长期无机有机肥配施对红壤性水稻土微生物群落多样性及酶活性的影响
陆海飞, 郑金伟, 余喜初, 周惠民, 郑聚锋, 张旭辉,刘晓雨, 程 琨, 李恋卿, 潘根兴*
(1南京农业大学农业资源与生态环境研究所,江苏南京 210095;2江西省红壤研究所,农业部重点野外观测站,江西进贤 331717)
长期无机有机肥配施对红壤性水稻土微生物群落多样性及酶活性的影响
陆海飞1, 郑金伟1, 余喜初2, 周惠民1, 郑聚锋1, 张旭辉1,刘晓雨1, 程 琨1, 李恋卿1, 潘根兴1*
(1南京农业大学农业资源与生态环境研究所,江苏南京 210095;2江西省红壤研究所,农业部重点野外观测站,江西进贤 331717)

微生物群落结构; 多样性; 酶活性; 红壤水稻土; 长期施肥
国际科学界早就提出,合理管理下土壤固碳具有减缓气候变化与提高农田生产力的双赢作用[1]。我国科学家也已证明,有机无机肥配合施用是提高稻田生产力和土壤固碳的关键农田管理措施[2-3];不同施肥以及有机物料的投入与否可通过影响土壤养分、基本性质来影响土壤有机质含量及其稳定性,进而影响利用这种有机质的微生物区系的丰度及结构变化以及微生物生物活性的变化。施肥也可通过影响土壤养分和作物生长而对土壤中微生物丰度、结构及其功能产生深刻影响[4-7]。土壤微生物量及其多样性和土壤酶活性是土壤质量和生态系统健康的指标[8-9]。有研究表明,不同施肥影响了土壤微生物总丰度的细菌/真菌的相对丰度[10-12];施有机物料或有机无机配施可以提高土壤多种酶的活性[13-15]。尽管单一的土壤酶活性作为土壤健康的指标可能存在偏差[16],但土壤总体酶活性指标[17]和归一化酶活性强度[18]可以用来表征土壤的总体酶活性。有机无机肥配合施用下农田生产力和土壤固碳作用的提高是否对土壤微生物区系、土壤生物活性和生态系统健康有明显的改善作用,仍有待进一步研究。
本文以江西省进贤市红壤试验站的长期定位试验中不同施肥处理的表土(0—15 cm)为研究对象,通过对土壤微生物群落的研究和土壤总体酶活性的分析,探讨长期不同施肥对土壤微生物多样性及土壤酶活性的影响,进一步认识有机无机肥配合施用条件下水稻土的固碳对生产力及生态系统的功能效应。
1 材料与方法
1.1 试验地概况

1.2 试验设计
试验始于1981年,设不施肥对照(CK)、单施氮肥(N)、常规施氮磷钾肥(NPK)、氮磷钾配施有机肥(NPKM)4个处理,每个处理3个重复,小区面积46.67 m2,随机区组排列[20]。各处理施肥量为: N处理每季作物施尿素N 90 kg/hm2;NPK处理每季水稻施尿素N 90 kg/hm2、P2O545 kg/hm2、K2O 75 kg/hm2;NPKM处理每季施氮、磷、钾肥的量与NPK处理一致,另外,早稻施紫云英(鲜重)22500 kg/hm2,晚稻施猪粪(鲜重)22500 kg/hm2。种植制度为双季稻。
1.3 测定项目和方法
2011年于晚稻收获时采集土壤样品。采样时各小区按“S形”随机取5 点采集0—15 cm土壤样品,混合后装袋,放入保鲜箱内带回实验室,部分鲜样置于-80℃冰箱中保存,剩余样品除去植物残体和石块后风干,过0.15 mm筛备用。
1.3.1 土壤基本理化性状的测定 土壤有机碳、全氮含量的测定采用元素分析仪(ElementarVariomax CNS Analyser,德国Elementary公司); 土壤pH值的测定参照《土壤农化分析》相关方法[21]。
1.3.2 土壤酶活性的测定 称取相当于1.0000 g烘干土的风干土样,放入500 mL的玻璃烧杯中,加入灭菌后冷却的醋酸缓冲液125 mL,于磁力搅拌器上均质10 min后,用移液枪吸取土壤悬液,枪头去掉尖端。采用改进的Marx等[22]的荧光微孔板检测技术测定土壤几丁质酶、α-葡萄糖苷酶、β-葡萄糖苷酶、纤维素酶、酸性磷酸单脂酶和木聚糖酶活性(土壤样品12次重复,标准6次重复,空白3次重复);用改进的Williams等[23]的紫外分光光度计法测定土壤过氧化物酶活性(土壤样品16次重复,空白12次重复)。所用试剂购自Sigma-Aldrich Co.Ltd公司,用无菌水配置后于4℃冰箱短暂保存待用。
1.3.3 土壤微生物分子生态分析
1) 基因组DNA的提取 取0.25 g新鲜土样,使用DNA快速提取试剂盒(Power Soil,Mo Bio公司),按操作说明进行,提取的DNA用1.5%琼脂糖凝胶电泳验证。
2) 荧光实时定量PCR 分析 土壤细菌定量PCR分析的分子标靶基因为16S rRNA基因V3可变区,采用引物为338F和518R,目标片段200 bp,扩增程序: 95℃预变性3 min,95℃变性1 min,56℃退火1 min,72℃延伸1 min,40个循环[24]。土壤真菌标靶基因为18S rRNA基因,所用引物为NS1和Fung,目标片段300 bp,扩增程序:95℃预变性3 min,95℃变性1 min,57℃退火1 min,72℃延伸1 min,40个循环[25]。通过TA克隆的方法,获得上述基因的重组质粒,以10倍梯度稀释得到各自的标准曲线。每个样品3个重复。定量反应体系为20 μL,包括扩增酶混合物SYBR®PremixEXTaqTM(Takara Shuzo, Shinga, Japan) 10 μL,DNA模板1 μL,10 μmol/L上、下游引物各0.4 μL,无菌水8.2 μL。
3)聚合酶链反应(PCR) 用引物968F和1401R扩增细菌16S rRNA基因V6区片段,目标片段430 bp,扩增程序: 94℃预变性10 min,94℃变性1 min,56℃退火1 min,72℃延伸1 min,30个循环[26]。选取引物NS1和Fung扩增土壤真菌18S rRNA,目标片段300 bp,扩增程序: 95℃预变性5 min,95℃变性1 min,57℃退火1 min,72℃延伸1 min,30个循环[25]。GC夹[24]加在上游引物的5′端。PCR采用50 μL反应体系:包括扩增酶混合物Go Taq® Green Master Mix(Promega, USA) 25 μL,DNA模板1 μL,10 μmol/L上、下游引物各1 μL,无菌水22 μL。PCR产物用2%琼脂糖凝胶电泳验证。

5)切胶、测序和系统进化树构建 凝胶拍照后对明显的条带进行切割,对PCR产物纯化后,酶连转化,挑取插入目标片段的克隆子,在上海华大基因公司完成测序。测序结果用BioEdit软件进行检验和比对,得到目标序列后,输入国际生物技术信息中心网站(NCBI)比对,挑选相似度最高并有代表性的序列。同时这些序列提交GenBank数据库,登录号分别为KJ021730-KJ021749(细菌)和KJ021750-KJ021766(真菌)。使用MEGA软件5.1版本,邻接法(Neighbor-joining analysis)构建系统发育树,距离矩阵按照p-distance参数模型计算,Bootstrap检验进行1000次取样。
1.4 数据处理
单个土壤酶活性值计算参照DeForest[27]的方法,归一化酶活性值计算采用靳振江等[18]的方法。即采用多属性决策法对酶活性归一化处理,首先将4个处理的7种酶活性分别进行归一化,得到单个处理的归一化酶活性值,计算公式为:
然后将每个处理归一化的7个酶活性值相加后取算术平均数,得到单个处理的酶活性值。微生物丰富度指数和香农指数计算采用Hedrick等[28]的方法。
试验数据用Excel 2003进行处理,用单因素方差分析检验不同处理间的差异显著性。
2 结果与分析
2.1 土壤有机碳、全氮含量和土壤酶活性

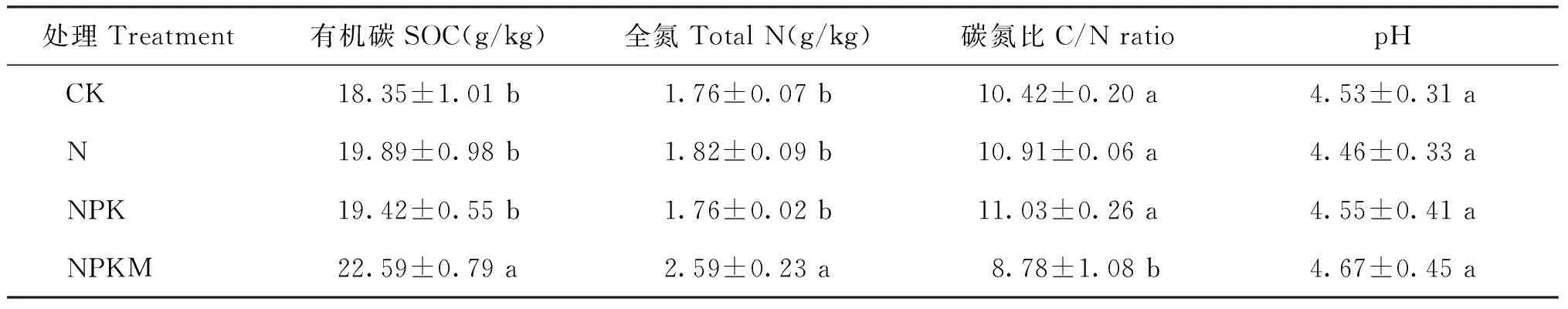
表1 长期施肥处理下土壤的理化性质
注(Note): 同列数据后不同字母表示处理间差异达5%显著水平 Values followed by different letters in a column are significant among treatments at the 5% level.
表2显示,N处理与CK相比土壤几丁质酶活性显著提高,其余6种酶活性与CK无明显差异。与CK相比,NPK处理的土壤几丁质酶和α-葡萄糖苷酶活性显著增强,其余酶活性无显著变化;NPK处理的α-葡萄糖苷酶和β-葡萄糖苷酶活性均显著高于N处理; NPKM处理的土壤纤维素酶、过氧化物酶和几丁质酶活性均显著高于CK、N和NPK处理,α-葡萄糖苷酶活性显著高于CK和N处理,β-葡萄糖苷酶活性显著高于N处理,酸性磷酸单酯酶和木聚糖酶活性各处理间无显著差异。计算得出的归一化酶活性值也表现为NPKM处理显著高于CK和其他施肥处理。
2.2 土壤细菌和真菌丰度

2.3 土壤微生物群落结构及多样性
图1A显示,NPKM处理下存在新的条带1和2。图1B聚类分析结果表明,CK和N处理处于一个独立簇,NPK处理形成另一个独立簇,NPKM处理聚成一簇,与CK、N和NPK处理的一簇相似度仅为66%。由此说明,NPKM处理下土壤细菌群落结构与其他处理存在显著的差异。从图2A可以看出,土壤真菌显示出较大的变异性。条带1、4、5、8、9、10、11是所有泳道共有的条带。由图2B的聚类分析看出,CK和N、NPK 3个处理未被分开,形成交错的一簇,而NPKM处理构成另一簇。说明只有NPKM处理明显改变了真菌的群落结构。对比表3的结果可以看出, N和NPKM处理的土壤细菌香农指数和丰富度指数均明显高于CK,而且NPKM处理的两个指数均较高;NPK处理与CK无显著差异。CK、N、NPK和NPKM处理的土壤真菌香农指数和丰富度指数均没有明显差异。
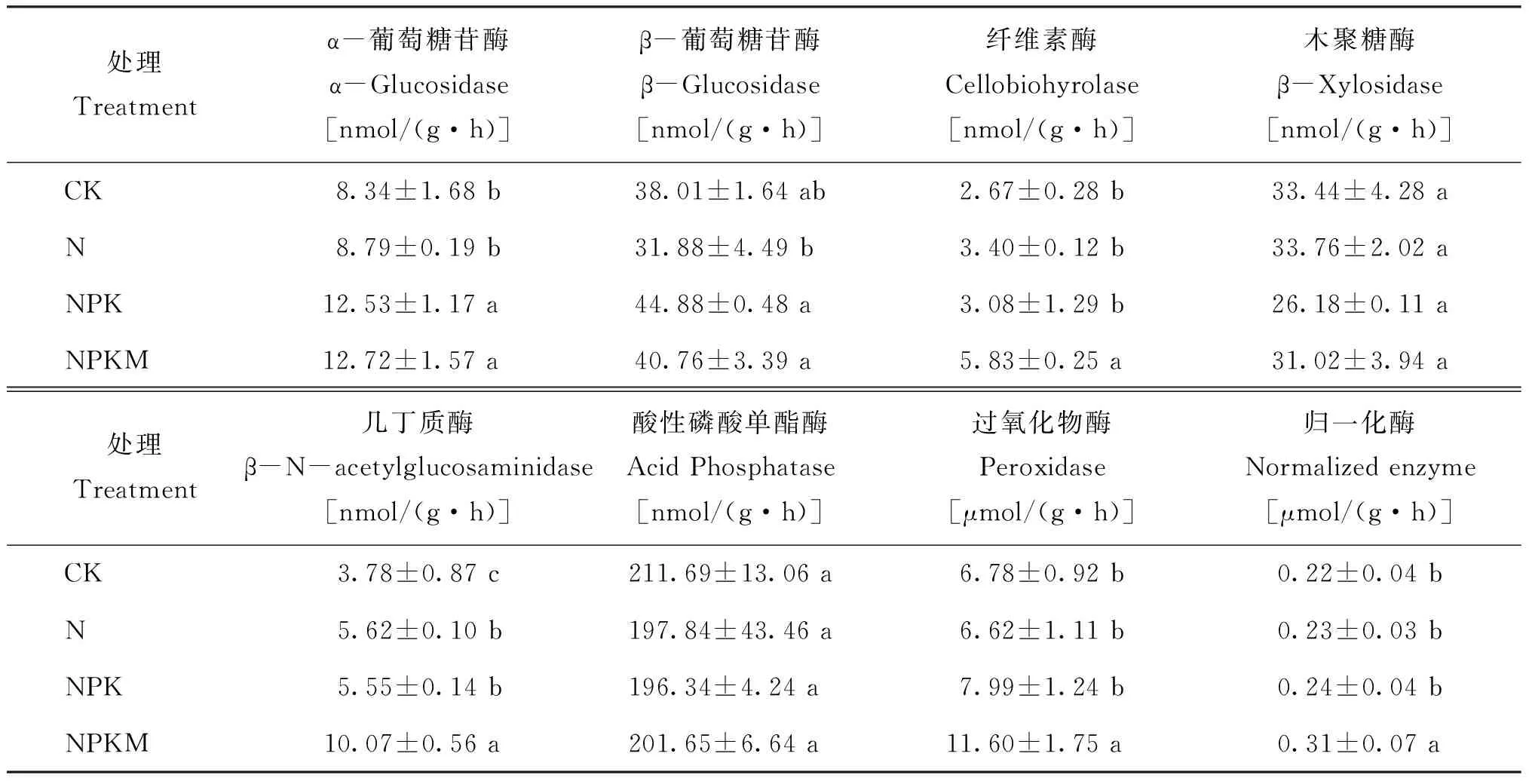
表2 长期施肥处理下土壤的酶活性
注(Note): 同列数据后不同字母表示处理间差异达5%显著水平 Values followed by different letters in a column are significant among treatments at the 5% level.
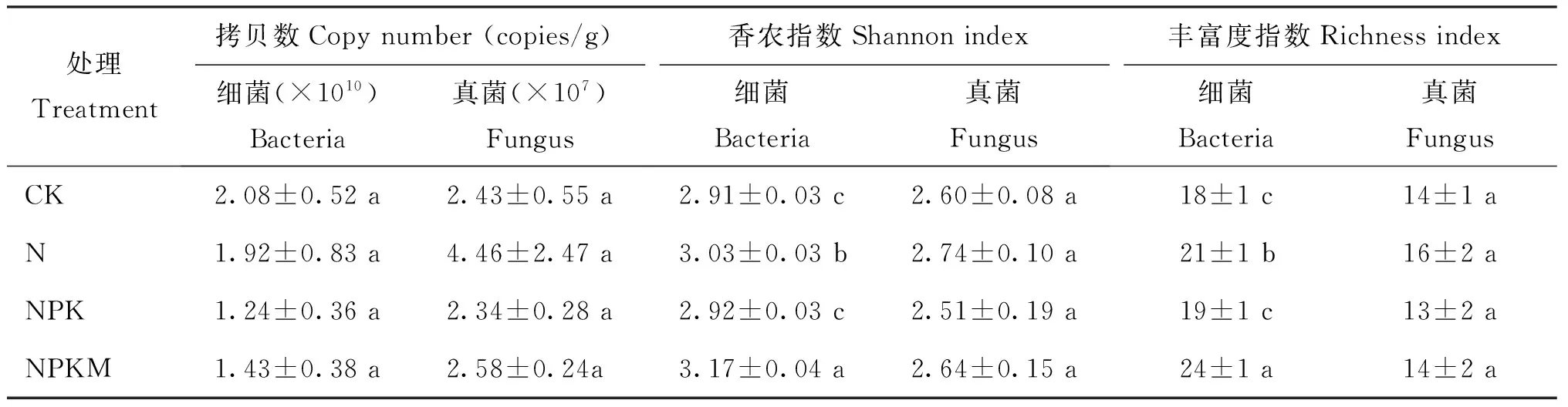
表3 长期施肥处理下土壤微生物丰度和多样性指数
注(Note): 同列数据后不同字母表示处理间差异达5%显著水平 Values followed by different letters in a column are significant among treatments at the 5% level.
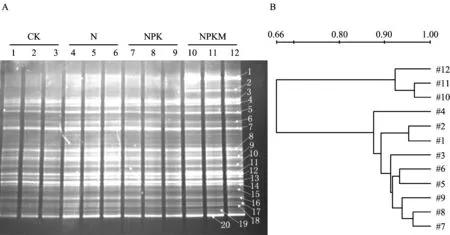
图1 16S rRNA V6片段PCR产物的电泳图(A)及聚类分析(B)Fig.1 DGGE profile(A) of amplified 16S rRNA fragments and cluster analysis(B)
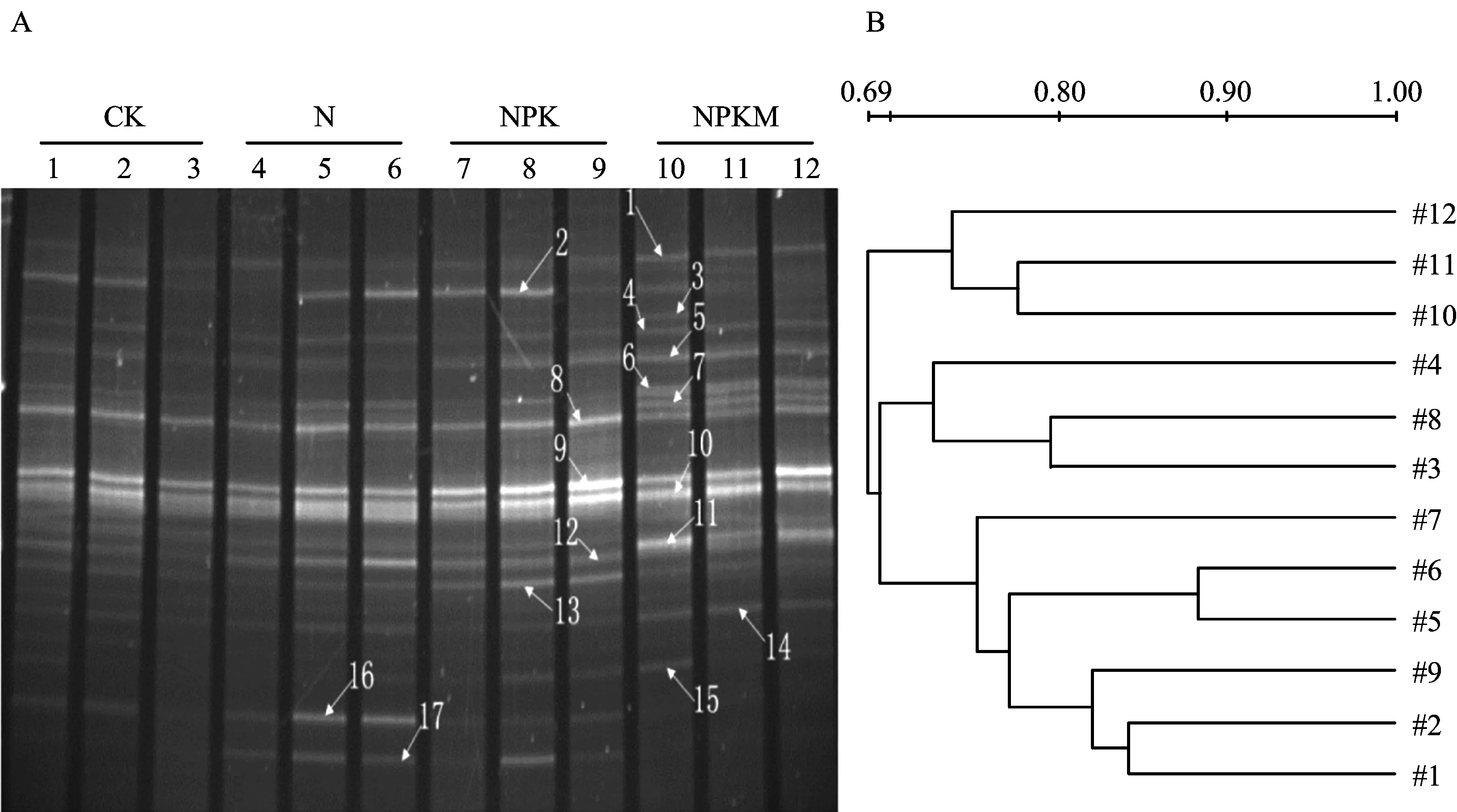
图2 18S rRNA PCR产物的电泳图(A)及聚类分析(B)Fig.2 DGGE profile(A) of amplified 18S rRNA fragments and cluster analysis(B)
2.4 土壤微生物群落组成

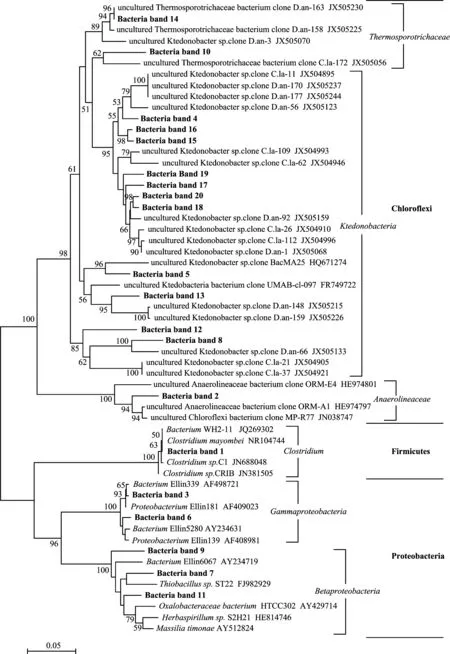
图3 土壤细菌系统进化树Fig.3 Phylogenetic tree of soil bacteria[注(Note): 克隆来自图1A电泳图中的标注条带Clones are obtained from the DGGE bands in Fig.1A. Bootstrap values are shown at branch points(>50%); 图中显示大于50%的引导值,标尺设置为0.05 The scale bar represents 0.05 substitutions per nucleotide.]
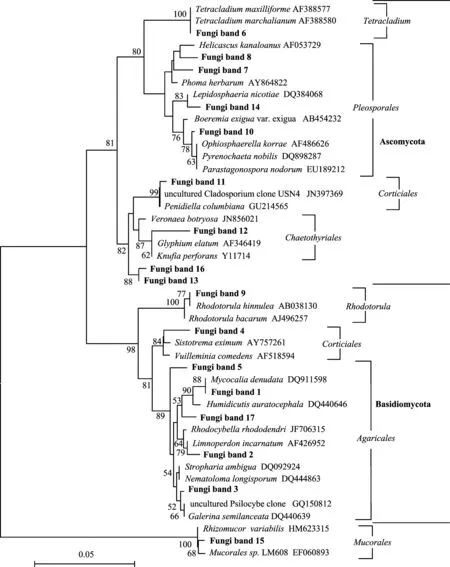
图4 土壤真菌系统进化树Fig.4 Phylogenetic tree of soil fungus[注(Note): 克隆来自图2A电泳图中的标注条带Clones are obtained from the DGGE bands in Fig.2A. 图中显示大于50%的引导值 Bootstrap values are shown at branch points(>50%);标尺设置为0.05 The scale bar represents 0.05 substitutions per nucleotide.]
2.5 相关性分析
由表4看出,土壤归一化酶活性值与土壤有机碳、全氮含量呈显著正相关关系。细菌的香农指数、丰富度指数与土壤有机碳含量显著正相关,与全氮含量和归一化酶活性值无显著相关关系。而真菌香农及丰富度指数与土壤有机碳、全氮含量和归一化酶活性值均无显著相关关系。
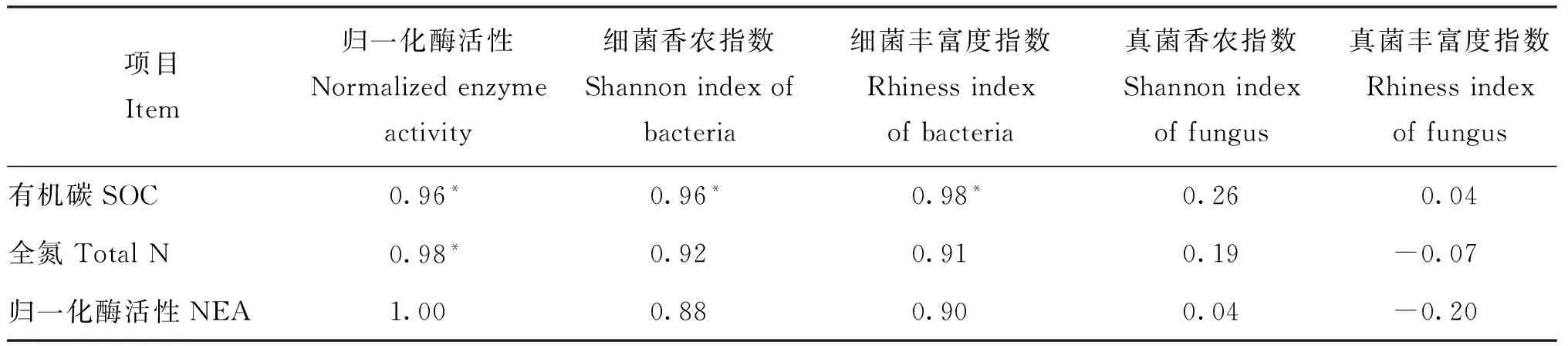
表4 土壤酶活性、微生物香农及丰富度指数与土壤性质的相关分析
注(Note): SOC—Soil organic carbon; NEA—Normalized enzyme activity. *—P<0.05.
3 讨论
3.1 长期施肥对土壤酶活性的影响
本研究结果表明,施肥均提高了土壤几丁质酶活性,而土壤中几丁质酶活性与土壤全氮含量显著正相关,同时由根际细菌产生的几丁质酶对植物病原菌的生物控制有重要作用[29]。对比3个施肥处理,纯氮(N)处理下, α-葡萄糖苷酶和β-葡萄糖苷酶活性显著低于氮磷钾平衡施肥(NPK)和有机无机配施(NPKM)处理,NPK和NPKM处理下,微生物可能更强地分解土壤中的纤维素。只在NPKM处理下测出土壤纤维素酶和过氧化物酶活性增加,因此有机无机肥配施可促进土壤中葡萄糖的水解。为了反映土壤的总体酶活性,我们计算了土壤的归一化酶活性值,结果显示,NPKM处理下,归一化酶活性值高于CK和N、NPK处理,并且土壤归一化酶活性值与土壤有机碳、全氮含量呈显著正相关关系(表4)。由此得出,有机无机肥配施优于单施化肥,可显著增强土壤酶活性,这与郑勇等[30]的研究结果一致。另外,刘骅等[14]和李晨华等[15]对灰漠土长期施肥的研究也指出,化肥配施有机物料增加了土壤酶活性,且与单施化肥相比更具优势。同时,根据袁颖红等[31]对同一试验地的研究指出,长期施用肥料,特别是有机无机肥配施能提高土壤微生物量碳、潜在可矿化碳和可溶性有机碳含量,从而提高土壤碳库质量。Zheng等[32]对该试验地土壤的室内培养试验表明,有机无机肥配合施肥下土壤有机碳含量提高,同时降低了有机碳分解性,降低了有机碳分解对升温的响应。因而认识到,有机无机配合施肥可促进土壤固碳,并增强土壤碳库的稳定性。
3.2 长期施肥对土壤微生物群落结构和多样性的影响
微生物群落在土壤有机质分解和营养元素循环中有着至关重要的作用,因此,土壤微生物群落的数量、结构和活性被认为与生态系统功能密切相关[33]。而张平究等[4]对太湖地区水稻土长期试验的研究也指出,长期化肥配施有机肥显著提高了土壤细菌的多样性。刘恩科等[34]指出化肥配施有机物料改变了土壤细菌的群落结构,而长期施化肥对土壤细菌群落结构的影响不大。袁红朝等[35]采用T-RFLP技术研究了同地带的水稻土不同施肥下的微生物群落变化,提出氮磷钾配施和化肥配施有机物料均可显著提高土壤细菌多样性,且化肥配施有机物料处理的多样性指数最高。使用同样的测试方法,Ge等[36]对河南封丘的潮湿雏形土(aquic cambosols)长期施肥试验的研究观察到无机肥配施有机物料显著提高了土壤细菌的多样性。本研究的结论与上述研究类似,长期有机无机肥配施显著提高了土壤细菌的多样性,并且土壤细菌的香农指数和丰富度指数与土壤有机碳含量显著正相关。因此,长期无机有机肥配施可通过增加土壤有机碳含量,为土壤细菌提供了更多的碳源,从而提高土壤细菌的多样性。不过,本试验中三种施肥处理对土壤真菌的多样性无显著影响,这与魏巍等[7]的研究结果一致。不同的是,在魏巍等[7]的研究中,相比不施肥处理,施肥处理下土壤细菌多样性水平降低,可能是黑土基础肥力较高,施化肥的影响可能与红壤性水稻土不同。本研究中通过聚类分析得出,长期有机无机肥配合施用改变了土壤细菌和真菌的群落结构。张平究等[4]也发现,化肥配施有机肥改变了土壤细菌的群落结构。由表1看出,NPKM处理相对CK、N和NPK处理显著提高了土壤有机碳和全氮含量,这给土壤微生物带来了更多碳源和营养元素,微生物活动变得更为活跃,群落结构也可能因此改变。
3.3 长期施肥对土壤微生物群落组成的影响
土壤微生物的群落组成直接影响土壤微生物的功能,不同类群的微生物对土壤的生理生化过程有不同的影响,因此,了解土壤微生物的群落组成就变得十分重要。利用克隆文库技术,袁红朝等[35]对湖南望城和王霞等[37]对湖南常德的红壤长期施肥试验土壤进行了微生物群落组成研究,其水稻土的优势菌群均为Proteobacteria(变形菌门)细菌,在秸秆还田后其丰度显著增加。而本研究中,通过对明显条带的切胶测序得出,供试酸性红壤水稻土中Chloroflexi(绿弯菌门)和Proteobacteria(变形菌门)为细菌的优势菌群,且以Chloroflexi(绿弯菌门)细菌占主导。值得注意的是,在本研究中,有机碳增加最多的NPKM处理土壤中,隶属于Clostridum(梭菌属)和Anaerolineaceae(厌氧绳菌科)的细菌显著增加。已有研究表明,Clostridum(梭菌属)作为纤维素降解菌的优势菌群广泛存在于秸秆沼气工程发酵系统中[38];并且Clostridum(梭菌属)作为产氢产乙酸菌的一类,是有机物厌氧消化过程中酸化阶段的主要微生物[39]。另根据研究,Anaerolineaceae(厌氧绳菌科)作为Chloroflexi(绿弯菌门)的代表类群,是猪粪厌氧消化过程中起降解作用的一类主要微生物[40]。因此,增加的这两类细菌有利于有机物的分解,促进营养元素的循环。本试验各处理的土壤真菌则主要属于Ascomycota(子囊菌门)和Basidiomycota(担子菌门),这与He等[41]的研究基本一致,但没有表现出随施肥处理的显著变化。
4 结论
与不施肥对照(CK)相比,长期有机无机肥配施(NPKM)显著提高了土壤有机碳和全氮含量,显著增强了土壤酶活性,相应地土壤细菌多样性也显著提高。同时,供试水稻土中Chloroflexi(绿弯菌门)和Proteobacteria(变形菌门)为细菌的优势菌群,但有机无机肥配施处理中隶属于Clostridum(梭菌属)和Anaerolineaceae(厌氧绳菌科)的两类细菌显著增加。因此,有机无机肥配施在促进土壤有机碳固定的同时,也提高了微生物的多样性,进而改善了生态系统的健康和生产力。
[1] Lal R. Soil carbon sequestration impacts on global climate change and food security[J]. Science, 2004, 304(5677): 1623-1627.
[2] Pan G, Smith P, Pan W. The role of soil organic matter in maintaining the productivity and yield stability of cereals in China[J]. Agriculture, Ecosystems & Environment, 2009, 129(1): 344-348.
[3] Wang C J, Pan G X, Tian Y Getal. Changes in cropland topsoil organic carbon with different fertilizations under long-term agro-ecosystem experiments across mainland China[J]. Science China Life Sciences, 2010, 53(7): 858-867.
[4] 张平究, 李恋卿, 潘根兴, 张俊伟. 长期不同施肥下太湖地区黄泥土表土微生物碳氮量及基因多样性变化[J]. 生态学报, 2005, 24(12): 2818-2824. Zhang P J, Li L Q, Pan G X, Zhang J W. Influence of long-term fertilizer management on topsoil microbial biomass and genetic diversity of a paddy soil from the Tai Lake region, China[J]. Acta Ecologica Sinica, 2005, 24(12): 2818-2824.
[5] He J Z, Zheng Y, Chen C Retal. Microbial composition and diversity of an upland red soil under long-term fertilization treatments as revealed by culture-dependent and culture-independent approaches[J]. Journal of Soils and Sediments, 2008, 8(5): 349-358.
[6] Shen J P, Zhang L M, Guo J Fetal. Impact of long-term fertilization practices on the abundance and composition of soil bacterial communities in Northeast China[J]. Applied Soil Ecology, 2010, 46(1): 119-124.
[7] 魏巍, 许艳丽, 朱琳, 等. 长期施肥对黑土农田土壤微生物群落的影响[J]. 土壤学报, 2013, 50(2): 372-380. Wei W, Xu Y L, Zhu Letal. Effect of long-term fertilization on soil microbial communities in farmland of black soil[J]. Acta Pedologica Sinica, 2013, 50(2): 372-380.
[8] Kennedy A C. Bacterial diversity in agroecosystems[J]. Agriculture, Ecosystems & Environment, 1999, 74(1): 65-76.
[9] Dick R P, Pankhurst C, Doube B Metal. Soil enzyme activities as integrative indicators of soil health[J]. Biological Indicators of Soil Health, 1997: 121-156.
[10] 颜慧, 钟文辉, 李忠佩, 等. 长期施肥对红壤水稻土磷脂脂肪酸特性和酶活性的影响[J]. 应用生态学报, 2008, 19(1): 71-75. Yan H, Zhong W H, Li Z Petal. Effects of long-term fertilization on phospholipid fatty acids and enzyme activities in paddy red soil[J]. Chinese Journal of Applied Ecology, 2008, 19(1): 71-75.
[11] 白震, 张明, 闫颖, 等. 长期施肥对农田黑土微生物活力与群落结构的影响[J]. 土壤学报, 2009, 46(1): 107-116. Bai Z, Zhang M, Yan Yetal. Effect of long-term fertilization on activity and community structure of soil microbein farmland mollisol[J]. Acta Pedologica Sinica, 2009, 46(1): 107-116.
[12] 卜洪震, 王丽宏, 尤金成, 等. 长期施肥管理对红壤稻田土壤微生物量碳和微生物多样性的影响[J]. 中国农业科学, 2010, 43(16): 3340-3347. Bu H Z, Wang L H, You J Cetal. Impact of long-term fertilization on the microbial biomass carbon and soil microbial communities in paddy red soil[J]. Scientia Agricultura Sinica., 2010, 43(16): 3340-3347.
[13] Bhattacharyya P, Chakrabarti K, Chakraborty A. Microbial biomass and enzyme activities in submerged rice soil amended with municipal solid waste compost and decomposed cow manure[J]. Chemosphere, 2005, 60(3): 310-318.
[14] 刘骅, 林英华, 张云舒, 等. 长期施肥对灰漠土生物群落和酶活性的影响[J]. 生态学报, 2008, 28(8): 3898-3904. Liu Y, Lin Y H, Zhang Y Setal. Effects of long-term fertilization on biodiversity and enzyme activity in grey desert soil[J]. Acta Ecologica Sinica., 2008, 28(8): 3898-3904.
[15] 李晨华, 贾仲君, 唐立松, 等. 不同施肥模式对绿洲农田土壤微生物群落丰度与酶活性的影响[J]. 土壤学报, 2012, 49(3): 567-574. Li C H, Jia Z J, Tang L Setal. Effect of model of fertilization on microbial abundance and enzyme activity in oasis farmland soil[J]. Acta Pedologica Sinica, 2012, 49(3): 567-574.
[16] 林天, 何园球, 李成亮, 等. 红壤旱地中土壤酶对长期施肥的响应[J]. 土壤学报, 2005, 42(4): 682-686. Lin T, He Y Q, Li C Letal. Response of soil enzymes to long-term fertilization in upland red soil[J]. Acta Pedologica Sinica, 2005, 42(4): 682-686.
[17] 和文祥, 谭向平, 王旭东, 等. 土壤总体酶活性指标的初步研究[J]. 土壤学报, 2010(6): 1232-1236. He W X, Tan X P, Wang X Detal. Study on total enzyme activity index in soils[J]. Acta Pedologica Sinica, 2010(6): 1232-1236.
[18] 靳振江, 邰继承, 潘根兴, 等. 荆江地区湿地与稻田有机碳, 微生物多样性及土壤酶活性的比较[J]. 中国农业科学, 2012, 45(18): 3773-3781. Jin Z J, Tai J C, Pan G Xetal. Comparison of soil organic carbon, microbial diversity and enzyme activity of wetlands and rice paddies in Jingjiang area of Hubei, China[J]. Scientia Agricultura Sinica, 2012, 45(18): 3773-3781.
[19] 龚子同. 中国土壤系统分类: 理论·方法·实践[M]. 北京: 科学出版社, 1999. Gong Z T. Soil taxonomic classification of China: Theory, Methodology and Applications[M]. Beijing: Science Press, 1999.
[20] 李辉信, 袁颖红, 黄欠如, 等. 不同施肥处理对红壤水稻土团聚体有机碳分布的影响[J]. 土壤学报, 2006, 43(3): 422-429. Li H X, Yuan Y H, Huang Q Retal. Effects of fertilization on soil organic carbon distribution in various aggregates of red paddy soil[J]. Acta Pedologica Sinica, 2006, 43(3): 422-429.
[21] 鲁如坤. 土壤农业化学分析方法[M]. 北京: 中国农业科技出版社, 2000. Lu R K. Methods of soil and agricultural chemistry [M]. Beijing: China Agricultural Scientech Press, 2000.
[22] Marx M C, Wood M, Jarvis S C. A microplate fluorimetric assay for the study of enzyme diversity in soils[J]. Soil Biology and Biochemistry, 2001, 33(12): 1633-1640.
[23] Williams C J, Shingara E A, Yavitt J B. Phenol oxidase activity in peatlands in New York State: response to summer drought and peat type[J]. Wetlands, 2000, 20(2): 416-421.
[24] Muyzer G, De Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA[J]. Applied and Environmental Microbiology, 1993, 59(3): 695-700.
[25] May L A, Smiley B, Schmidt M G. Comparative denaturing gradient gel electrophoresis analysis of fungal communities associated with whole plant corn silage[J]. Canadian Journal of Microbiology, 2001, 47(9): 829-841.
[26] Muyzer G, Smalla K. Application of denaturing gradient gel electrophoresis(DGGE) and temperature gradient gel electrophoresis(TGGE) in microbial ecology[J]. Antonie van Leeuwenhoek, 1998, 73(1): 127-141.
[27] DeForest J L. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA[J]. Soil Biology and Biochemistry, 2009, 41(6): 1180-1186.
[28] Hedrick D B, Peacock A, Stephen J Retal. Measuring soil microbial community diversity using polar lipid fatty acid and denaturing gradient gel electrophoresis data[J]. Journal of Microbiological Methods, 2000, 41(3): 235-248.
[29] 林先贵. 土壤微生物研究原理与方法[M]. 北京: 高等教育出版社, 2010. Lin X G. Principles and methods of soil microbiology research[M]. Beijing: Higher Education Press, 2010.
[30] 郑勇, 高勇生, 张丽梅, 等. 长期施肥对旱地红壤微生物和酶活性的影响[J]. 植物营养与肥料学报, 2008, 14(2): 316-321. Zheng Y, Gao Y S, Zhang L Metal. Effects of long-term fertilization on soil microorganisms and enzyme activities in an upland red soil[J]. Plant Nutrition and Fertilizer Science, 2008, 14(2): 316-321.
[31] 袁颖红, 李辉信, 黄欠如, 等. 长期施肥对红壤性水稻土活性碳的影响[J]. 生态环境, 2007, 16(2): 554-559. Yuan Y H, Li H X, Huang Q Retal. Effects of long-term different fertilization on labile organic carbon in red paddy soil[J]. Ecology and Environment, 2007, 16(2): 554-559.
[32] Zheng J, Li L, Pan Getal. Potential aerobic C mineralization of a red earth paddy soil and its temperature dependence under long-term fertilizer treatments[J]. Soil Use and Management, 2012, 28(2): 185-193.
[33] Zeller V, Bardgett R D, Tappeiner U. Site and management effects on soil microbial properties of subalpine meadows: a study of land abandonment along a north-south gradient in the European Alps[J]. Soil Biology and Biochemistry, 2001, 33(4): 639-649.
[34] 刘恩科, 赵秉强, 李秀英, 等. 不同施肥制度土壤微生物量碳氮变化及细菌群落 16S rDNA V3 片段 PCR 产物的 DGGE分析[J]. 生态学报, 2007, 27(3): 1079-1085. Liu E K, Zhao B Q, Li X Yetal. Microbial C and N biomass and soil community analysis using DGGE of 16SrDNA V3 fragment PCR products under different long-term fertilization systems[J]. Acta Ecologica Sinica, 2007, 27(3): 1079-1085.
[35] 袁红朝, 秦红灵, 刘守龙, 等. 长期施肥对红壤性水稻土细菌群落结构和数量的影响[J]. 中国农业科学, 2011, 44(22): 4610-4617. Yuan H C, Qin H L, Liu S Letal. Response of abundance and composition of the bacterial community to long-term fertilization in paddy soils[J]. Scientia Agricultura Sinica., 2011, 44(22): 4610-4617.
[36] Ge Y, Zhang J, Zhang Letal. Long-term fertilization regimes affect bacterial community structure and diversity of an agricultural soil in northern China[J]. Journal of Soils and Sediments, 2008, 8(1): 43-50.
[37] 王霞, 陈哲, 袁红朝, 等. 应用 16S rDNA克隆文库技术研究长期稻草还田对水稻土细菌多样性的影响[J]. 生态学报, 2010, 30(13): 3865-3874. Wang X, Chen Z, Yuan H Cetal. Effect of long-term fertilization by the application of rice straw on bacterial diversity in paddy soil[J]. Acta Ecologica Sinica., 2010, 30(13): 3865-3874.
[38] 崔文文. 规模化秸秆沼气反应器中微生物群落结构分析[D]. 北京: 中国农业科学院硕士学位论文, 2013. Cui W W. Analysis on microbial community of plant-scale biogas digestor with straw as substrate[D]. Beijing: Ms thesis of Chinese Academy of Agriculture Sciences, 2013.
[39] 钱泽澍, 闵航. 沼气发酵微生物学[M]. 杭州: 浙江科学技术出版社, 1986. Qian Z S, Min H. Microbiology of biogas fermentation[M]. Hangzhou: Zhejiang Science and Technology Press, 1986.
[40] Cardinali-Rezende J, Pereira Z L, Sanz J Letal. Bacterial and archaeal phylogenetic diversity associated with swine sludge from an anaerobic treatment lagoon[J]. World Journal of Microbiology and Biotechnology, 2012, 28(11): 3187-3195.
[41] He J Z, Zheng Y, Chen C Retal. Microbial composition and diversity of an upland red soil under long-term fertilization treatments as revealed by culture-dependent and culture-independent approaches[J]. Journal of Soils and Sediments, 2008, 8(5): 349-358.
Microbial community diversity and enzyme activity of red paddy soil under long-term combined inorganic-organic fertilization
LU Hai-fei1, ZHENG Jin-wei1, YU Xi-chu2, ZHOU Hui-min1, ZHENG Ju-feng1, ZHANG Xu-hui1, LIU Xiao-yu1,CHENG Kun1, LI Lian-qing1, PAN Gen-xing1*
(1InstituteofResources,EcosystemandEnvironmentofAgriculture,NanjingAgriculturalUniversity,Nanjing210095,China;2JiangxiInstituteofRedSoil/FieldObservationStationofMinistryofAgriculture,Jinxian331717,China)
【Objectives】 Amendment of soil with organic and inorganic fertilizers has been considered an important source for carbon sequestration. However, it needs further research on influences of carbon sequestration to soil microbial health and reasons of improving soil biological activity. Soil samples were collected from Jiangxi Institute of Red Soil’s experimental fields. The aim of our study was to discuss the influences to microbial community diversity and enzyme activity of soil causing by different fertilizations. 【Methods】 Soil samples were collected after the harvest of rice, and total soil DNA was extracted. Soil microbial community diversity, composition and abundance were investigated using polymerase chain reaction combined with denaturing gradient gel electrophoresis(PCR-DGGE), cloning combined with sequencing and quantitative real-time PCR(qPCR). And the target genes for qPCR and PCR-DGGE were V3 and V6 partial bacterial 16S rRNA or partial fungal 18S rRNA. The DGGE analysis of using an 8% polyacrylamide gel with a denaturant gradient of 35%-65% for bacteria and 20%-40% for fungi. 96-well microplate fluorimetric was applied to analysis activities of β-N-acetylglucosaminidase, α-glucosidase, β-glucosidase, cellobiohydrolase, acid phosphatase and β-xylosidase of soil, and ultraviolet spectrophotometry was used for investigating activity of soil peroxidase. 【Results】 The Shannon index and richness index of soil bacteria are increased significantly under the treatment of organic manure plus chemical fertilization(NPKM) compared to the control without application of fertilizer(CK), while there are no changes in the two indexes of soil fungi among different treatments based on the PCR-DGGE analysis. Moreover the clustering analysis demonstrates that the NPKM treatment alters the community structure of soil bacteria and fungi. The results of sequencing indicate that soil bacteria is affiliated with Chloroflexi, Proteobacteria and Firmicutes, while soil fungi is belonged to Basidiomycota and Ascomycota. The bacterial phyla belonged toClostridiumand Anaerolineaceae is stimulated under the NPKM treatment, and the community of fungi is not changes significantly. The results of qPCR show that the copies of soil bacteria and fungi are not altered under different treatments. The soil enzyme assay indicates that comparing to CK, the treatment of nitrogen(N) significantly improves the activity of soil β-N-acetylglucosaminidase, the treatment of nitrogen, phosphorus and potassium(NPK) enhances the activities of soil β-N-acetylglucosaminidase and α-glucosdiase, and the NPKM treatment increases the activities of β-N-acetylglucosaminidase, cellobiohydrolase and peroxidase of soil. The activities of soil acid phosphatase and β-xylosidase are not altered among different treatments. Meanwhile, the normalized enzyme activity is increased under the NPKM treatment compared to the CK, N and NPK treatments. 【Conclusions】 The findings of this study conclude that the long-term fertilization treatment of NPKM significantly increases soil bacterial diversity, changes soil microbial community structure and improves soil enzyme activity. Therefore, the combined inorganic/organic fertilization can enhance the productivity of agricultural ecosystem and improve the health of soil ecosystem.
microbial community structure; diversity; enzyme activity; red paddy soil; long-term fertilization
2014-01-26 接受日期: 2014-07-13 网络出版日期: 2015-02-04
国家自然科学基金重点项目(40830528)资助。
陆海飞(1990—),男,江苏靖江人,硕士研究生,主要从事土壤碳氮循环与微生物过程研究。E-mail: lhf903@163.com * 通信作者 E-mail: pangenxing@aliyun.com
S154.3; S147.2
A
1008-505X(2015)03-0632-12

