Modified activated carbons with amino groups and their copper adsorption properties in aqueous solution
Mohammad Hassan Mahaninia *,Paria Rahimian ,Tahereh Kaghazchi
1 Department of Chemistry,University of Saskatchewan,Saskatoon,Saskatchewan,Canada
2 Department of Textile Engineering,Amirkabir University of Technology,Tehran,Iran
3 Department of Chemical Engineering,Amirkabir University of Technology,Tehran,Iran
Keywords:Activated carbon Amino groups Cu(II)adsorption Freundlich model
ABSTRACT Activated carbons were prepared by two chemical methods and the adsorption of Cu(II)on activated carbons from aqueous solution containing amino groups was studied.The first method involved the chlorination of activated carbon following by substitution of chloride groups with amino groups,and the second involved the nitrilation of activated carbon with reduction of nitro groups to amino groups.Resultant activated carbons were characterized in terms of porous structure,elemental analysis,FTIR spectroscopy,XPS,Boehm titration,and pHzpc.Kinetic and equilibrium tests were performed for copper adsorption in the batch mode.Also,adsorption mechanism and effect of pH on the adsorption of Cu(II)ions were discussed.Adsorption study shows enhanced adsorption for copper on the modified activated carbons,mainly by the presence of amino groups,and the Freundlich model is applicable for the activated carbons.It is suggested that binding of nitrogen atoms with Cu(II)ions is stronger than that with H+ions due to relatively higher divalent charge or stronger electrostatic force.
1.Introduction
Activated carbon is a conventional porous adsorbent with unique physical properties such as relatively large surface area,high porosity,and variable pore size distribution and also contains oxygen functional groups,which have significant influences on the adsorption properties of this material[1-5].Activated carbon has recently attracted great attention in water and wastewater treatment processes due to its cost effectiveness,abundance,and suitable adsorptive properties[6-9].Chiefly,one of the most important reasons for suitability of this adsorbent in such applications is its high ability tow ardsorganic substance removal,which makes it suitable for water purification[10-12].However,this adsorbent has lower sorption affinity towards inorganic substances.
Heavy metals are among the most toxic pollutants entering the environment in vast quantities through waste waters of different industrial plants such as mining operations,electronics,tanneries,electroplating and petrochemical industries,as well as textile mill plants[13-15].Specially,copper is commonly used in electric and electroplating industries and agricultural poisoning[15]and can be found in the wastewater effluents of these industries.When present with high concentrations in the human body,Cu(II)ions can cause liver and kidney damages.The legislation limits for discharge of copper are recommended to be less than 2 mg·L−1by the World Health Organization[15].
There are many processes for treatment of Cu(II)from contaminated waste waters,including chemical precipitation,membrane filtration,reverse osmosis,ion exchange,and adsorption.Among various treatment technologies,activated carbon adsorption is commonly used due to its easy operation,porous surface structure and harmlessness to the environment.However,in general,activated carbons are more effective for the adsorption of organic compounds rather than metal ions and inorganic pollutants,so developing methods for modification of activated carbons towards these materials should be considered.
Usually,original activated carbons contain some functional groups(mostly oxygen groups),and several techniques have been used to improve their structure by introducing other heteroatom-containing functional groups.For example,many investigations have been carried out on activated carbon to introduce functional groups,and it is expected to improve the adsorption of adsorbent towards specific adsorbate[16-19].
Amination of activated carbon,as one of the ways for modification of its structure,has been investigated in several researches,through the reaction with NH3at elevated temperatures[20,21],but amination through chemical reaction at low temperature has been rarely reported[22,23].Considering the limited researches about using these kinds of activated carbons for copper removal,the amination of activated carbon and its influence on copper adsorption capacity are investigated in the present work.In this context,it is attempted to modify the surface of activated carbon by two methods,both introducing amino groups on activated carbon at low temperature.The first innovative method is based on the chlorination of activated carbon and then substitution of chloride groups with amino groups.The second method has been reported elsewhere[24],based on nitrilation of activated carbon and reduction of nitro groups to amino groups.
Eventually,the adsorption capability of the modified adsorbents towards Cu(II)in aqueous phase is determined in the batch mode.Kinetic and equilibrium experiments are carried out in order to determine and compare the rate and capacity of Cu(II)adsorption and provide reasonable mechanisms involved in the copper adsorption by the newly designed sorbents.
2.Materials and Methods
2.1.Activated carbon sample
Commercially activated carbon(prepared from Norit)with 425 kg·m−3apparent density and 15 mm particle size(D50)was used as the carrier adsorbent for introduction of nitrogen groups,denoted as SAE.SAE was dried at 90°C for 24 h after washing with 10%HCl and deionized water separately in a Soxhlet apparatus for 24 h to remove impurities.
2.2.Amination of activated carbon
2.2.1.First method:Chlorination of activated carbon and substitution of chloride groups with amino groups
Fig.1 shows the mechanisms of surface modification by the two methods.In the first method,amino groups are introduced to the activated carbon surface in a three-step procedure.The first step involves the oxidation of carbon to increase oxygen functional groups.10 g of carbon was oxidized with 100 ml of nitric acid(5 mol·L−1)at 84 °C for 5 h.Then the mixture was filtered and washed with deionized water in a Soxhlet apparatus till neutral pH was attained.The second step involves the chlorination of activated carbon and transformation of oxygen groups into chloride groups.10 g oxidized activated carbon was treated with 25 ml concentrated thionyl chloride at 84°C and refluxed for 24 h.Then the mixture was filtered and washed with deionized water in the Soxhlet apparatus till neutral pH was attained.In the final step,chloride groups were substituted into amino groups by another reaction;10 g of chlorinated carbon along with 50 ml of aqueous ammonia(28%)was placed in a 1000 ml flask and refluxed for 24 h.Then the mixture was filtered and washed with deionized water in the Soxhlet apparatus till neutral pH was attained.It is necessary to note that after each step,the product was dried at 90°C.This carbon sample is hereafter abbreviated to ACN1.
2.2.2.Second method:Nitrilation of activated carbon and reduction of nitro groups to amino groups
The second method for the amination of activated carbon includes two stages.The first step involves a reaction for the nitrilation of activated carbon.At0 °C(an ice bath),50 ml of concentrated(18 mol·L−1)sulfuric acid(H2SO4)was added slowly to 50 ml of concentrated(15.7 mol·L−1)nitric acid(HNO3).Then,10 g activated carbon was slowly added to this acid mixture and stirred for 50 min in the ice bath.The mixture was filtered and washed with deionized water in the Soxhlet apparatus.The product was then dried at 90°C.The second step was the reduction of nitrobenzene to aniline by FeCl2.Reduction of nitrated activated carbon proceeded in a 1000 ml flask containing 100 ml of hydrochloric acid,2 g of powdered iron,and 8 g of carbon with stirring at 80°C for 24 h.The aminated activated carbon obtained was dried at 90°C after the separation of additional powdered iron with magnet and washing with deionized water in the Soxhlet apparatus.This carbon sample is hereafter abbreviated to ACN2.
3.Characterizations
3.1.Point of zero charge measurements
Point of zero charge for produced carbons was measured according to the method suggested by Noh and Schwarz[25],which requires recording of the equilibrium pH after shaking of suspensions of carbon samples in distilled water for 24 h.The initial pH of the suspensions was selected in the range of 2-11.The fixed equilibrium value of pH was taken as the pHzpc.
3.2.Boehm titration
The method introduced by Boehm[26]was used for the determination of the amount of acidic functional groups available on the activated carbon surface.In this method,0.5 g activated carbon sample was weighed into three 100 ml conical flasks.Then 30 ml of aqueous solutions of sodium hydrogen carbonate(0.1 mol·L−1),sodium carbonate(0.1 mol·L−1),and sodium hydroxide(0.1 mol·L−1)was added into the flasks.Suspended solutions were kept at20°Cfor24 h.After filtration,10 ml of withdrawn aliquots of products was titrated with 0.1 mol·L−1hydrochloric acid.The amount of acidic surface functional groups such as carboxylic groups and phenolic hydroxyl groups was calculated using the titration data.
3.3.Elemental analysis
Elemental analysis of the produced samples was performed by an elemental analyzer(model Carlo Erba 1106).The sample was weighed accurately on an aluminum foil and put into the instrument.Prior to the flash combustion process,the system was purged with helium carrier gas.Flash combustion was then performed at 2073 K,and the gaseous combustion products were quantified using a thermal conductivity detector.Results were obtained as percentages of carbon,hydrogen,and nitrogen,and the oxygen content was determined by difference.Also the amounts of volatile matter of all samples were measured.Samples were weighed,placed in a covered crucible,and heated in a furnace at(1173±15)K.The samples were cooled and weighed.Loss of mass represents moisture and volatile matter.The remainder is coke( fixed carbon and ash).

Fig.1.Synthesis of aminated activated carbon by two different methods.
3.4.Fourier transform infrared spectroscopy(FTIR)
The surface organic FTIR spectra were taken using a Perkin Elmer spectrophotometer instrument(model Paragon 1000PC).Data acquisition was performed automatically using an interfaced computer and a standard software package.The samples were dried first under vacuum at 150°C,ground with KBr salt followed by compression between two stainless steel cylinders to form a thin transparent solid film.The spectrometer collected 64 spectra in the range of 400-4000 cm−1,with a resolution of 4 cm−1and 100 scans.
3.5.Determination of porous properties
The textural parameters of all samples were determined by nitrogen adsorption experiments at liquid nitrogen temperature(77 K)with a Quantachrome NOVA 1000 instrument equipped with a commercial software of analysis and calculation.The samples were first out gassed at 523 K for 3 h under the vacuum prior to the N2adsorption/desorption tests.The specific surface area was calculated according to the Brunauer-Emmett-Teller(BET)equation,assuming a nitrogen molecule surface area of 0.162 nm2.The total pore volume and the volume and surface area of micro-and mesopores were also determined.
3.6.X-ray photoelectron spectroscopy measurement
A Shimadzu XPS(AXIS-HS type)was employed to measure changes in surface functional groups before and after surface modification.The AlKRline was used as the exciting X-ray source(1486.6 eV).
3.7.Copper adsorption experiments
The capacities of commercially activated carbon(SAE)and the aminated carbons(ACN1 and ACN2)to adsorb Cu(II)ions from aqueous solutions were determined through a series of batch mode adsorption experiments by a flame atomic adsorption spectrometer(GBC 906,Australia).The stock solution of 1000 mg·L−1Cu(II)was prepared by dissolving 1 g CuCl2in deionized water acidified with 5 ml of concentrated HCl and diluting to a 1 L volume.The kinetic and equilibrium experiments were carried out in order to determine and compare the rate and capacity of Cu(II)adsorption for the produced activated carbons.In the kinetic tests,0.01 g of adsorbent was added to a number of 50-ml glass flasks containing 30 ml Cu(II)solution with the initial concentration of 40 mg·L−1.The suspensions were shaken at 150 r·min−1and 25°C,and after 2,5,10,30 and 60 min,and then after every 60 min(until 1440 min),4 ml of the solution was sampled by a 10 ml plastic syringe and filtered by a What man NO.42 filter,and then analyzed for Cu(II)concentration using an atomic absorption spectrophotometer.The equilibrium isotherm of Cu(II)adsorption was established by adding adsorbents into a series of100-mlglass bottles with the initial pH of~7.0.The suspensions were shaken at150 r·min−1and 25 °C for24 h,and then the solutions were filtered and analyzed using an atomic absorption spectrophotometer.All the above-mentioned adsorption experiments were repeated three times and the average values were reported.
3.8.pH study
The uptake of Cu(II)as a function of pH was determined in the pH range of 2-6.The initial pH of solutions was adjusted by adding the required amount of dilute NaOH and HCl solutions(0.1 mol·L−1).The suspensions were shaken at 200 r·min−1for 24 h under 25 °C.
4.Results and Discussion
4.1.Physico-chemical characterization
The textural and chemical characteristics of the starting carbon and the samples after amination are presented in Table 1.The amination of SAE carbon by each procedure leads to a little decrease in the BET surface area of the modified carbons(ACN1 and ACN2)and the amounts of this decrease in both amination methods are almost the same and insignificant.This suggests that the aromatic hydrocarbon employed reactions to introduce amino groups on the carbon surface,but did not block the pores opening on the adsorbent surface.The value of pHpzcin the carbons containing an amino group is higher than the virgin one.The amount of basic sites available on the surface of modified activated carbons determined by the Boehm titration was higher than that of the virgin sample and all of these results indicate that the addition of aminated groups raisesthe surface basicity significantly,suggesting the introduction of basic amino groups into the carbon surface.It clearly derivates from the results of Boehm titration that the amounts of lactone,hydroxyl and carboxyl groups decrease in the modified samples compared to SAE perhaps due to exchange between hydroxyl and amino groups in the modified samples.

Table 1 Textural and chemical characteristics of activated carbon samples
Table 2 gives the results of elemental analysis for all samples.The aminated samples present less volatile matter than the SAE(original carbon)due to the surface reactions.The results show increases in the nitrogen content of modified carbons compared to the virgin sample.It can be seen that both procedures have a relatively good ability to introduce nitrogen into the structure of activated carbons and can be considered as the efficient methods for the amination of materials.Comparison of the two amination methods shows that the ACN1 sample has more nitrogen content than ACN2,so that the first method is more successful in the amination of carbons.

Table 2 Elemental analysis of the activated carbons
Fig.2 shows the surfaces of the modified activated carbons with amino groups and the virgin one studied using FTIR analysis and spectra.In order to get better comparison,the spectra are divided into several subregions and shown separately.Some peaks appear in the first sub-region,concerning C=O stretching(1750 cm−1),C=C stretching of aromatic rings and C-N stretching(1000-1350 cm−1)[27].Unfortunately,no direct evidence could be observed for N-H stretching,because the most diagnostic signal from N-H stretching is expected at 3200-3400 cm−1,and in the present spectra it is actually lost within the broad O-H stretching bands in the main panel.

Fig.2.The FTIR spectra of virgin(SAE)and aminated activated carbons(ACN1&ACN2).
Fig.3 shows the X-ray photoelectron spectroscopy(XPS)measurements on N(1s).A peak assigned to the N-O bond and another one assigned to C-N-H are found at about 405 and 399 eV,respectively[28].
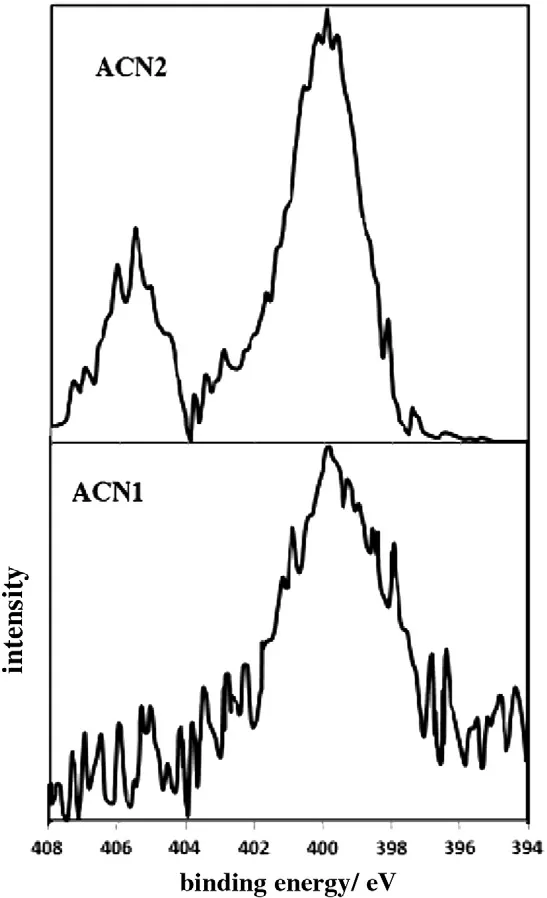
Fig.3.N(1s)XPS spectra of modified carbons.
Table 3 shows the calculation results on the ratio of area under each of the peaks to that under the peak for C(1s)of the samples.The intensity at the peak assigned to the N-O bond is very low in ACN1,whilethat at the peak assigned to the N-H bond increases for both modified activated carbons.This confirms that amination with the second method introduces nitro groups onto the activated carbon surface and they change to amino groups through its reduction.

Table 3 N/C values of modified carbons measured by XPS
4.2.Copper adsorption

Fig.4.Adsorption capacity of Cu(II)as a function of solution pH.
Fig.4 illustrates the effect of solution pH on the copper adsorption capacity of activated carbons.-NH2binds with Cu(II)ions by coordination rather than the ion-exchange process.The pH of a solution is an important evidence to be considered when using an activated carbon containing amino groups as a sorbent material.The efficiency of Cu(II)ion removal by activated carbon is dependent on p Kaof the ligand and the stability constant of the metal-ligand complex as well as the pH of the solution[29].All samples with weak adsorption of copper species at acidic pHs may be justified to the existence of large amount of hydronium ions(H3O+)that compete with the positively charged Cu(II)for the surface adsorbing sites.Thus the adsorption of Cu(II)will be decreased.The pH value cannot exceed 6 since precipitation of copper hydroxide will be accrued.The adsorption capacities of Cu(II)ions increased significantly as the pH value increased from 2 to 4 and remained approximately at0.05 mmol·g−1(equal around 3.177 mg·g−1)as the pH increased to 5.8.NH2-AC was able to remove 30%-90%of Cu(II)at pH 2-4,whereas the virgin activated carbon could remove a mere amount of Cu(II)in the same pH range.The pH-dependent adsorption could be interpreted by the pH effects on the association/dissociation of surface functional groups,surface charges,formation of ion species,and interactions between functional group and metal ions,which was discussed in details[30,31].At low pH,the electrical repulsion between Cu(II)ions and positively charged function groups on the carbon surface would be responsible for the low Cu adsorption while at high solution pH,the carbon surface became more negatively charged due to the dissociation of functional groups,which could enhance the electrostatic interactions of Cu(II)ions with negative function groups and therefore make the copper adsorption convenient.With these explanations,other adsorption experiments were carried out at the initial pH of solution of 6.0 to reach the maximum copper adsorption capability.
Fig.5 shows the percentage of Cu(II)(aq)removal from aqueous phase as a function of time.The initial adsorption rate of the ACN1 and ACN2 samples is higher than that of the SAE sample,although SAE has the highest BET surface area.Thus it may be concluded that the porous structure is not the most important factor determining the adsorption capacity of these activated carbons towards copper,and their surface chemistry and functionalities can also have a role.Amino groups present on the carbon surface substantially enhance the adsorption of Cu(II)(aq)under neutral or slightly basic conditions,with the carbon of highest nitrogen content(ACN1)having greater adsorption capacity.It can be seen that during the first hour of the process,almost 90%of removal capacity is achieved and then the adsorption gradually reaches a steady state in all of the samples.

Fig.5.Cu(II)adsorption as a function of contact time(operating conditions:pH 6.0;T=25°C).
In order to determine the Cu(II)sorption capacity of the activated carbons,the equilibrium adsorption tests were also carried out.The experimental results are shown in Fig.6,by plotting the equilibrium adsorption capacity of adsorbents qe[=V(C0−Ce)∕M]versus Ce,where C0and Ceare the initial and equilibrium concentrations of Cu(II)ion in the solution,V is the volume of the solution and M is the mass of adsorbent.It can be seen that the amount of Cu(II)adsorbed by the ACN1 and ACN2 samples at equilibrium conditions is much higher than that by the virgin sample.The higher adsorptive capacity of these samples can be related to the formation of strong Cu-bands with the-NH2functional group because of the basic property of nitrogen groups.It has been found that the NH2group has a large capacity to make a complex with metalions.Therefore,activated carbon produced by the first method has a significant effect on the capacity to attract aqueous Cu(II)ions.The higher the nitrogen content of adsorbent,the larger the equilibrium adsorption capacity towards Cu(II)ions.Thus the adsorption capacity of ACN1 resulted from higher nitrogen content is higher than that of the other samples.In the case of the ACN2 sample,as it is noted before,using nitric acid in the modification process reduces the pHzpcvalue and increases the surface acidity of the sample,both having reverse effects on the adsorption capacity of ACN2.
The experimental data on equilibrium study for the adsorption of Cu(II)onto the adsorbents are fitted with the most common 2-parameter adsorption isotherm model for adsorption from liquid liquids:Freundlich model.

The mechanism and the rate of adsorption are functions of constants 1/n and KF.
The values of parameters and the correlation coefficients for two different temperature conditions(15 and 25°C)are given in Table 4.According to the value of R2,it can be concluded that the fit of the Freundlich equation for the adsorption of copper on all adsorbents is good,while the fitting for the aminated samples is better than the virgin sample.Fora good adsorbent with a favorable adsorption,0.2<1/n<0.8,and the values obtained for all samples situate in this range.The other constant of the Freundlich model(KF)is indicative of the adsorption capacity of adsorbent towards adsorbate,and the aminated samples have higher values than the virgin sample.The higher the adsorption temperature,the higher the value of KF.

Table 4 Constants and correlation coefficients of Freundlich plots
4.3.Copper adsorption mechanisms
Avariety of interactions between metalions and the carbon surface is possible,for example,formation of surface complexes[32],ion-exchange processes with the participation of strong surface acidic groups[33],and redox reactions with a change of metal valence[34].
In the case of the modified carbon,more specifically with the presence of amino groups,nitrogen atoms of the amino groups have free ion pairs of electrons that can potentially bind with Cu(II)ions or H+ions to form a coordination complex through an electron pair sharing as illustrated in Fig.7.Binding of nitrogen atoms with Cu(II)ions is speculated to be stronger than that with H+ions due to relatively higher divalent charge or stronger electrostatic force[35,36].It is believed that a combination of more negatively charged surface and the presence of amino groups as induced by the modification could be responsible for enhanced Cu(II)ion adsorption by ACN1 and ACN2.
It seems necessary to notice that besides ion exchange,physical adsorption in micro-and especially in meso-pores of the produced activated carbon has an important role in the adsorption of copper species.

Fig.6.Equilibrium isotherms of Cu(II)adsorption by SAE,ACN2 and ACN1.(a)t=24 h,pH 6.0,T=15 °C;(b)t=24 h,pH 6.0,T=25 °C.

Fig.7.Formation of coordinating complex between amine groups and copper species.
5.Conclusions
In this work,introduction and characterization of amino groups(-NH2)onto the activated carbon structure were investigated through two different chemical reactions.The porous characterization of the modified carbons has shown a slight decrease in surface area with an efficient method for modification.Elemental analysis results,FT-IR spectra and also results of XPS substantiate that both procedures increase the nitrogen content and introduce amino groups on the surface of activated carbon successfully,while the first method introduces more amino groups on the activated carbon surface.Proved by the Boehm titration and pHzpc, fixed amino groups on the surface of adsorbent give it a basic characteristic,with which modified carbons are much more effective than the unmodified AC in sequestering Cu(II)over the pH range from 2.0 to 5.8.This basic nature could strengthen the reaction of activated carbon surface with Cu(II)ions according to the acid-base Lewis theory.For the applicability of the amino-activated carbons for the adsorption of copper from aqueous phase,it is found that the introduction of amino groups onto the carbon increases the adsorption capacity of Cu(II)ions considerably,because of a strong interaction of basic amino groups with Cu(II)ions.
 Chinese Journal of Chemical Engineering2015年1期
Chinese Journal of Chemical Engineering2015年1期
- Chinese Journal of Chemical Engineering的其它文章
- Power consumption and flow field characteristics of a coaxial mixer with a double inner impeller☆
- Drag-induced breakup mechanism for droplet generation in dripping within flow focusing microfluidics☆
- Lattice Boltzmann simulation of double diffusive natural convection in a square cavity with a hot square obstacle
- Numerical simulation of steady flow past a liquid sphere immersed in simple shear flow at low and moderate Re☆
- Effect of sol size on nanofiltration performance of a sol-gel derived microporous zirconia membrane☆
- Mass transfer performance of structured packings in a CO2 absorption tower☆
