Neofusicoccum parvum causing canker of seedlings of Juglans regia in China
••••
ORIGINAL PAPER
Neofusicoccum parvum causing canker of seedlings of Juglans regia in China
Zhongdong Yu1•Guanghui Tang1•Shaobin Peng1•Hui Chen1•Meizhi Zhai1
©Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2015
Cankered,dying seedlings ofJuglans regiawere observed in Shaanxi province in the northwest region of China.Neofusicoccum parvumwas isolated from these cankered tissues,with the identif i cation based on morphology and an ITS-nrDNA sequence.In order to demonstrate how cultures ofN.parvumcould cause the expected symptoms,artif i cial infection,using these isolates and re-isolation of the pathogen,was used.This is the f i rst report on this taxon as a walnut canker pathogen in China.
Forest tree disease⋅ITS-nr-DNA⋅Koch’s postulates⋅Neofusicoccum parvum⋅Walnut
Introduction
Walnut(Juglans regia),is an indigenous tree species in China.In Shaanxi province in the northwest region of the country,market demand has stimulated the importation of new cultivars from adjacent provinces(Xinjiang,Gansu, Henan,Sichuan,Yunnan),as well as from the US and the EU.It was in this context that cultivar‘Xiangling’was brought into the northwest region from Xinjiang province. Seedlings ofJuglans regiaare typically grown to serve as rootstocks for scions of walnut cultivars.In 2010,disease symptoms,including lesions girdling the base of the stems, were observed on one-to three-year-old seedlings grafted with cv.‘Xiangling’as scions.Cankers followed and these were accompanied by brown and red-brown exudates,Fig.1a.Finally,the seedlings died.
The only known canker diseases affecting walnuts in China areValsaandBotryosphaeriacanker.The disease caused byValsa juglandisis called locally known as‘Black Water Disease’and often leads to plantation failure.Botryosphaeriacanker caused byB.dothidea(anamorph:Fusicoccum aesculi)appears later on the same trees affected byValsa.The species ofBotryosphaeriaassociated with this walnut canker do vary around the world (Hagsag et al.2007;Rumbos 1987;Yu et al.2010).But in all cases,only adult trees are affected,and its pathogen is believed to beBotryosphaeria dothidea;while in Egypt the pathogen isBotryodiplodia theobromae(Hagsag et al. 2007)and in Greece,it isB.ribis(Rumbos 1987).Some authors considerB.ribisas a synonym ofB.Dothidea(von Arx and Muller 1975;Maas and Uecker 1984),while others think not(Phillips et al.2002;Yu et al.2010). However,both are reported occurring only on adult trees.
Materials and methods
Morphology and pathogenesis analysis
Tissue samples from 10 walnut seedling stems were cultured on medium of potato,dextrose and agar(abv.PDA), following surface-sterilization in 1/1000 g/L mercurybichloride for 30 s followed by washing in sterile water. The samples were then incubated at 25°C in a dark room. Other samples from adult trees in different loci were treated the same way but the pathogens from adult trees were only used for phylogeny study.When clones developed well,subcultures were purif i ed and bacteria clones were also subcultured using scaled-line culture methods. Culturing traits and clone morphology on medium were dated until a sporocarp matured.

Fig.1 SymptomsandmorphologyofNeofusicoccumparvum. a Seedlings with lesions girdling the base stem.b Yellow pigment ringing the colony c Microconidia&Macroconidia.d Aseptate and septate macroconidia before germination.e Conidiogenous cells producing conidia holoblastically.f Conidiogenous cells with 1–3 indistinct percurrent proliferations
Isolates from seedlings were further used for pathogenesis investigation using artif i cial inoculation.Mycelial plugs(2 mm in diameter)were applied to bark wounds of equal size on the base of the stems of seedlings ofJuglans regiacv.‘Shangluo’.Controls were inoculated with sterile PDA plugs.Bacterial inoculation was also carried out by puncturing the bark with a needle.Each inoculum was applied to f i ve stems and covered by f i lms for 2–3 weeks, as was the control.The inoculation was carried out under controlled conditions in a chamber(temperature 25°C, relative humidity 75%,and 150 μm-2 s-1 light for 16 h per day).DNA extraction,Sequencing and Blasting.
Mycelium on medium of PDA were scraped off into 1.5 mL tube and immersed into 2%CTAB at 65°C, granted deliberately for few minutes and then incubated at 65°C for about 1 h,followed by adding equivalent volumes of chloroform isopropanol(24:1)for proteins erasing at 8000 rpm for 10 min.The upper aqueous layer was transferred to a fresh eppendorf tube,1/3 volume of NH4COOH(10 M)and 2.5×volume ethanol added,and the mixture was put at-20°C for 60 min.After centrifugation for 15 min at 12,000 rpm,4°C,the DNA pellet was washed with 70%(v/v)ethanol for 2 times,and dried in a Vacuum Pump.The dried pellet was resolved in 100 μl of TE-buffer(0.01 mM Tris–HCl,pH 8.0,containing 0.1 mM EDTA),dissolved at 65°C and then stored at -20°C until use.Internal translate space(ITS)fragment DNA was amplif i ed with universal primes ITS1and ITS4(White et al.1990).PCR reactions were carried out in a total mixture volume of 25 μL containing a f i nal concentration of 1.5 mM MgCl2,0.5 mM dNTPs(Applied Biosystems),10 pmol of each primer,1.0 U AmpliTaq®DNA Polymerase(Applied Biosystems),and 20 ng of genomic DNA.Samples were incubated in a thermal cycler (PTC-100,MJ Research,USA)and followed the reaction protocol:94°C for 3 min pre-denature,94°C for 2 min denature,53°C anneal for 1 min 30 s,72°C extension for 1 min 30 s,after 10 cycles,continued as following:93°C for 1 min,51°C for 55 s and 72°C for 1 min,25 cycles later,with a f i nal extension at 72°C for 10 min 30 s,and then stop reaction at 25°C for 10 min.PCR products were electrophoresis on 1.5%agar gel(Melt tempt.36–37°C), and then purif i ed with the ZymocleanTM Gel DNA Recovery Kit(Zymo Research Corp.)by following manufacturer’s instructions.Samples were then sequenced using ITS1and ITS4primers and the sequencing reaction products were cleaned following differential precipitation with ethanol.Sequences were determined by an ABI 3130 xl automated sequencer(Applied Biosystems)anddeposited in GenBank(Table 1).Nucleotide BLAST search(unmodif i ed options)was used to compare our sequences to those presented in the GenBank.
Phylogeny tree construction
DNA sequences were aligned f i rst with Clustal X 1.81 (Thompson et al.1997),and then adjusted based on the algorithm of Nei and Kumar(2000).All positions containing gaps and missing data were completely eliminated from the dataset.Data was analyzed by maximum parsimony(MP)using the Close-Neighbor-Interchange algorithm(Nei and Kumar 2000)as implemented in MEGA 4.0 (Tamura et al.2007).Support for the branches based on parsimony criteria was estimated by bootstrap analysis using 1000 replicates.
Results and discussion
Fungalcolonies and 2 bacterialcolonies were dated and subcultured after 1 week.Sub-cultures of fungi quickly developed cottony,aerial mycelium.Colonies were initially pale to white on PDA,but diffused yellow pigment into agar around the colony 5 dayslater,Fig.1b.After10 days,aerial mycelium became grey,and submerged mycelium became dark violaceous.Conidiomata were unilocular pycnidia and conidia began to disperse from the ostioles within 10–15 days.Conidiomata were papillate,partially immersed in the medium,and covered with dark olive-green,appendage-like hyphae.Macroconidia were hyaline,thin-walled, aseptate,fusiform,with asubobtuseapex and atruncatebase, Fig.1c.One or two light yellow septa developed as spores begin matured,Fig.1d.The mean size of 50 macroconidiawas 22.3±0.8×5.1±0.3 μm;mean length/width ratios were 3.8±0.1 μm.Microconidia were rod-shaped to ovoid with rounded ends and thick walls,and they measured 3–5×1–2 μm,Fig.1c.Conidiophores were hyaline,unbranched,and cylindrical,Fig.1e,f.On walnut stems, conidiomata were dark grey to black,eustromatic,separate, uni-ormultilocular.Loculeswere up to 200 μm in diameter, immersed in the bark,and sub-epidermal.Conidiophores were the same as that in PDA medium but longer, 7.3–21.5×4–6 μm,with 1–3 indistinct percurrent proliferations,Fig.1f.Conidia were similar shape to those produced in PDA medium but more variable in size,and ranged from 12–25.5×4.5–8.2 μm,with a mean length/width ratio of 2.75±0.6(100 conidia).Bacteria clone were identif i ed asBacillus subtilisby 16SrDNA(accession no.KF591602-KF591603)and were reported in another paper(Yu et al. 2015).

Table 1 Accessions and isolates for phylogenetic tree
Inoculation experiment showed the putative pathogen can infect via wounded bark.After 2–3 weeks,all seedlings inoculated with the putative fungi pathogen showed the expected symptoms,with cankers and copious exudates at inoculation sites,Fig.2.Symptoms were not visible in the control or in the stems inoculated with bacteria.No fungus was reisolated from controls.The putative pathogen was re-isolated from all stems.

Fig.2 Artif i cial inoculation.The upper site is the control inoculated by sterilized PDA plug,and the two sites below show pathogenesis of the isolate NW11-016 after 2 weeks
Based on the symptoms,cultural morphologies,and conidial characters,the fungus was tentatively identif i ed asNeofusicoccum parvumaccording to(Slippers et al.2004).Neofusicoccum parvumhas previously been reported as causing canker symptoms ofVitis viniferain Spain(Martos et al.2011;Moral et al.2010),andMangifera indicain Australia(Slippers et al.2005),andEucalyptusin Uruguay (Perez et al.2010),and walnut in California(Michalidis et al.2012).It was reported occurring onPopulus,VitisandEucalyptus(Yu et al.2009)in recent years in China,and to our knowledge,it is the f i rst report ofN.parvumas a canker pathogen of seedlings ofJuglans regiain China. The isolates used in this study are maintained in the culture collection of the Forestry College,Northwest A&F University,China,as culture collection number NW11-016 and NW11-014.
However,N.luteum,B.dothideaandN.ribisare similar toN.parvum.Pennycook and Samuels(1985) describedN.luteumand separated it fromN.parvumandB.dothideaaccording to the yellow pigment diffused in young PDA culture.Phillips et al.(2002)indicated that this is not a reliable character unless the cultural conditions are controlled carefully.Instead,they described the unilocular conidiomata ofN.luteumas distinct fromN. parvumandB.dothidea.However,this is also inf l uenced by culturing conditions.(Slippers et al.2005)foundN. ribisandN.parvumboth diffused yellow pigment in young culture and suggested conidium dimensions and aerial mycelium of aged colonies as being important characters to distinguishN.parvum,N.luteum,N.ribisandB.dothidea.
To determine the systematic placement of the putative pathogen in this paper,the ITS rDNA locus was amplif i ed and sequenced with the universal primers ITS1and ITS4(White et al.1990),followed by a BLAST search in GenBank and a phylogenetic study,Table 1.
Sequences of the putative pathogen(NW11-016)and a similar culture(NW11-014)from cankered seedlings showed 100%sequence identity withN.parvum(Fig.3). All isolates(Table 1)from adult stems and branches ofJuglans regiawere in theB.dothideaclade,which is consistent with the report of Tang et al.(2012).B.dothideawas thought a complex species(Slippers et al.2004;Pennycook and Samuels 1985),and can be found in a wide host range(Smith 1934).There were many cryptic species associated withB.dothidea,now it can be distinguished by help of molecular technologies(Slippers et al.2004; Denman et al.2003).N.luteumin this analysis was monophyletic.N.ribisandN.parvumare clearly closelyrelated(Sakalidis et al.2011)and this was conf i rmed here, and it is confusing to f i nd bothN.ribis(Urbez-Torres et al. 2012)andN.parvum(Martos et al.2011)ever described as canker pathogen ofVitis vinfera.
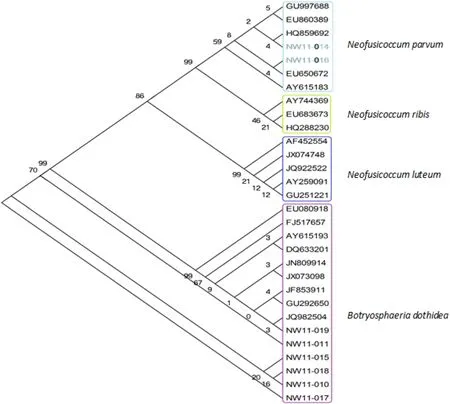
Fig.3 Phylogenetic relationships between selected Botryosphaeria species and isolates from walnut.Isolates with capital letters‘NW’and JN809914 were collected from walnut in China by the author.The others are from GenBank;JF835911 is from walnut in China
It is also important to note thatN.ribishas been reported as a walnut pathogen(Rumbos 1987),andB.ribis(anamorph:N.ribis)was ever thought as a synonym ofB. dothidea(Michailidis 1991;Whitch and Clayton 1963). Since mostBotryspaheriaspecies are cryptic,other coding sequence genes may be useful for further identif i cation (Inderbitzin et al.2010).In this paper,we reported this seedling pathogen wasNeofusicoccum parvumnewly occurred on walnut tree.
AcknowledgmentsI would thank deeply George Newcombe in University of Idaho for his English reviewing and suggestion.This study was supported by the program for tacking key problem of Shaanxi agricultural scientif i c and technological extent(2015NY124), Project NSFC(31270690),Project PCSIRT(NO.IRT1035),special funding for basic s&t work of Ministry of Science and Technology (2009FY210100)of China.
Denman S,Crous PW,Groenewald JZ,Slippers B,Wingf i eld BD, Wingf i eld MJ(2003)Circumscription ofBotryosphaeria speciesassociated withProteaceaebased on morphology and DNA sequence data.Mycologia 95(2):294–307
Hagsag WM,Rayya A,Kasim NE(2007)First report of a canker disease of walnut causedby Botryodiplodia theobromaein Egypt.Plant Dis 91(2):226
Inderbitzin P,Bostock RM,Trouillas FP,Michailides TJ(2010)A six locus phylogeny reveals high species diversity inBotryosphaeriaceaefrom California almond.Mycologia 102:1350–1368
Maas JL,Uecker FA(1984)Botryosphaeria dothideacane canker of thornless blackberry.Plant Dis 68:720–726
Martos S,Torres E,Bakali MA,Raposo R,Gramaje D,Armengol J, Luque J(2011)Co-operational PCR coupled with dot blot hybridization for the detection ofPhaeomoniella chlamydosporaon infected grapevine wood.J Phytopathol 159(4):247–254
Michailidis TJ(1991)Pathogenicity,distribution,sources of inoculum,and infection courts ofBotryosphaeria dothideaon pistachio.Phytopathology 81:566–573
Michalidis TJ,Chen SF,Coates B,Morgan D,Puckett R,Hasey J, Anderson K,Buchner R,DeBuse C,Fichtner E,Bentley W (2012)Managing anthracnose blight andBotryosphaeriaandPhomopsiscankers of Walnut.Walnut,Research Reports, pp 367–381
Moral J,Munoz-Diez C,Gonzalez N,Trapero A,Michailides TJ (2010)Characterization and pathogenicity ofBotryosphaeriaceaespecies collected from olive and other hosts in Spain and California.Phytopathology 100(12):1340–1351
Nei M,Kumar S(2000)Molecular evolution and phylogenetics. Oxford University Press,New York
Pennycook SR,Samuels GJ(1985)BotryosphaeriaandFusicoccumspecies associated with ripe fruit rot ofActinidia deliciosa(kiwifruit)in New Zealand.Mycotaxon 24:445–458
Perez CA,Wingf i eld MJ,Slippers B,Altier NA,Blanchette RA (2010)Endophytic and canker-associatedBotryosphaeriaceaeoccurring on non-nativeEucalyptusand nativeMyrtaceaetrees in Uruguay.Fungal Divers 41(1):53–69
Phillips AJL,Fonseca F,Povoa V,Castilho R,Nolasco G(2002)A reassessment of the anamorphic fungusFusicoccum luteumand description of its teleomorphBotryosphaeria luteasp.nov. Sydowia 54:59–77
Rumbos IC(1987)Twig and branch dieback of walnut trees induced byBotyosphareria ribis.Plant Pathol 36:602–605
Sakalidis ML,Hardy GEJ,Burgess TI(2011)Endophytes as potential pathogens of the baobab speciesAdansonia gregorii:a focus on theBotryosphaeriaceae.Fungal Ecol 4:1–14
Slippers B,Crous PW,Denman S,Coutinho TA,Wingf i eld BD, Wingf i eld MJ(2004)Combined multiple gene genealogies and phenotypic characters differentiate several species previously identif i ed asBotryosphaeria dothidea.Mycologia 96:83–101
Slippers B,Johnson GI,Crous PW,Coutinho TA,Wingf i eld BD, Wingf i eld MJ(2005)Phylogenetic and morphological reevaluation of theBotryosphaeriaspecies causing diseases ofMangifera indica.Mycologia 97(1):99–110
Smith CO(1934)Inoculations showing the wide host range ofBotryosphaeria ribis.J Agric Res 49:467–476
Tamura K,Dudley J,Nei M,Kumar S(2007)MEGA4:Molecular Evolutionary Genetics Analysis(MEGA)software version 4.0. Mol Biol Evol 24(8):1596–1599
Tang W,Ding Z,Zhou ZQ,Wang YZ,Guo LY(2012)Phylogenetic and pathogenic analyses show that the causal agent of apple ring rot in China isBotryosphaeria dothidea.Plant Dis 96(4):486–496
Thompson JD,Gibson TJ,Plewniak F,Jeanmougin F,Higgins DG (1997)The CLUSTAL-X windows interface:f l exible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Urbez-Torres JR,Peduto F,Striegler RK,Urrea-Romero KE,Rupe JC,Cartwright RD,Gubler WD(2012)Characterization of fungal pathogens associated with grapevine trunk diseases in Arkansas and Missouri.Fungal Divers 52(1):169–189
von Arx JA,Muller E(1975)Re-evaluation of bitunicate ascomycetes with keys to families and genera.Study Mycol 9:1–159
Whitch W,Clayton CN(1963)Blueberry stem blight caused byBotryosphaeria dothidea(B.ribis).Phytopathology 53:705–712
White TJ,Bruns T,Lee S,Taylor J(1990)Amplif i cation and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In:Innes MA,Gelfand DH,Sninsky JJ,White TJ(eds)PCR Protocols:a guide to methods and applications.Academic Press, San Diego
Yu L,Chen XL,Gao LL,Chen HR,Huang Q(2009)First report ofBotryosphaeria dothideacausing canker and shoot blight ofEucalyptusin China.Plant Dis 93(7):764
Yu ZD,Zhao GC,Dan JY,Ren ZZ(2010)Phylogeny ofBotryosphaeriaspecies based on ITS-nrDNA sequences. Mycosystema 29:285–293
Yu ZD,Wang JL,Tang GH,Zhai MZ(2015)Exploring of endophyticBacillus subtilisas an agent of biocontrol for walnut canker. Biotechnol Indian J 11(11):419–425
4 November 2013/Accepted:28 January 2014/Published online:11 August 2015
Project funding:This study was supported by the program for tacking key problem of Shaanxi agricultural scientif i c and technological extent(2015NY124),Project NSFC(31270690),Project PCSIRT (NO.IRT1035),special funding for basic S&T work of Ministry of Science and Technology(2009FY210100)of China.
The online version is available at http://www.springerlink.com
Corresponding editor:Chai Ruihai
✉Zhongdong Yu
yuzhongdong001@nwsuaf.eud.cn;yu-10083@163.com1Forestry College,Northwest A&F University, Yangling 712100,Shaanxi,People’s Republic of China
 Journal of Forestry Research2015年4期
Journal of Forestry Research2015年4期
- Journal of Forestry Research的其它文章
- Drone remote sensing for forestry research and practices
- Life cycle environmental impact assessment of biochar-based bioenergy production and utilization in Northwestern Ontario, Canada
- Growth rates of Eucalyptus and other Australian native tree species derived from seven decades of growth monitoring
- Effect of f i rst thinning and pruning on the individual growth of Pinus patula tree species
- The inf l uence of selective cutting of mixed Korean pine(Pinus koraiensis Sieb.et Zucc.)and broad-leaf forest on rare species distribution patterns and spatial correlation in Northeast China
- Modeling forest f i res in Mazandaran Province,Iran
