,Mn,P,O,Au and Al are indicated in Fig.4.Al and Au are introduced from the aluminum substrate for EDS study.From the P mapping,it is found that element P distributes uniformly on the surface of LiNi1/3Co1/3Mn1/3O2.Owing to the existence of the P element,it can be concluded that the compound composition of Ni3(PO4)2is synthesized.Also,the high-resolution transmission electron microscope(HRTEM)image of LiNi1/3Co1/3Mn1/3O2is shown in Fig.5.It is apparent in Fig.5 that there are some cotton-like particles deposited on the surface of LiNi1/3Co1/3Mn1/3O2.These particles are identified as Ni3(PO4)2and the Ni3(PO4)2layer reaches about 20 nm.Thus,the HRTEM can further demonstrate the existence of the surface coating of Ni3(PO4)2.Accordingly,it is reasonable that Ni3(PO4)2is distribution uniformly on the surface of LiNi1/3Co1/3Mn1/3O2.
3.3.Electrochemical performance
The cycle-life performances of the bare and Ni3(PO4)2-coated LiNi1/3Co1/3Mn1/3O2operated between 4.3 and 2.7 V at the rate of 0.5C,1C,2C and 5C are shown in Fig.6.The initial discharge capacity of the bare LiNi1/3Co1/3Mn1/3O2decreases gradually with cycling and finally reaches 56%at 5C rate of its initial discharge capacity after 40 cycles.But the cycle performance ofNi3(PO4)2-coated LiNi1/3Co1/3Mn1/3O2is more excellent than the bare LiNi1/3Co1/3Mn1/3O2,especially at high discharge rate.The specific capacity at 5C rate of Ni3(PO4)2-coated LiNi1/3Co1/3Mn1/3O2is 91.3 mA·h·g−1,while the bare LiNi1/3Co1/3Mn1/3O2is 80.5 mA·h·g−1.When the discharge rate increases from 0.5C to 5C,the discharge capacity of the Ni3(PO4)2-coated LiNi1/3Co1/3Mn1/3O2decreases from 146.3 to 91.3 mAh·g−1,which is 62%capacity retention and higher than the bare LiNi1/3Co1/3Mn1/3O2.The rate capability is an important factor for battery performance because the charge time of batteries in portable electric devices depends on the rate of Li+extraction and intercalation from and into the cathode.Thus,it can be concluded that the Ni3(PO4)2coating can obviously enhance the battery performance,especially for power battery.
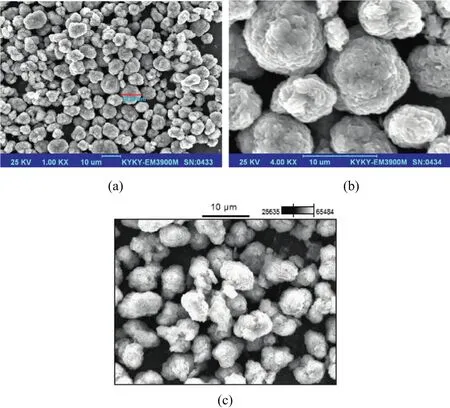
Fig.2.SEM images of LiNi1/3Co1/3Mn1/3O2 and the coated sample,(a)and(b):the pure LiNi1/3Co1/3Mn1/3O2,(c):Ni3(PO4)2 coated LiNi1/3Co1/3Mn1/3O2.
The cycle-life performances of the bare and Ni3(PO4)2-coated LiNi1/3Co1/3Mn1/3O2operated between 4.3 and 2.7 V at the rates of 0.5C,1C,2C and 5C are shown in Fig.7.Considering that the charge cut-off voltage is 4.6 V,this cycle-life characteristic is excellent compared with that of the bare LiNi1/3Co1/3Mn1/3O2.The discharge capacity of the bare LiNi1/3Co1/3Mn1/3O2is decreased gradually at the rates of 0.5C,1C and 2C,and decreased drastically at 5C.But Ni3(PO4)2-coated LiNi1/3Co1/3Mn1/3O2has an outstanding capacity retention,where 99%capacity retention after 10 cycles at 5C rate is observed.It is known that the LiPF6salt in electrolyte can generate HF via reaction with trace H2O,especially at high cut-off voltage the reaction would be more violent.The generated HF attacks the surface of the cathode and accordingly the metal ions in the surface(Ni,Co,Mn)dissolve into the electrolyte.The existence of Ni3(PO4)2coating can effectively prevent the fatigue of the particles during the continuously cycling at high voltage and keep the structural stability.Thus,the cycle performance is prominently improved.
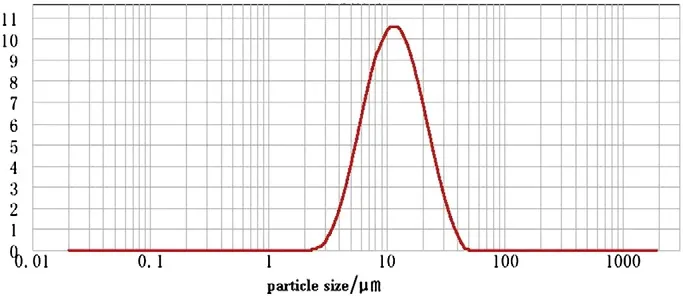
Fig.3.The size distribution of bare LiNi1/3Co1/3Mn1/3O2.
3.4.Electrochemical impedance spectra
The electrochemical impedance spectra of all the samples are illustrated in Fig.8.Each spectrum shows an intercept at high frequency for the resistance of the electrolyte“Re”on the realaxis Zre,followed by a semicircle in the high-to-middle frequency range and an inclined line in the low frequency range.The inclined line is attributed to the diffusion of the lithium ions into the bulk of the electrode material,the so-called Warburg diffusion.The impedance spectra can be described by the equivalent circuit presented in the inset picture,where Rctrepresents the charge transfer resistance,Rwand the Warburg element(W s)represent the Warburg impedance and the constant phase element CPE1represents the double layer capacitance.The size of Rctis reflected by the semicircle intercept on the Zreaxis.It can be seen that the value of Rctfor Ni3(PO4)2-coated LiNi1/3Co1/3Mn1/3O2is smaller than that of bare LiNi1/3Co1/3Mn1/3O2.Therefore,it is rational to conclude that the Ni3(PO4)2coating improves the properties of the LiNi1/3Co1/3Mn1/3O2particle surfaces,and restrains the reaction between HF and the cathode surface.Accordingly,the Rctdecreases obviously.The above result is in accordance with the cycle performance.


Fig.4.EDS spectrum and the element mappings,(a)the EDS spectrum,(b)O,(c)P,(d)Mn,(e)Co,(f)Ni.
The exchange current density is a parameter to indicate the reversibility of the electrode.The exchange current density(i0)of the samples is calculated from the equation where n is the number of electrons transferred per molecule during the intercalation,F is the Faraday's constant(96500 C·mol−1),R is the gas constant(8.314 J·mol−1·K−1)and T is the temperature(298.5 K).It can be seen that i0is inversely proportional to Rct.Thus,the i0of Ni3(PO4)2-coated LiNi1/3Co1/3Mn1/3O2is higher than that of bare LiNi1/3Co1/3Mn1/3O2,implying its best reversibility.

Fig.6.Cycle-life performances of the bare and Ni3(PO4)2-coated LiNi1/3Co1/3Mn1/3O2 operated between 2.7 and 4.3 V.

Fig.7.Cycle-life performances of the bare and Ni3(PO4)2-coated LiNi1/3Co1/3Mn1/3O2 operated between 2.7 and 4.6 V.

Fig.8.Electrochemical impedance spectra of the samples.
4.Conclusions
LiNi1/3Co1/3Mn1/3O2is first prepared by the controlled crystallization method and then coated with a Ni3(PO4)2nanolayer.SEM images show that the morphology of the particles is spherical and particle size distribution is located from 6 to 23 μm.The tap density of prepared LiNi1/3Co1/3Mn1/3O2and the coated sample is 2.3 g·cm−3.The current exchange density i0of the coated sample is higher than that of LiNi1/3Co1/3Mn1/3O2,which is beneficial to its electrochemical performances,including the discharge capacity,rate capability and cycle performance.The maximum discharge capacities of 176.6 mA·h g−1and 104.9 mA·h g−1at 1C and 5C rates have been demonstrated by Ni3(PO4)2-coated LiNi1/3Co1/3Mn1/3O2.It also exhibits good cycle ability at different cut-off voltages.
 Chinese Journal of Chemical Engineering2015年1期
Chinese Journal of Chemical Engineering2015年1期
- Chinese Journal of Chemical Engineering的其它文章
- Power consumption and flow field characteristics of a coaxial mixer with a double inner impeller☆
- Drag-induced breakup mechanism for droplet generation in dripping within flow focusing microfluidics☆
- Lattice Boltzmann simulation of double diffusive natural convection in a square cavity with a hot square obstacle
- Numerical simulation of steady flow past a liquid sphere immersed in simple shear flow at low and moderate Re☆
- Effect of sol size on nanofiltration performance of a sol-gel derived microporous zirconia membrane☆
- Mass transfer performance of structured packings in a CO2 absorption tower☆
