儿童胸膜肺母细胞瘤的影像学表现及临床病理分析
胡悦林,刘鸿圣,高 秋,黄 莉,鹿连伟,肖伟强,周 宁
(广州市妇女儿童医疗中心,广东 广州 510623)
儿童胸膜肺母细胞瘤的影像学表现及临床病理分析
胡悦林,刘鸿圣,高秋,黄莉,鹿连伟,肖伟强,周宁
(广州市妇女儿童医疗中心,广东 广州510623)
目的:探讨儿童胸膜肺母细胞瘤(PPB)的影像学及临床病理表现,提高对本病的认识。方法:回顾性分析经手术病理及免疫组化证实的12例PPB的影像学资料,根据病理切片、免疫组织化学按Dehner分型分为Ⅰ、Ⅱ、Ⅲ三型;根据不同分型的X线和CT特点,将影像表现与病理进行对照分析。结果:PPB X线表现为胸腔内巨大占位病变,肺及纵隔结构受压。CT表现:胸膜下肺外围非均匀性巨大软组织肿块,常伴有胸腔积液、肺不张。增强扫描肿瘤实性成分呈不均匀明显强化,囊性成分无强化。镜检:肿瘤主要由原始胚胎性的圆形或卵圆形细胞组成。免疫组化:Vimentin(+),Desmin(+)。结论:PPB影像学表现不典型,确诊需依靠病理及免疫组织化学检验相结合。
肺肿瘤;儿童;体层摄影术,螺旋计算机
胸膜肺母细胞瘤 (Pleuropulmonary blastoma,PPB)被认为是一种儿童早期胚胎发育不良或发育障碍的高侵袭性恶性肿瘤,多与肺和/或胸膜有关,好发于婴幼儿,多见于6岁以下儿童,性别上无明显差异。发病率占全部肺母细胞瘤的19%~30%[1]。有家族发病倾向[2]。儿童PPB出现症状较晚,极易发生周围浸润和转移而出现肺外症状,不易早期明确诊断。以往报道最小患者为出生后1 d,男女性别没有明显差异,近10年来有增加的趋势[1],国内也不断有个案报道。本文分析了我院12例经病理及免疫组织化学检验证实的小儿PPB,对其影像学表现及临床病理特征进行分析,提高对本病的认识。
1 材料与方法
1.1临床资料
患儿男5例,女7例,年龄最小3月,最大10岁,平均年龄2岁10月。因发热、咳嗽或气促、气喘、胸痛等呼吸道感染症状就诊,胸片发现胸腔占位后行CT平扫及增强检查,均有完整的临床资料及影像资料。根据病理切片、免疫组织化学按Dehner分型分为Ⅰ、Ⅱ、Ⅲ三型,其中Ⅰ型2例,Ⅱ型2例,Ⅲ型8例。
1.2检查方法
患儿均为X线胸片检查发现胸腔占位后行CT进一步检查。CT机型号为Philips Brilliance 64层螺旋CT。扫描前患儿均口服水合氯醛0.5 mL/kg镇静。扫描范围由胸廓入口至T12下缘。扫描参数:120 kV,20~25 mAs,FOV:24 cm×24 cm,层厚0.5 mm连续扫描;所有病例均行增强扫描,对比剂为优维显,用量3 mL/kg。
2 结果
2.1X线检查
2例Ⅰ型PPB均表现为巨大含气空腔,内见纤细分隔。10例Ⅱ型和Ⅲ型PPB表现为一侧胸腔内占位病变,3例伴有肺纵隔疝,3例病灶巨大者表现为一侧“白肺”,心缘消失,7例伴有胸腔积液,4例伴有气胸。
2.2CT表现
肿瘤位于右侧胸腔2例,左侧胸腔10例;Ⅰ型2例,Ⅱ型2例,Ⅲ型8例。肿物多见于胸膜下的肺实质,其中8例直径>8 cm,形态不规则,密度不均匀,均出现患侧肺不张及不同程度纵隔受压移位表现,其中3例病灶内可见斑点状钙化。Ⅰ型2例表现为单侧充满空气的局限性薄壁囊肿,内可见纤细分隔,增强后未见强化。Ⅱ型2例为囊实性混杂密度占位,1例病灶内可见多个长短不一气液平面,增强后实性成分强化显著,内可见多条迂曲粗大血管及斑片状强化,2例病灶均与纵隔分界清晰,纵隔受压移位,右肺受压1例为节段性不张,另1例全肺不张,1例伴有患侧胸腔积液。Ⅲ型8例平扫表现为密度不均实性肿块,增强可见肿块明显不均匀强化,其中6例肿块内见多发条索状、絮片状不均匀强化,肿块边缘强化显著。肿块均压迫支气管或周围肺组织导致肺不张,5例与纵隔分界不清,4例与肺动脉、主动脉粘连或侵入血管间隙。2例压迫肝脏上部使其左移,下腔静脉受压前移。肿块跨越中线7例,6例伴有胸腔积液,2例侵犯胸膜导致胸膜增厚。伴有纵隔淋巴结肿大5例,伴有肺门淋巴结肿大2例。
2.3病理表现
肿瘤大体标本Ⅰ型2例显示为囊性,表现为薄壁的含气囊肿,内可见细分隔。Ⅱ型2例为囊实性,囊壁较厚,切面大部分呈囊性变,见多个大囊,囊腔内见多灶性息肉样物。Ⅲ型8例均为实性,鱼肉状或息肉样,部分表面呈结节状,质软,有灶状出血坏死。Ⅱ型与Ⅲ型肿物中4例可见乳白色半透明的软骨组织,肿物周围多可见纤维组织包绕,外周为受压萎缩的肺组织,3例有完整包膜,与邻近组织界限明显,无周围浸润,其余均侵袭邻近组织(术中与肺脏、壁层胸膜及其它组织难以分辨)。显微镜下观察Ⅰ型囊壁含有发育较幼稚的原始小细胞及软骨小岛样结构,囊肿被覆上皮下可见胚芽细胞或有横纹肌肉瘤细胞分化现象。Ⅱ型肿瘤实性区主要由胚芽细胞或肉瘤样细胞组成,其中1例可见软骨小岛。Ⅲ型全部为胚胎性间叶成分,含横纹肌区和软骨样分化区。Ⅱ型及Ⅲ型部分胸膜、肺组织、肺门淋巴结活检镜下可见肿瘤浸润。
3 讨论
3.1PPB的分类
PPB是一种儿童时期罕见的胚胎性恶性肿瘤,与胚胎发育不良有关,由不同分化程度的肿瘤性上皮管腔和胚胎性间叶成分构成,其囊性成分可能被覆有纤毛的、良性化生性上皮,与经典的肺母细胞瘤(即成人肺母细胞瘤)有很大的不同,因此将其单独分类称为PPB,WHO新分类将其归入肺软组织肿瘤[3],将PPB按Dehner等[4]分型分为Ⅰ、Ⅱ、Ⅲ三型。Ⅰ型为囊性,无实性成分;Ⅱ型为囊实性,既有囊性成分又有实性成分;Ⅲ型为实性肿瘤,无上皮被覆的囊腔。Ⅰ型多见于婴儿(平均年龄10月),预后最好;Ⅱ、Ⅲ型多见于年长儿(平均2岁),预后差[5]。
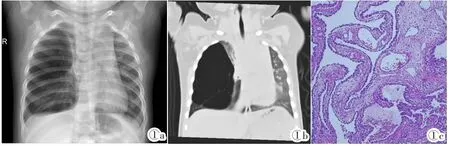
图1 Ⅰ型PPB。女,3月,咳嗽就诊。图1a:X线胸片:右胸腔巨大空腔样占位,右上肺纵隔疝。图1b:CT肺窗冠状窦组:空腔内可见条状分隔。图1c:光镜所见:HE,囊壁被覆肺泡上皮,可见发育幼稚的原始小细胞,灶性见软骨小岛样结构。Figure 1. Three-month female with cough.Figure 1a:Chest X-ray:the right pleural occupying huge cavity sample.The right upper lung hernia into mediastinal.Figure 1b:CT scan(lung window):strip separated was shown in the cavity.Figure 1c:Microscope examination(HE):the alveolar epithelial was covered on the cyst wall,and primitive small cells and cartilage island structure were visible in the lesion.
3.2PPB的诊断与鉴别诊断
儿童PPB的诊断主要依靠病理形态表现和免疫组化。因其临床表现无特异性,恶性程度高,侵袭性强,所以肿瘤早期诊断、完整切除、综合治疗是决定预后的关键。PPB胸部X线片和CT表现为在肺周边部胸膜下肺实质的巨大团块,本组病例左侧胸腔多见,与李航等[6]所报道的PPB左侧较多相符。CT平扫3例瘤体内可见少量斑点状钙化,与CT平扫肿瘤未见钙化灶不符合[7],考虑因本组病例部分病灶内含软骨成分所致。本组病例均显示胸部肋骨骨质结构完整,与文献报道病变容易侵犯肋骨不符合。肿块可以是囊性、实性或囊实性,常累及胸膜,侵犯纵隔,伴有胸腔积液、气胸、纵隔移位等胸腔占位病变表现,但并无特异性,Ⅰ型胸膜肺母细胞瘤形态学上貌似良性,预后较好,与先天性肺气道畸形术前在影像学上几乎无法鉴别,也难与其他先天性良性肺囊肿区分。故应对病理多取材,仔细观察,镜下寻找囊壁内原始间叶成分,以防遗漏Ⅰ型PPB。否则,切除囊肿后未能病理诊断Ⅰ型PPB,经过2~4年将进展为Ⅱ型或Ⅲ型PPB,预后更差。Ⅱ、Ⅲ型PPB病灶通常广泛累及胸膜,可伸入纵隔内血管间隙,导致大血管和心脏移位,也可累及邻近肺组织,侵犯胸膜出现胸腔积液,与肺脓肿合并脓胸,纵隔畸胎瘤、卵黄囊瘤、淋巴瘤等在影像表现上难以鉴别,肺脓肿合并脓胸,病史较长,呼吸道症状明显,抗炎治疗后可好转吸收,而PPB抗炎治疗无效。畸胎瘤瘤体内常出现脂肪和钙化或骨化。卵黄囊瘤因肿瘤能合成甲胎蛋白,因此,往往伴有AFP升高,可与本病鉴别。淋巴瘤为实性肿块,大部分密度均匀,增强扫描有助于鉴别,呈明显均匀强化,囊变及坏死少见,合并纵隔淋巴结肿大更为多见。最后诊断依靠病理检查,其组织学特征是肿瘤的间质成分中含胚胎性横纹肌肉瘤细胞和成熟或不成熟软骨小岛,并见到未分化的小圆形细胞、梭形细胞。免疫组化肿瘤细胞Vimentin阳性,肌源性标记物Desmin阳性。
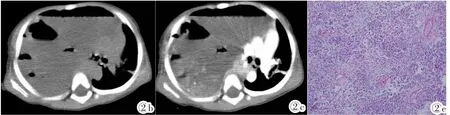

图2 Ⅱ型PPB。男,2岁3月,气促就诊。图2a:X线平片:右侧液气胸,胸腔占位待排除。图2b:CT平扫轴位:肿块中见多个液气平面。图2c:CT增强轴位:肿块不均匀明显强化,纵隔明显左移。图2d:CT增强冠状位:肿块内见多发索条状、斑片状强化,右肺受压不张。图2e:光镜所见:HE,见不成熟原始间叶细胞、横纹肌肉瘤成分,局部可见幼稚软骨成分。Figure 2. Two and three-month years old male with polypnea.Figure 2a:Chest X-ray:hydropneumothorax in the right,except for chest occupied.Figure 2b:Pre-contrasted enhancement scan:a mass with multiple gas-fluid levels.Figure 2c:Post-contrasted enhancement axial CT scan:a mass with heterogeneous enhancement,making mediastinal shift to the left.Figure 2d:Post-contrasted enhancement coronal CT scan:a mass within multiple strips and patchy enhancement,making the right lung electasis.Figure 2e:Microscope examination(HE):immature primitive mesenchymal,rhabdomyosarcoma components and partly immature cartilage components were seen within lesion.
3.3PPB发展的病理进程及免疫组化特点
根据病理、免疫组织化学,PPBⅠ型为含气的具有细间隔的单纯囊肿,良性上皮下可见原始间叶细胞,随后间叶细胞在细间隔过度生长,形成兼具囊性和实性成分的肿瘤即为PPBⅡ型,增殖的间叶细胞形成的实性结节完全取代囊性区,最终形成Ⅲ型PPB[8],随着时间的推移或由于手术切除不完全等因素,Ⅰ型PPB可向Ⅱ型或Ⅲ型PPB转变,由囊性逐渐演变为实性[9-10]。
PPB是一种高侵袭性的恶性肿瘤,横纹肌肉瘤样成分是Ⅱ、Ⅲ型PPB的显著特点,本组12例中均未发现恶性上皮成分,肿瘤成分中多为未分化的间叶细胞,核梭形,具有丰富的染色质,胞浆少或有少量粉染核胞浆,部分区域显示软骨、纤维或横纹肌肉瘤样分化。免疫组化示代表间叶组织或细胞分化的特异性标记物Vimentin阳性,Desmin和Myogenin阳性表达。代表上皮样结构的CK阴性,EMA阴性。
3.4PPB的治疗及预后
PPB的治疗是以手术为主的多学科治疗,未累及胸膜、横膈膜或纵隔的Ⅰ型PPB行手术切除,慎用化疗。Ⅱ型PPB需行手术和化疗。Ⅲ型PPB应在完整手术切除的基础上加术前术后化疗。必要时行术前活检确诊[9]。患儿的预后与其肿瘤的病理分型、肿瘤的大小、是否累及纵隔或胸膜、手术能否完整切除等有关[11-12]。国外报道5年生存率为42%,国内报道2年生存率为27.3%,5年生存率为9.1%[13]。其中有广泛囊性变者预后较好,Ⅰ型预后明显好于Ⅱ、Ⅲ型[14]。对于该病应当结合临床资料、影像学特点和病理学、免疫组化检查,尽早明确诊断,肿瘤完整切除后,联合放化疗,使患儿得以长期存活。
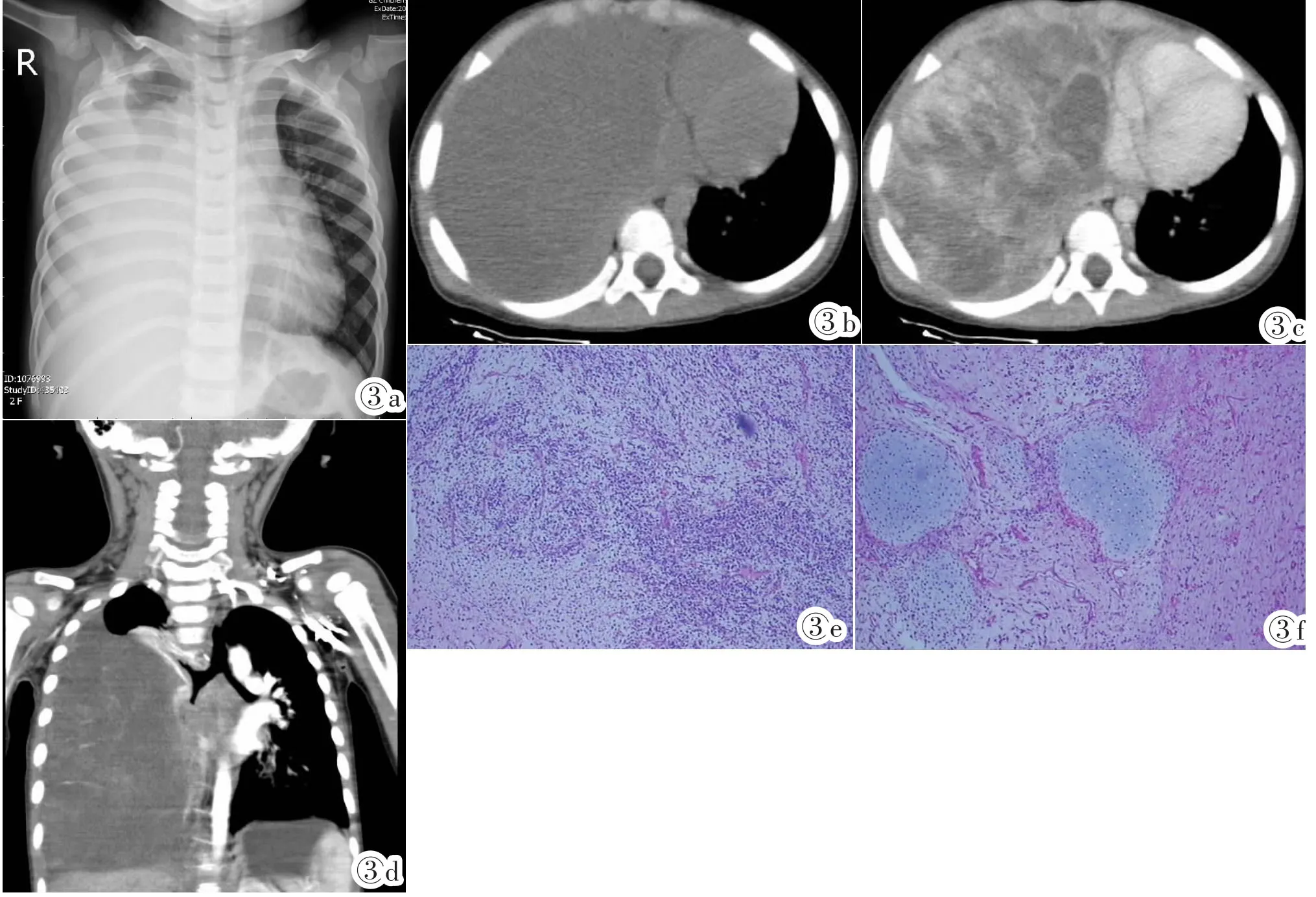
图3 Ⅲ型PPB。女,2岁3月,突发呼吸困难就诊。图3a:X线平片:右胸腔占位,右膈面消失。图3b:CT平扫轴位,右胸腔实性不均匀肿块。图3c:CT增强轴位,肿块实性成分明显强化,囊性部分无强化,边缘强化明显,纵隔轻度左移。图3d:CT增强冠状位,肿块内见多发条状血管,右支气管开口闭塞,肿瘤侵犯纵隔。图3e:光镜所见:HE,见原始未分化间叶细胞、短梭形肿瘤呈片状排列,肿瘤细胞核深染,异型性明显,核分裂易见,部分区域可见幼稚软骨小岛。图3f:HE,幼稚③*软骨小岛,S-100(+)。Figure 3. Two and three-month years old female with sudden breathing difficulty.Figure 3a:Chest X-ray:the right thorax was occupied and the right diaphragmatic surface was disappeared.Figure 3b:Pre-contrasted enhancement axial CT scan:a heterogeneous solid mass was occupied in the right thorax.Figure 3c:Post-contrasted enhancement axial CT scan:the solid component with obvious enhancement,the cystic component with no enhancement and obvious enhancement margin,making mediastinal shift to the left slightly.Figure 3d:Post-enhancement coronal CT scan:multiple blood vessels inside the mass,making the right bronchus occlusion and mediastinum invaded.Figure 3e:Microscope examination(HE):immature primitive mesenchymal cells and short spindle shaped cell were covered in the tumor which was shown with nuclear hyperchromatic,obvious atypia,nuclear fission and some immature cartilage islands. Figure 3f:Immature cartilage island,S-100(+)(HE).
[1]Gupta K,Vankalakunti M,Das A,et al.An autopsy report of a rate pediatric lung tumor:pleuropulmonary blastoma[J].Indian J Pathol Microbiol,2008,51(2):225-227.
[2]Fosdal MB.Pleuropulmonary blastoma[J].J Pediatr Oncol Nurs,2008,25(6):295-302.
[3]Brambilla E,Travis WD,Colby TV,et al.The new World Health Organization classification of lung tumors[J].Eur Respir J,2001,18(6):1059-1068.
[4]Dehner LP,Watterson J,Priest J.Pleuropulmonary blastoma:a unique intrathoracic pulmonary neoplasm of childhood[J].Perspect Pediatr Pathol,1995,18(4):214-226.
[5]DougIas N,Miniati C,Claire L,et al.Prenatal presentation and outcome of children with plouropulmonary blastoma[J].J Pediatr Surg,2006,41(1):66-71.
[6]李航,孙国强,曾骐,等.小儿胸膜肺胚细胞瘤的CT表现[J].中华放射学杂志,2005,39(5):513-515.
[7]李小会,唐文伟,管红梅,等.儿童胸膜肺母细胞瘤五例的CT表现[J].中华放射学杂志,2013,47(10):945-947.
[8]HillDA, JarzembowshiJA, PriestJR,etal.Type1 pleuropulmonaryblastoma:pathologyandbiologystudyof51 cases from the international pleuropulmonary blastoma registry[J]. Surg Pathol,2008,32(2):282-295.
[9]ShivastavaR,SahaA,MeheraB,etal.Pleuropulmonary blastoma:transition from type I(cystic)to typeⅢ(solid)[J]. Singapore Med J,2007,48(7):190-192.
[10]PriestJR, HillDA, WilliamsGM, etal.Typein pleuropulmonaryblastoma:areportfromtheinternational pleuropulmonary blastoma registry[J].J Clin Oncol,2006,24(27):4492-4498.
[11]高欣凤,郭志平,李宗凯,等.小儿胸膜肺母细胞瘤临床特点及病理分析[J].临床小儿外科杂志,2013,12(4):317-319.
[12]Indolfi P,Casale F,Carli M,et al.Pleuropulmonary blastoma. Management and prognosis of 11 cases[J].Cancer,2000,89(6):1396-1401.
[13]lndolfi P,Bisogno G,Casale F,et al.Prognostic factors in pleuropulmonary blastoma[J].Pediatr Blood Cancer,2007,48(3):318-323.
[14]曾骐,周春菊,贺延儒,等.小儿胸膜肺母细胞瘤[J].中华胸心血管外科杂志,2001,17(6):343-345.
[15]Kusafuka T,Kuroda S,Inoue M,et al.P53 gene mutations in pleuropulmonary blastomas[J].Pediatr Hematol Oncol,2002,19(2):117-128.
Imaging features and clinical pathological analysis of pleuropulmonary blastoma in children
HU Yue-lin,LIU Hong-sheng,GAO Qiu,HUANG Li,LU Lian-wei,XIAO Wei-qiang,ZHOU Ning
(Guangzhou Women and Children's Medical Centre,Guangzhou 510623,China)
Objective:To explore the images and clinical pathologic features of pleuropulmonary blastoma(PPB)and to improve the understanding of this disease.Methods:The imaging findings of 12 cases of PPB confirmed by pathology were analyzed retrospectively.They were divided into theⅠ,Ⅱ,Ⅲ types according to Dehuer calssfication.According to X-ray and CT examination in PPB of different types,the images and pathological features were analyzed.Results:A large mass of PPB was shown on X-ray image in thoracic cavity,which could oppress lung and mediastinal.A huge heterogeneous density mass beneath the pleura lung periphery was shown on CT image,often associated with pleural effusion,atelectasis.In post-contrasted enhancement image,the solid component showed obvious enhancement,while the cystic component showed no enhancement.Primitive embryonal round or oval cells were mainly composed of the tumor by microscopic examination.Vimentin and Desmin were shown positive in the immunohistochemical study.Conclusion:The diagnosis of PPB should be based on both the pathological and immunohistochemical evidences duing to lacking of photography features.
Lung neoplasms;Child;Tomography,spiral computed
R743.2;R743.3;R814.42
A
1008-1062(2015)07-0469-04
2014-12-22;
2015-01-14
胡悦林(1977-),女,河南人,主治医师。

