Biogas by two-stage microbial anaerobic and semi-continuous digestion of Chinese cabbage waste☆
Xiaoying Dong ,Lijie Shao ,Yan Wang ,Wei Kou ,Yanxin Cao ,Dalei Zhang ,*
1 College of Engineering,Shenyang Agriculture University,Shenyang 110000,China
2 Liaoning Institute of Energy Technology,Yingkou 115000,China
3 MAHLE Engine Components Co.,Ltd.Piston Ring Plant,Yingkou 115000,China
Keywords:Anaerobic digestion Semi-continuous Two-stage Biogas Chinese cabbage waste Microbial ecology
A B S T R A C T Anaerobic digestion of Chinese cabbage waste was investigated through a pilot-scale two-stage digester at a mesophilic temperature of 37°C.In the acidification digester,the main product was acetic acid,with the maximum concentration of 4289 mg·L−1 on the fourth day,accounting for 50.32%of total volatile fatty acids.The oxidation reduction potential(ORP)and NH4+-N level decreased gradually with hydraulic retention time(HRT)of acidification.In the second digestion phase,the maximum methanogenic bacterial concentration reached 9.6 × 1010 ml−1 at the organic loading rate(OLR)of 3.5–4 kg VS·m−3,with corresponding HRT of 12–16 days.Accordingly,the optimal biog as production was 0.62 m3·(kg VS)−1,with methane content of 65%–68%.ORP and NH4+-N levels in the methanizer remained between−500 and −560 m V and 2000–4500 mg·L−1,respectively.Methanococcus and Methanosarcina served as the main methanogens in the anaerobic digester.
1.Introduction
Chinese cabbage,a staple vegetable of Northern China in winter,has a giant annual production of 500 million tons in Liaoning Province[1].Due to excess production,low market price or other reasons,large amounts of cabbage are discarded,causing economic loss and environmental problems.Biog as produced by biomass fermentation is regarded as a substitute for natural gas and anaerobic digestion of this biodegradable waste will provide a solution to recycle value-added products[2,3].Anaerobic digester(AD)is very promising due to low cost and ease of operation in absence of light and inorganic electron acceptors,such as oxygen[4].Two-stage AD has more applications in biog as productivity and organic matter removal compared with conventional one-phase digesters.Because the phase separation in two-stage AD provides the optimal conditions for acidogens and methanogens in turn,acidifying and methanizing organisms in their separate environs are assured.The production and quality of biog as can be substantially improved.By means of sufficient acidification-phase regulation,an efficient methane-producing effect has been realized,a key technology for improving overall efficiency of two-stage anaerobic digestion[5–8].One of our subjects,Methanosarcina,belongs to archaea,which is difficult to isolate and culture.Compared to the traditional method,molecular biology provides method to determine the composition of microbial community during AD processes.16S rDNA library-based analyses provide an accurate overview of the diversity within microbial biocoenoses because of greater sequence divergences between different species at 16S rDNA level[9].Real-time polymerase chain reaction(RT-PCR)and 454 pyrosequencing are usually used in the detection of microbial diversity,but 454 pyrosequencing is especially expensive.Nextgeneration sequencing has revolutionized genome sciences[10].We have performed our sequencing by improving the method using MiSeq sequencer,designated here as quantitative MiSeq(qMiSeq),for accurate quantification of libraries on large-scale sequencing projects,based on the Illumina platform with the advantages of speed and low cost[11].How ever,little work has been reported on application of MiSeq on mtGenome analysis.Several reports have provided quality metrics supporting the strength of Illumina MiSeq as a candidate for mtDNA analysis[12].
In this study,a pilot-scale two-stage AD is adopted in a semicontinuous feeding mode to treat Chinese cabbage waste(CCW)and biogas production is continuous.We present an investigation on optimal organic loading rate,biogas production and methane content.We will analyze methanizing archaea and general bacteria during coproduction by novel molecular method(miseq)to investigate predominant methanogens and related functional bacteria.
2.Materials and Methods
2.1.Anaerobic digestion reactor
To investigate the tw o-stage anaerobic digestion for CCW,a 10 L acidification digester and a 100 L methanization digester were used,as shown in Fig.1.Both digesters was heated to(37 ± 1)°C in a water bath system(24 h).A screw pump fed 100 L·h−1of the material from the acidification digester once a day to the methanization digester,and then the same volume of effluents was discharged to the return tank.3–5 L of effluents in return tank was used for reflux to adjust pH in the methanizer after periodic checks.A w et gas flow meter was used to measure the daily biogas production.

Fig.1.Semi-continuous two-stage digester.1—acidifier;2—methanizer;3—screw pump;4—gas flow meter;5—agitator;6—water bath;7—heating rod;8—interlayer;9—sampling mouth;10—feed port;11—over flow port;12—gasoutlet port;13—return tank;14—outlet.
2.2.Substrate composition
The composition of substrates is listed in Table 1.
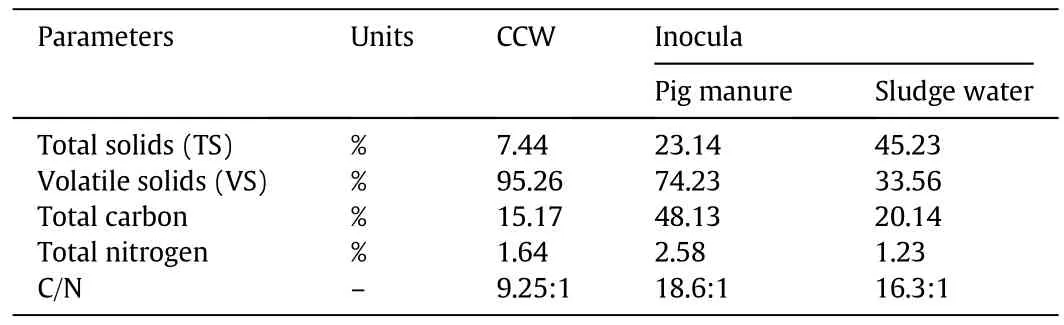
Table 1Average chemical composition of raw materials
2.3.Experimental procedures
CCW was collected from vegetable market and shredded by machine.To investigate the optimal acidification parameters,the slurry of CCW(0.2–0.7 kg VS·d−1)was introduced into the first digester for acidification.With 40%(by volume)fresh pig manure and 20%(by volume)sludge water,the methanogenesis phase was inculcated,left about one month to culture methanogens.When the concentration of methanogens was stable,the effluent from acidifiers was fed to the second digester daily at the feed rate of 3–11 kg CCW for more than two months.The feed was increased at a constant rate and each feed rate was employed for one w eek.
2.4.Chemical analysis
The liquid samples of total volatile fatty acids(VFAs)in the acidification and methane phase were centrifuged at 10000 r·min−1for 10 min,then filtered through a 0.45 μm membrane,and finally assessed using a capillary DB-WAX column(30 m×0.53 mm×1 μm)in a gas chromatograph(GC-7820A)with nitrogen as the carrier gas.Temperatures of injector and detector were 250 °C.The temperature of oven was 100–220 °C,heating at a rate of 15 °C·min−1.Sample volumes of 0.4 μl were injected via the detector(TCD:250°C).An oven was used to dry TS and VS and K2Cr2O method was applied to soluble chemical oxygen demand(SCOD)[13].The biogas production was tracked with a wet gas meter.Using a PHS-25 meter(Shanghai Leichi Instrumentation Factory),the pH value and ORP were measured.Ammonia nitrogen(NH4+-N)level was determined by pay reagent luminosity law[13]and methanogen concentration was determined by fluoroscope TYU-30C(Shanghai Yuguang Factory).
2.5.Microbial community analysis
2.5.1.DNA extraction
When the system was operated under steady conditions,the anaerobic digested sludge in the methanizer was sampled.After extracting total bacterial DNA from the sample by DNA isolation kit(Takara,Dalian,China),a PCR amplifier(Flexcycler,German)was applied according to the conditions described by Jonathan et al.[11]and the product was stored at−20 °C.
2.5.2.High-throughput sequencing
A library was constructed on the combined V4 region of 16S r DNA district based on Illumina Miseq technology.For the archaea,primer pairs 5′-CAGYMGCCRCGGKAAHACC-3′and 3′-GGACTACNSGGGTMTC TAAT-5′were used.For the bacteria,primer pairs 5′-GTGCCAGCMGC CGCGGTAA-3′and 3′-GGACTACHVGGGTWTCTAAT-5′were employed.The design of primers and sequencing were provided by Novogene Co.,Ltd.in Beijing.
3.Results and Discussion
3.1.Acidification
The four VFAs(acetic,propionic,isobutyric and butyric acid)increased with acidification HRT to 8058 mg·L−1in the first five days[Fig.2(a)].Acetic acid(HAc)reached 4289 mg·L−1on day 4,accounting for 53.2%of total VFAs.Isobutyric(HIBu)and butyric(HBu)acid were high,reaching 1894 and 2015 mg·L−1on days 5 and 6,respectively.These three VFAs composed the main substrate for biogas production.Unlike other studies,the production of HIBu in this experiment was relatively high,possibly due to the properties of cabbage.The specific metabolic mechanism of HIBu,how ever,needs further study.Accumulated at a certain concentration,HPa is usually an inhibitory substrate,with highest content at 721 mg·L−1on the 7th day.All VFAs were degraded by microbes to HAc before converting to CH4,with their conversion sequence being HAc>HEt>HBu>HPa[14].Other VFAs,valeric and caproic acids,were also detected,accounting for 2%–4%and 7%–9%of total volatile fatty acids(tVFAs),respectively.The tVFAs in the acidifiers attained a concentration of 9750 mg·L−1.Dinsdale et al.performed acidification tests with fruit and vegetable waste and achieved tVFAs of 6100 mg·L−1[15].Siegert and Banks found that VFA concentrations above 2000 mg·L−1inhibited cellulose degradation,while those above 4000 mg·L−1feebly inhibited glucose degradation[16].Most VFAs remain in the solution,while a small amount of VFAs convert to gases such as H2,H2S,CH4,and escape from the reactor[17,18].VFAs produced in the separate acidification digester have the advantage in obtaining a high yield while avoiding the inhibition of methanogen growth.The initial pH of acidifier is 5.65,decreases to a low point of 3.72 on day 4,and increases after the production of VFAs reduces.Acidifying bacteria exhibit some tolerance of pH variation and the limit for acidifying bacterial enzyme activity is pH 5.0–6.0[19].The pH value between 5 and 7 is beneficial for the hydrolysis of particulate organic matter.ORP is used to indicate oxygen content in anaerobic environment.Suitable redox potential is a basic condition for methane production in normal activity.Microorganisms can grow under a relatively wide facultative condition in an AD reactor.In our experiments,the redox potential of acidifiers gradually decreases with acidification HRTFig.2(b).The reason may be that oxygen content decreases with vegetable waste decomposition,with the low point at−395 mv on day 9.NH4+-N level in the acidifier also gradually decreases from 1498 mg·L−1to 387 mg·L−1.The cause may be the continuous conversion of NH4+-N,nitrate or N2by nitrobacteria and denitrifying bacteria.In studying bio-denitrification,Mulder et al.have found anaerobic ammonium oxidate,and anammox[20].When the microbes are under anaerobic conditions,they use NH4+as electron donor and NO3or NO2as electron acceptor,and then transfer ammonium nitrogen and nitrate nitrogen to N2.

Fig.2.Main parameters in the acidifier.
3.2.Methanation
Biog as production was observed daily with the material from the first digester at different feed rates in the range of 0.15–0.55 kg VS·d−1,without additional buffer.The main deficiency of anaerobic digestion of CCW is the tendency to low pH,which inhibits methanogens.Therefore,the digester was initially inoculated with 40%pig manure and 20%sew age water to provide sufficient capacity buffers in the second digester to prevent start-up failure due to acidification.Fig.3(a)depicts that initial daily biogas production increases with feed rate.When feed rate attains 0.4 kg VS·d−1,the average biogas production reaches its maximum value of 0.248 m3·L−1·d−1and the organic loading rate(OLR)achieves 4.0 kg VS·m−3·d−1,with the highest biogas production of 0.62 m3·(kg VS)−1.Total solid content reaches 15.98%at this OLR and most of the TS are converted to biogas.After the starting period,the methane content of the biogas is always over 50%.The maximal methane content is 70%at the feed rate of 0.35 kg VS·d−1,while methanogen concentration reaches a maximum of 9.6×1010m·L−1[Fig.3(d)].The maximal biogas production does not correspond to the maximum methane content,w hich may be due to the predominance of methanogenic bacteria at OLR of 3.5 kg VS·m−3·d−1.Other bacteria may be more competent as OLR increases,decreasing methane content.Bouallagui et al.applied a methanogenic inclined two-stage tubular AD to fruit and vegetable wastes,operated at 30°C,and found the optimal OLR to be 5.7 kg VS·m−3·d−1,yielding 0.37 m3·(kg VS)−1of biogas[21].Two coupled anaerobic sequencing batch reactors were operated at mesophilic temperature for anaerobic digestion of fruit and vegetable waste and 0.32 m3CH4·(kg CODinput)−1was obtained[14].Rachma et al.used orange peel waste to produce biogas and achieved a maximum production of 0.217 m3CH4·(kg VS)−1[32].Our yields are competent strongly with these experiments.How ever,above the optimal OLR,both the biogas yield and methane content decrease due to excess accumulation of VFAs or insufficient buffering capacity.Inhibition with VFAs concentrations exceeding 10.0 g·L−1was reported[22].Because of excess acidification product from the first phase,methanogens are restrained by adverse environment,decline and die,resulting in an imbalance between production and consumption.
Generally,when ORP falls below−350 m V,anaerobic digestion commences.The methanizer works steadily at ORP of−454 m V to−560 m V,a suitable environment for anaerobic bacteria.NH+4-N accumulates from 2024 mg·L−1to 4506 mg·L−1in the feed rate range 0.15–0.35 kg VS·d−1and does not inhibit methanogens.NH+4-N concentration decreases to 3408 mg·L−1at the optimal OLR because ammonia nitrogen is consumed as an important nutrient for microbial growth.When the OLR rises above 4.0 kg VS·m−3·d−1,the environment is not optimal for substrate conversion and NH+4-N accumulates again[Fig.3(b)].The digester may be operated at 3 kg VS·m−3·d−1,with a concentration of total ammonia nitrogen at 4500 mg·L−1[23].The concentration of NH+4-N in the methanizer is much higher than that in the acidifier because the inoculum(pig manure)has an abundance of nitrogenous organic compounds,which are unstable and readily decompose into ammonia nitrogen.Reflux addition of fermentation liquor from the methanizer also increases the accumulation of NH+4-N.
VFA concentration in the methane digester becomes extremely low at steady state[Fig.3(c)],with most of it converting to biogas by organisms.At the feed rate between 0.15–0.4 kg VS·d−1,the concentration of acetic acid decreases from initial 1120 mg·L−1to 213 mg·L−1from the 3rd to the 42nd day.Isobutyric and butyric acids,how ever,initially increase to 315 and 378 mg·L−1on the 9th day,respectively,then decrease to a nadir of 136 and 121 mg·L−1on day 41.The concentration of propionic acid in the experiment is410 mg·L−1at the end of fermentation and is considered to be an inhibitor in the digestion process.Inhibition of methanogens by HPa is not obvious in the w hole experimental process.Barredo and Evison indicate that the number of methanogens as a secondary index decreases when HPa is between 1500 and 2220 mg·L−1[24].Wang et al.have found that as HPa concentration increases to 900 mg·L−1,significant inhibition appears[25].When the feed rate exceeds 0.4 kg VS·d−1,all the VFAs begin to accumulate.The pH value is held over 7.0 in the steady biogas production,ranging between 7.0 and 8.0 within 42 days in the methanizer.When the OLR exceeds the optimum,pH drops.Suitable pH for methanogens is 6.5–7.8.Activities of methanogens in the system are inhibited when the pH values below 6 or above 8.5[26].Furthermore,in order to prevent acidification in the methanizer and loss of microorganisms,reflux effluent is pumped to adjust pH.

Fig.3.Main parameters in the methanizer.
Table 2 presents the general methanizer performance at different OLRs.When the highest methane yield in the digester reaches 161.2 L·d−1,the corresponding optimal OLR is 3.5 kg VS·m−3·d−1,the HRT is 12 days,VS removal is 65%,and SCOD removal is 93%.Over-extended residual time yields low biogas production due to substrate shortage.On the contrary,substrate will be discharged without full utilization if residual time is too short.High levels of VFAs can be fully converted to methane in the methanizers in relatively short residual time,whereas one-stage AD requires a long starting period to degrade complex substrates.Thus the two-stage AD is more suitable for the removal of a wide range of substrates and has extensive applications.
We investigated the archaea and bacteria community in the methanizer at steady state using high-throughput sequencing technology(miseq),as shown in Fig.4.Methanococcus and Methanosarcina are the main methanogens in the digester.The former is a strictly acetotrophic genus while the latter is facultative.They constitute 91%of the main methanogenic population.A small amount of strict hydrogenotrophic genus Methanoculleus is also detected.To study the archaeal community in biogas production,Ros et al.usedANAEROCHIP micro-array and real-time PCR[27],which showed that the species Methanosaeta and Methanosarcina are important in the AD process.It is found that hydrogenotrophic methanogenic genus Methanobacteriales constitutes more than 93%of archaea in the digester through the real-time quantitative PCR detecting system technology(QPCR),with remainder being Methanobacterium and Methanobacterium congolense[28].Michihiko et al.have reported that Methanosarcina and Methanobacterium/Methanobrevibacter are found mostly in the AD system[29].Certain archaea species utilize various substrates to synthesize methane as an end product:(1)species employing acetate exclusively,acetoclastic or acetotrophic methanogens;(2)species using H2/CO2or formate(hydrogenotrophic methanogens);(3)those catalyzing methyl compounds[30].Proteobacteria is the largest phylum in AD,accounting for 40.12%of non-methane bacteria,including many pathogenic bacteria,such as Escherichia coli,Salmonella,Vibrio cholerae,Helicobacter pylori and other famous species.In addition,non-methane bacteria contain 19.07%Acidobacteria species,such as Koribacter(50.2%of phylum total)and Solibacter(49.8%).Acidobacteria metabolizes the residuals that are not fully acidified.We have detected 3.75% Nitrospira,a genus that transfers nitrite to nitrate and 4.75%belonging to Rhodoplane,a photosynthetic bacterium genus,w hich can take H2S or organic matter as hydrogen donor for photosynthesis with the production of H2.Rhodoplane also transfer nitrate to N2by nitrite breathing.Chloroflexi(1.84%)is a phylum that produces energy through photosynthesis,also know n as green sulfur bacteria.Typical Chloroflexi bacteria are linear,moveby gliding,are facultative anaerobic microbes,do not produce oxygen during photosynthesis and cannot fix nitrogen.Chloroflexi fix carbon dioxide through 3-hydroxypropionic acid instead of the Calvin pathway.Bacteroidetes and Firmicutes communities show unexpected stability in the biogas reactor when fed with various defined substrates[31].We have found them in abundance in our digester.
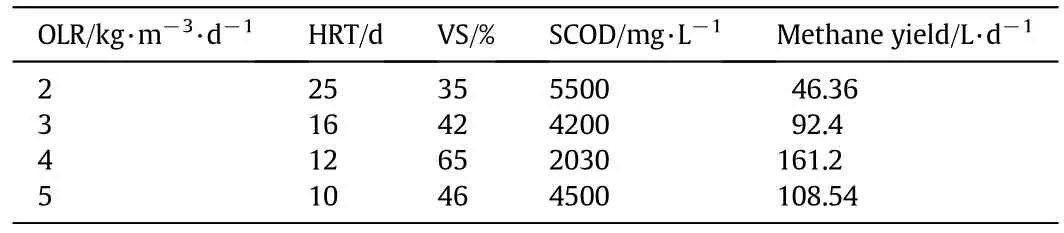
Table 2Average methanizer parameters at different OLRs at steady state

Fig.4.Phylum(a)and genus(b)level distribution of bacterial populations in the methanizer operated at steady state.
4.Conclusions
CCW degrades very well through two-stage AD technology operated at mesophilic temperature,inoculated with pig manure and sludge water.We achieved high biogas and methane productivity in a methanizer w ithout additional materials,such as nitrogen substrate and bicarbonate.Acetic acid is the main product of the acidifier,with the maximum concentration of 4289 mg·L−1on the fourth day,accounting for 50.32%of tVFAs.The optimal OLR is 3.5–4 kg VS·m−3·d−1with corresponding HRT of 12–16 days.Peak biogas production is 0.62 m3·(kg VS)−1,with a methane content of 65%–68%,indicating a good performance of the tw o-stage AD.At optimal OLR,methanogen concentration reaches 9.6 × 1010ml−1.The main methanogens are Methanococcus,according to high-throughput sequencing(miseq).Tw o-stage AD has the advantages of fast gas production and stable operation.In summary,the volume of digesters in this study is of reasonable proportion,and we have achieved empirically-determined continuous operation of stage connection and efficient production at 0.39 m3CH4·(kg VS)−1.The results of anaerobic digestion of CCW suggest that the two-stage AD is a promising technology in term of degradation yield and methane productivity,which could provide the guidance for the large biogas project.
 Chinese Journal of Chemical Engineering2015年5期
Chinese Journal of Chemical Engineering2015年5期
- Chinese Journal of Chemical Engineering的其它文章
- Key factors governing alkaline pretreatment of waste activated sludge☆
- Crystal structure and thermal decomposition kinetics of 1-(pyridinium-1-yl)propane-(1-methylpiperidinium)bi[bis(trifluoromethanesulfonyl)imide],[PyC3Pi][NTf2]2☆
- Synthesis of magnetically modi fi ed palygorskite composite for immobilization of Candida sp.99–125 lipase via adsorption☆
- Nitrogen removal characteristics of heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis C16☆
- Simultaneous heterotrophic nitrification and aerobic denitrification at high initial phenol concentration by isolated bacterium Diaphorobacter sp.PD-7☆
- Experimental study by online measurement of the precipitation of nickel hydroxide:Effects of operating conditions☆
