NO3-/NH4+ratios affect plant growth,chlorophyll content, respiration rate,and morphological structure in Malus hupehensis seedlings
•••
ORIGINAL PAPER
NO3-/NH4+ratios affect plant growth,chlorophyll content, respiration rate,and morphological structure in Malus hupehensis seedlings
Yu Dong1,2•Huan-huan Zhi2•Qian Zhao1,2•Jun-feng Guan1
©Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2015
Malus hupehensis(Pamp.)Rehd.is a widely cultivated rootstock in China.We studied the effect of threeratios(100/0,50/50,and 0/100,molar basis)at total nitrogen(N)concentration of 8 mmoL L-1in a nutrient solution onM.hupehensisseedlings.Plant biomass,concentration,chlorophyll content,respiratory rate,and cellular structure were investigated.M.hupehensisseedlings at theratio of 50/50 had the highest level of fresh weight,dry weight, shoot length,and chlorophyll(a,b,anda+b)content,but the lowest respiration rate in the leaves and roots.In addition,thickness and numbers of palisade and spongy tissue cells of the leaves were greater with this treatment than with other treatments.At the NO3-/NH4+ratio of 100/0,the leaves and roots had higher NO3-concentration and lower NH4+concentration.However,the opposite trend occurred at the NO3-/NH4+ratio of 0/100.Chlorophyll(a,b,anda+b)content was lowest at the NO3-/ratio of 100/0 than at the other ratios.At theratio of 0/100,oxygen(O2)consumption increased in the leaves and roots,and irregular epidermis and cortex cells were observed in the root apical meristematic and mature region.Our results indicated that theratio at 50/50 was suitable for growth ofM.hupehensisseedling to achieve the highest biomass production and eff i ciency.
Cellular structure⋅Chlorophyll content⋅Malus hupehensis(Pamp.)Rehd.Respiratory rate
Introduction
The nitrogen(N)requirement in plants can be supplied by nitrate(NO3-)and ammonium(NH4+)assimilation(Lasa et al.2002).Nitrogen metabolism affects many physiological processes such as uptake,transport,assimilation, amino acid biosynthesis,carbohydrate metabolism,and ultimately plant growth regulation(Lawlor 2002).In the N uptake processes,NO3-and NH4+are the two major inorganic N forms for plant absorption in a soil solution. Plants can uptake both ions.However,the physiological, biochemical,and molecular features of NO3-and NH4+differ in metabolism(Jackson et al.2008;Patterson et al. 2010).Nitrate can be accumulated into the vacuoles in the roots and transported to the leaves for assimilation,and dissimilated to alleviate the toxicity of a high NO3-concentration(Black et al.2002;Xu et al.2012).In the N assimilation process,NO3-is f i rst reduced to nitrite (NO2-)by nitrate reductase(NR)in the cytoplasm,and then converted to NH4+by nitrite reductase(NiR)in the plastids and in the cytoplasm.However,NH4+derivedfrom NO3-or by uptake is further assimilated into amino acids via the glutamine synthetase/glutamate-2-oxoglutarate aminotransferase(GS/GOGAT)pathway(Xu et al. 2012).Shortened roots and reduced shoot biomass are clearly two phenotypic traits that occur at high levels of NH4+(Chaillou et al.1994;Loque´and von Wire´n 2004).Malus hupehensis(Pamp.)Rehd.has been widely cultivated in China as an important rootstock for apple production.However,it remains unknown how the mechanisms of the uptake and transport of NO3-and NH4+respond to the availability of different NO3-/NH4+ratios.
For most plants,the energy cost for uptake and assimilation of NH4+is less than for NO3-(Guo et al.2007).In addition,a high level of NH4+affects many important physiological processes(e.g.rhizosphere acidif i cation, nutrient imbalance,damage to photosynthesis,and carbohydrate limitation)(Britto and Kronzucker 2002;Chen et al.2013).Excess NO3-can induce root branching (Celis-Ara´mburo et al.2011),whereas excess NH4+can inhibit the length of the root apical meristematic and elongation region(Liu et al.2013).However,few studies have reported morphological responses of leaves and roots to different NO3-/NH4+ratios.To explore the regulative mechanism of N absorption on plant growth,it is thus necessary to determine the relationship between NO3-and NH4+uptake,chlorophyll content,O2consumption,and morphological structure.
This aims of this study were to assess the growth and development responses ofM.hupehensisseedlings to three NO3-/NH4+ratios(100/0,50/50,and 0/100)in a nutrient solution and to evaluate the effects of NO3-and NH4+concentration,chlorophyll content,respiration rate,and morphological structure.
Materials and methods
Plant material and treatments
Malus hupehensisseedlings were germinated in vermiculite,irrigated with distilled water,and grown for approximately 10 days.The temperature was maintained at 25±2°C with a 14/10 h(light/dark)cycle.Uniform seedlings were subsequently selected and transferred to a plastic pallet(40×40×15 cm)f i lled with 5 L fullstrength Hoagland’s nutrient solution.After cultivation for approximately 14 days in the nutrient solution,the seedlings were transferred to an N-free nutrient solution (Hoagland and Arnon 1950)for 48 h to generate N-deprived seedlings.The seedlings were then treated with the three NO3-/NH4+ratios at a total N concentration of 8 mmoL L-1.1.0 L of nutrient solution were added the macronutrients at three NO3-/NH4+ratios of 100/0,50/50, and 0/100(Table 1).One milliliter of micronutrients contained 2.86 g L-1of H3BO3,0.08 g L-1of CuSO45H2O, 0.22 g L-1of ZnSO47H2O,1.81 g L-1of MnCl24H2O, 0.09 g L-1of H2MoO44H2O,and 2.0 mL of Fe-EDTA containing 5.56 g L-1of FeSO47H2O and 7.46 g L-1of C10H14N2O8Na22H2O.The nutrient solutions were adjusted to pH 6.3 with 1 N NaOH or HCl,based on the method of Chang et al.(2010).
All treated seedlings were incubated in a 14/10 h (light/dark)cycle,at a temperature of 26/16°C(day/night) and 60–70%relative humidity under artif i cial light of 1000 μmoL m-2s-1.The nutrient solutions were replaced at 3-day intervals to provide a stable total N concentration in the nutrient solution.Leaves from the third true leaf and the whole roots were removed at 3-day intervals,and frozen in liquid nitrogen for analyses of NO3-and NH4+concentration and chlorophyll(a,b,anda+b)content. For observation of the leaf and root structure,the third true leaf and the taproot were removed at 6-day intervals and rinsed.All three treatments were applied to three replicates, and 30 plants from each replicate were taken at each sampling point.
Biomass measurements
The fresh weight(FW)was recorded at 3-day intervals by using a standardized weight procedure.Each plant was separated into shoot and root components.The length of the longest shoot and root were then measured.The plants were dried in an oven at 65°C for 24 h to determine the dry weight.
Determination of NO3-and NH4+concentration
NO3-concentration was quantif i ed as described by Patterson et al.(2010).The f i ne powder(0.1 g)of the leaves or roots was extracted in 1.0 mL deionized water at 45°C for 1 h.After centrifugation at 8,000×gat 20°C for 15 min, the supernatant was used for NO3-quantif i cation.The supernatant(0.1 mL)was mixed with 0.4 mL of 5%(w/v) salicylic acid in H2SO4.After incubating at room temperature for 15 min,9.5 mL of 2 N NaOH was added and set at room temperature for 30 min.The NO3-concentration was determined by measuring the absorbance at 410 nm with a spectrophotometer(UNICO UV-2100,United Products and Instruments Inc.,USA).NO3-concentration was calculated from a standard curve obtained by known concentrations of KNO3.
NH4+concentration was quantif i ed as described by Bra¨utigam et al.(2007).The f i ne powder(0.1 g)of the leaves or roots was extracted in 1.0 mL of 0.1 M HCl and 500 μL of chloroform.The samples were shaken at 4°Cfor 15 min,and then centrifuged at 12,000×gat 4°C for 10 min.The aqueous phase was transferred to a 2.0 mL tube.Fifty milligrams of activated charcoal was added,and then centrifuged at 12,000×gat 4°C for 5 min.The supernatant(20 μL)was placed into the cavities of a 96-well microplate with the addition of 110 μL of 1%(w/ v)phenol–0.005%(w/v)sodium nitroprusside solution in water and 110 μL of 1%(v/v)sodium hypochlorite–0.5% (w/v)sodium hydroxide solution in water.After the microplate was incubated at 37°C for 30 min,the NH4+concentration was determined by measuring the absorbance at 620 nm with a microplate photometer(Multiskan MK3,Thermo Scientif i c,Finland).The NH4+concentration was calculated from a standard curve that was obtained by known concentrations of(NH4)2SO4.

Table 1 The components of macronutrients at three NO3-/NH4+ratios at the total N concentration of 8 mmoL L-1
Determination of chlorophyll content
For the determination of chlorophyll(a,b,anda+b)content,the f i ne powder(0.1 g)of leaves was homogenized in 10 mL of 80%acetone,and then centrifuged at 12,000×gfor 10 min.Chlorophyll(a,b,anda+b)content was determined by measuring absorbance at 663 and at 646 nm with a spectrophotometer(UNICO UV-2100,United Products and Instruments Inc.,USA). Chlorophyll(a,b,anda+b)content was calculated using the equations of Porra et al.(1989),as follows:(1) chlorophylla(μg mL-1)=12.25A663-2.55A646;(2) chlorophyllb(μg mL-1)=20.31A646-4.91A663;and (3)chlorophylla+b(μg mL-1)=17.76A646+7.34A663.
Measurement of respiration rate
The respiration rate of the leaves and roots was determined by the method of Balkos et al.(2010).The fresh weight (0.5 g)of the leaves or roots was measured at 3-day intervals and the O2consumption was measured using an electrode in a liquid phase oxygen measurement system (Oxygraph,Hansatech Instrument Ltd.,UK).The third true leaf and the whole root were collected,rinsed,and immediately cut into 2 mm2and 2 mm sections,respectively, using a razor blade.The samples were placed into an oxygen electrode chamber at 20°C,which contained 2.0 mL of 5 mM MES and 2 mM CaCl2(pH 7.2).The chamber was then sealed.The decline in the O2concentration was monitored in the dark for 15 min with the initial,linear,and decline rates used to calculate the O2depletion rates.
Observation of cellular structure
Tissue samples of the leaves and roots were observed by the method of Aparicio and Marsden(1969).The third true leaf was cut into 2 mm2sections and 1 cm of the root tip was removed from the taproot using a razor blade.Samples were f i xed in 2.5%(v/v)glutaraldehyde in 0.1 M potassium phosphate buffer(pH 7.4)at 4°C for 24 h.After washing in the same buffer,the samples were post-f i xed in 1%(w/v)osmium tetroxide in 0.1 M potassium phosphate buffer(pH 7.4)at 4°C for 3 h,and then dehydrated in a graded alcohol series and embedded in EPON812.For structural observation,1.5 μm thickness semi-thin sections were prepared with a glass knife on an ultramicrotome (Leica Ultracut R,Leica,Germany)and stained with 1% (w/v)methylene blue containing 1%(w/v)borax solution in water.After washing with deionized water,the semi-thin sections were observed under a f l uorescent microscope (Olympus BX51,Olympus Optical Co.Ltd.,Japan)that was equipped with a DP-72 digital camera under the transmit light(100×magnif i cation).
Statistical analysis
Data were subjected to analysis of variance(ANOVA).The signif i cance of differences between the means at the level ofP<0.05 was determined by Duncan’s multiple comparison test.
Results
Effects of NO3–/NH4+treatments on plant biomass
The fresh and dry weight per plant increased with N supply.During plant growth,the change of fresh weight was similar to change of dry weight.Fresh weight,dry weight, and shoot length at NO3-/NH4+ratios of 100/0 and 50/50 were signif i cantly higher than at the NO3-/NH4+ratio of 0/100(Fig.1a–c).Root length was highest at the NO3–/ NH4+ratio of 50/50 and lowest at the NO3–/NH4+ratio of 100/0(Fig.1d).
Effects of NO3-/NH4+treatments on NO3-and NH4+concentration
NO3-concentration gradually increased in leaves and roots at the NO3-/NH4+ratio of 100/0,whereas the opposite trend occurred at the NO3-/NH4+ratio of 0/100.The lowest NO3-concentration in the roots at the NO3-/NH4+ratio of 0/100 surprisingly occurred after 3 days of treatment and remained at a low level during plant growth.At the NO3-/NH4+ratio of 50/50,the NO3–concentration in the leaves and roots reached a peak after 3 days of treatment,and then declined,but subsequently increased after 12 days of treatment(Fig.2a,b).
NH4+concentration decreased in leaves at NO3-/NH4+ratios of 100/0 and 50/50 during plant growth.The lowest NH4+concentration occurred at the NO3-/NH4+ratio of 100/0.However,at the NO3-/NH4+ratio of 0/100,the NH4+concentration in leaves decreased after 3 days of treatment,and then gradually increased and was higher than at the other ratios(Fig.2c).NH4+concentration in roots increased during plant growth at NO3–/NH4+ratios of 50/50 and 0/100.The highest concentration occurred at the NO3-/NH4+ratio of 0/100.However,at the NO3–/NH4+ratio of 100/0,the NH4+concentration in the roots decreased after 6 days of treatment,and then increased,but remained lower than at the other ratios(Fig.2d).
Effects of NO3-/NH4+treatments on chlorophyll content of leaves
At NO3-/NH4+ratios of 50/50 and 0/100,chlorophyll(a,b,anda+b)content gradually increased and the highest chlorophyll(a,b,anda+b)content occurred at the NO3-/NH4+ratio of 50/50.However,chlorophyll(aanda+b)content at the NO3-/NH4+ratio of 100/0 slowlyincreased and no signif i cant difference was found in chlorophyllbcontent during plant growth(Fig.3).
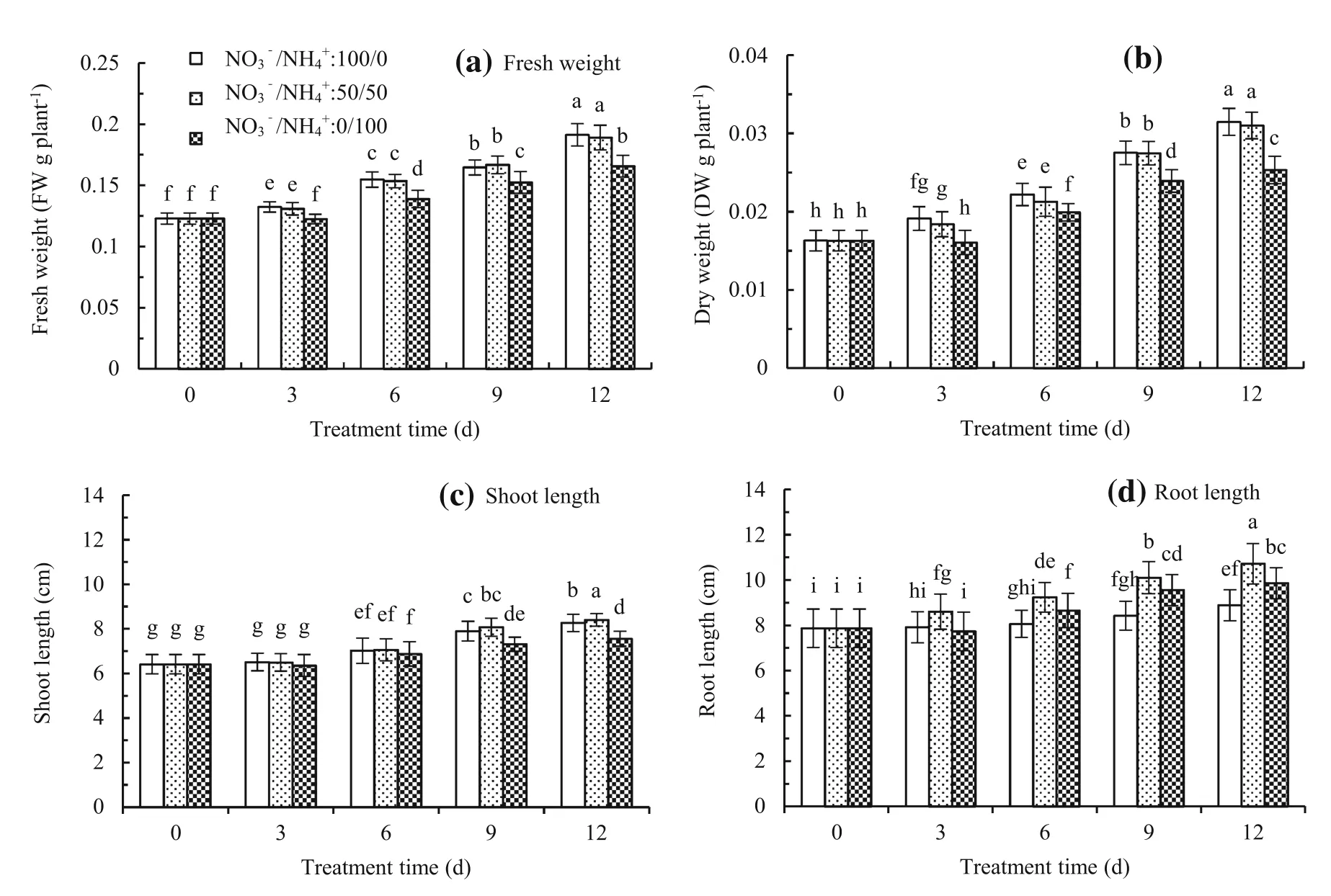
Fig.1Fresh weight(a),dry weight(b),shoot length(c),and root length(d)ofMalus hupehensisseedlings treated at NO3–/NH4+ratios of 100/0,50/50,and 0/100.Thebarindicates the mean±the standard error(n=3).The different letters on the bars indicate signif i cant difference at P<0.05
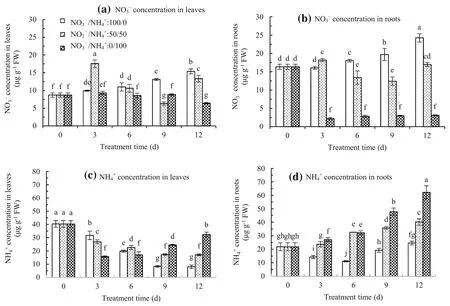
Fig.2 The NO3-and NH4+concentration in the leaves(a,c)and the roots(b,d)of Malus hupehensis seedlings treated at the NO3-/NH4+ratios of 100/0,50/50,and 0/100.The bar indicates the mean±the standard error(n=3).The different letters on the bars indicate signif i cant difference at P<0.05
Effects of NO3-/NH4+treatments on respiration rate
O2consumption at the NO3-/NH4+ratio of 0/100 increased and remained at a high level in leaves and roots, whereas lower O2consumption occurred at the NO3-/ NH4+ratios of 100/0 and 50/50(Fig.4).At NO3-/NH4+ratios of 100/0 and 50/50,O2consumption in leaves and roots decreased after 3 days of treatment,and then increased after 6 days of treatment;O2consumption subsequently decreased in leaves(Fig.4a).However,roots maintained stable O2consumption(Fig.4b).
Effects of NO3-/NH4+treatments on morphological structure
In comparison to the other ratios,the number of palisade and spongy cells and the thickness of the leaves were greater at the NO3-/NH4+ratio of 50/50 after 12 days of treatment(Fig.5e,f).The size of the palisade cells was greater at the NO3-/NH4+ratio of 100/0 than at the other ratios(Fig.5c).
After 6 days of treatment at the NO3-/NH4+ratio of 100/0,the root apical meristematic region showed just one epidermal layer.During plant growth,7–9 epidermal layers were observed after 12 days of treatment;however,the epidermis cells were squeezed together and cells in the root apical meristematic region were fewer at this ratio than at the other ratios(Fig.6b,c).At the NO3-/NH4+ratio of 50/50,3–4 epidermal layers and numerous cells in the root apical meristematic region were observed after 6 and 12 days of treatment,and the cells in the aforementioned region were closely arranged as a circle or rectangle (Fig.6e,f).At the NO3-/NH4+ratio of 0/100,no epidermal layer was observed after 6 days of treatment,whereas epidermal layers were present after 12 days of treatment, and the membrane of epidermal cells were broken down and mixed together.Cells in the root apical meristematic region at the NO3-/NH4+ratio of 0/100 were larger, compared to those treated with the other NO3-/NH4+ratios (Fig.6h,i).
In the root apical mature region,the root structure is composed of the epidermis,cortex,and vascular cylinder (Fig.7).At the NO3-/NH4+ratio of 50/50,f i ve cortex layers were observed after 12 days of treatment and the cortex cells were larger than at the other ratios.The vascular cylinder had three xylem strands,whereas just twoxylem strands were observed at NO3-/NH4+ratios of 100/0 and 0/100(Fig.7c,f,i).At the NO3-/NH4+ratio of 0/100,the epidermal and the cortical cells of the root apical mature region were irregularly shaped and wrinkled after 6 days and 12 days of treatment.However,compared to the other ratios,the vascular cylinder cells maintained cell integrity at this ratio(Fig.7h,i).
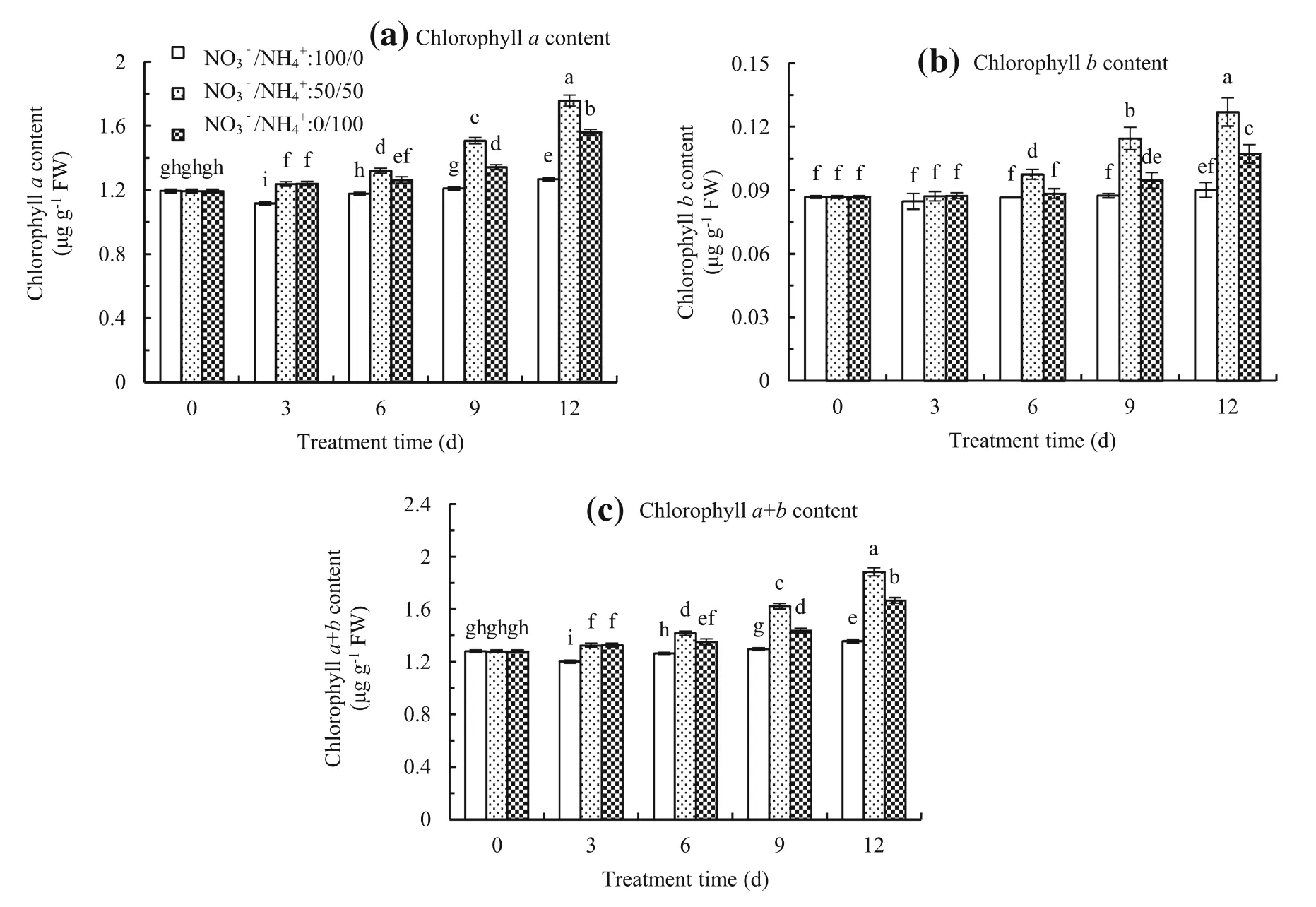
Fig.3 Chlorophyll a content(a),chlorophyll b content(b),and chlorophyll a+b content(c)in leaves of Malus hupehensis seedlings treated at NO3-/NH4+ratios of 100/0,50/50,and 0/100.The bar indicates the mean±the standard error(n=3).The different letters on the bars indicate signif i cant difference at P<0.05

Fig.4 Respiration rate in leaves(a)and roots(b)of Malus hupehensis seedlings treated at the three NO3-/NH4+ratios of 100/0,50/50,and 0/100.The bar indicates the mean±the standard error(n=3).The different letters on the bars indicate signif i cant difference at P<0.05
Discussion
For most plants,supply of mixed NO3-and NH4+is superior to either NO3-or NH4+supplied alone(Zhang et al.2007).The optimal proportions of NO3-/NH4+depends on the plant species,environmental conditions, developmental stage,and the concentration of supplied N(Claussen 2002;Tylova-Munzarova et al.2005).M. hupehensisseedlings grew well with greater grow that the NO3-/NH4+ratio of 50/50,whereas the biomass and shoot length at the NO3-/NH4+ratio of 0/100 was suppressed and decreased(Fig.1).Sotiropoulos et al.(2005)also reported that apple rootstock had a higher fresh mass with the supply of NH4NO3as the N source.The f i nding that biomass was limited by a sole NH4+supply was similar to that reported by Zhou et al.(2007),who found thatM. hupehensisseedlings had lower fresh weight under a sole NH4+supply.

Fig.5 The third true leaf structure of Malus hupehensis seedlings grown in a nutrient solution at NO3-/NH4+ratios of 100/0,50/50,and 0/100.PC palisade cells,SC spongy cells

Fig.6 Cell structure of the meristematic zone in the root tip of Malus hupehensis seedlings grown in a nutrient solution at NO3-/NH4+ratios of 100/0,50/50,and 0/100.EC epidermis cells,MRC meristematic region cells

Fig.7 Cell structure of the maturation zone in the root tip of Malus hupehensis seedlings grown in a nutrient solution at NO3-/NH4+ratios of 100/0,50/50,and 0/100.EC epidermis cells,CC cortex cells, VCC vascular cylinder cells
Plant morphology and growth rate often depend on nitrogen metabolism.The rate of NO3-and NH4+uptake is associated with properties of the root structure and the fl ux from the soil to the roots(Brix et al.2002).In this study,NO3-concentration gradually increased in the roots at the NO3-/NH4+ratio of 100/0,whereas the same trend occurred for NH4+uptake in the roots at the NO3-/NH4+ratio of 0/100(Fig.2b,d).This result was consistent with previous studies showing thatArabidopsis thalianaseedlings displayed a higher uptake of NO3-or NH4+in roots under a sole NO3-or NH4+supply(Patterson et al.2010). In addition,our data showed that the NO3-concentration in roots remained very low during plant growth at the NO3-/NH4+ratio of 0/100.NO3-concentration in leaves remained stable after 9 days of treatment,but then decreased(Fig.2a,b).These results suggested that in the absence of NO3-supply,NO3-in the roots was transported faster to the leaves and no NH4+was converted by oxidation to NO3-in the roots or in the leaves.Earlier studies indicated that NH4+taken up from soil was directly assimilated or stored in root cells(Husted et al.2000; Tobin and Yamaya 2001).NH4+concentration at the NO3-/NH4+ratio of 0/100 increased in roots during plant growth,decreased in leaves during the 3 days of treatment, and then gradually increased(Fig.2c,d).These results suggest that the processes of NH4+absorption,assimilation,and transport from the roots to the leaves took some time to achieve.
Chlorophyll is a photosynthetic pigment in all higher plants.Nitrogen often affects leaf pigment concentration and photosynthesis(Zhao et al.2003).It has been demonstrated that NO3-assimilation requires substantial energy and a substantial portion of the products of the electron chain(Gao et al.1992).In our results,chlorophyll (a,b,anda+b)content was signif i cantly inhibited and leaf thickness was thinner at the NO3-/NH4+ratio of 100/0 than that at the NO3-/NH4+ratio of 50/50(Figs.3,5c). Tobacco plants with a sole NH4+supply similarly had smaller cells and increased numbers of chloroplasts per unit leaf area(Walch-Liu et al.2000).Lower chlorophyll (a,b,anda+b)content and thinner leaves consistentlyoccurred at the NO3-/NH4+ratio of 0/100 during plant growth.
Previous studies reported that respiration rate is strongly stimulated by an excess of NH4+in the leaves and roots (Guo et al.2007;Chen et al.2013).The reason might be an increase in the adenosine triphosphate(ATP)requirement for cytosolic sucrose synthesis,which is accompanied by a high rate of mitochondrial oxidative phosphorylation under an excess NH4+supply(Guo et al.2005).Chen et al. (2013)indicated that high NH4+enhanced root respiration, and suppressed root biomass and total root length in two rice cultivars.Our results similarly showed that the leaves and roots at the NO3-/NH4+ratio of 0/100 consumed large amounts of O2in response to the accumulation of NH4+and the inhibition of plant biomass(Figs.1,2,4).Therefore, our studies highlight that it is necessary to investigate the root morphological structure in response to a high level of NH4+.After staining cells of the root tip ofArabidopsis thalianawith Evans blue,Qin et al.(2008)found that the cells gradually die off under treatment with increased NH4+concentration.Our results revealed that,at the NO3-/NH4+ratio of 0/100,the epidermal and cortical cells in the root apical meristematic region and mature region were irregularly shaped and wrinkled,and cell death resulted(Figs.6h,i,7h,i).This structural change was similar in the roots of rice[where the NADH-dependent glutamate synthase(NADH-GOGAT)protein is localized in the epidermis layer]under a high level of NH4+treatment(Ishiyama et al.1998).It is clear that the supply of excess NH4+increased respiration rate,interfered with NO3-uptake,and impacted the morphological structures of leaves and roots.
We concluded thatM.hupehensisseedlings showed differential responses to the three NO3-/NH4+ratios (100/0,50/50,and 0/100)during plant growth.Compared to the NO3-/NH4+ratios of 100/0 and 0/100,seedlings at the NO3-/NH4+ratio of 50/50 had greater plant biomass, chlorophyll(a,b,anda+b)content,leaf thickness,and root size,but had a lower respiration rates in leaves and roots.At the NO3-/NH4+ratio of 100/0,the seedlings exhibited a higher uptake of NO3-and a lower NH4+concentration in leaves and roots;however,the opposite trend was observed at the NO3-/NH4+ratio of 0/100. Furthermore,at the NO3-/NH4+ratio of 0/100,we found higher O2consumption in leaves and roots,and death of the epidermal and cortical cells in the root apical meristematic region and mature region.These results suggest thatM.hupehensisseedlings slowed biomass accumulation and chlorophyll synthesis,increased respiration,and inhibited and damaged morphological structure development in the leaves and roots under a sole NO3-or NH4+supply.
Aparicio SR,Marsden P(1969)A rapid methylene blue-basic fuchsin stain for semi-thin sections of peripheral nerve and other tissues. J Microsc 89:139–141
Balkos KD,Britto DT,Kronzucker HJ(2010)Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza saticaL.cv.IR-72).Plant Cell Environ 33:23–34
Black BL,Fuchigami LH,Coleman GD(2002)Partitioning of nitrate assimilation among leaves,stems and roots of poplar.Tree Physiol 22:717–724
Bra¨utigam A,Gagneul D,Weber APM(2007)High-throughput colorimetric method for the parallel assay of glyoxylic acid and ammonium in a single extract.Anal Biochem 362:151–153
Britto DT,Kronzucker HJ(2002)NH4+toxicity in higher plants:a critical review.J Plant Physiol 159:567–584
Brix H,Dyht-Jensen K,Lorenzen B(2002)Root-zone acidity and nitrogen source affectsTypha latifoliaL.growth and uptake kinetics of ammonium and nitrate.J Exp Bot 53:2441–2450
Celis-Ara´mburo TJ,Carrillo-Pech M,Castro-Concha LA,Miranda-Ham ML,Martı´nez-Este´vez M,Echevarrı´a-Machado I(2011) Exogenous nitrate induces root branching and inhibits primary root growth inCapsicum chinenseJacq.Plant Physiol Biochem 49:1456–1464
Chaillou S,Rideout JW,Raper CD,Morot-Gaudry JF(1994) Responses of soy bean to ammonium and nitrate supplied in combination to the whole root system or separately in a split root system.Physiol Plant 90:259–268
Chang J,Liu D,Cao HQ,Chang SX,Wang XY,Huang CC,Ge Y (2010)NO3-/NH4+ratios affect the growth and N removal ability ofAcorus calamusandIris pseudacorusin a hydroponic system.Aquat Bot 93:216–220
Chen G,Guo SW,Kronzucher HJ,Shi W(2013)Nitrogen use eff i ciency(NUE)in rice links to NH4+toxicity and futile NH4+cycling in roots.Plant Soil 369:351–363
Claussen W(2002)Growth,water use eff i ciency,and proline content of hydroponically grown tomato plants as affected by nitrogen scource and nutrient concentration.Plant Soil 247:199–209
Gao Y,Motosugi H,Sugiura A(1992)Rootstock effects on growth and f l owering young apple trees grown with ammonium and nitrate nitrogen.J Am Soc Hortic Sci 117:446–452
Guo SW,Schinner K,Sattelmacher B,Hansen U(2005)Different apparent CO2compensation points in nitrate-and ammoniumgrownPhaseolus vulgarisand the relationship to non-photorespiratory CO2evolution.Physiol Plant 123:288–301
Guo SW,Zhou Y,Shen QR,Zhang FS(2007)Effect of ammonium and nitrate nutrition on some physiological processes in higher plants—growth,photosynthesis,photorespiration,and water relations.Plant Biology 9:21–29
Hoagland DR,Arnon DI(1950)The water culture method for growing plants without soil.Calif Agric Exp Stn Circ 347:1–32
Husted S,Hebbern CA,Mattsson M,Schjoerring JK(2000)A critical experimental evaluation of methods for determination of NH4+in planttissue,xylemsap and apoplasticf l uid.PhysiolPlant109:167–179
Ishiyama K,Hayakawa T,Yamaya T(1998)Expression of NADH-dependent glutamate synthesis protein in the epidermis and exodermis of rice roots in response to the supply of ammonium ions.Planta 204:288–294
Jackson LE,Burger M,Cavagnaro TR(2008)Roots nitrogen transformations,and ecosystem services.Annu Rev Plant Biol 59:341–363
Lasa B,Frechilla S,Aparicio-Tejo PM,Lamsfus C(2002)Alternative pathway respiration is associated with ammonium ion sensitivity in spinach and pea plants.Plant Growth Regul 37:49–55
Lawlor DW(2002)Carbon and nitrogen assimilation in relation to yield:mechanisms are the key to understanding production systems.J Exp Bot 53:773–787
Liu Y,Lai NW,Gao K,Chen FJ,Yuan LX,Mi GH(2013) Ammonium inhibits primary root growth by reducing the length of meristem and elongation zone and decreasing elemental expansion rate in the root apex inArabidopsis thaliana.PLoS ONE 8:e61031
Loque´D,von Wire´n N(2004)Regulatory levels for the transport of ammonium in plant roots.J Exp Bot 55:1293–1305
Patterson K,Cakmak T,Cooper A,Lager I,Rasmusson AG,Escobar MA(2010)Distinct signalling pathways and transcriptome response signatures differentiate ammonium-and nitrate-supplied plants.Plant Cell Environ 33:1486–1501
Porra RJ,Thompson WA,Kriedemann PE(1989)Determination of accurate extinction coeff i cients and simultaneous equations for assaying chlorophyllaandbextracted with four different solvents:verif i cation of the concentration of chlorophyll standards by atomic absorption spectroscopy.Biochim Biophys Acta 975:384–394
Qin C,Qian WQ,Wang WF,Wu Y,Yu CM,Jiang XH,Wang DW, Wu P(2008)GDP-mannose pyrophosphorylase is a genetic determinant of ammonium sensitivity inArabidopsis thaliana. Proc Natl Acad Sci USA 105:18308–18313
Sotiropoulos TE,Mouhtaridou GN,Thomidis T,Tsirakoglou V, Dimassi KN,Therios IN(2005)Effects of different N-sources on growth,nutritional status,chlorophyll content,and photosynthetic parameters of shoots of the apple rootstock MM 106 cultured in vitro.Biol Plant 49:297–299
Tobin AK,Yamaya T(2001)Cellular compartmentation of ammonium assimilation in rice and barley.J Exp Bot 52:591–604
Tylova-Munzarova E,Lorenzen B,Brix H,Votrubova O(2005)The effects ofon growth,resource allocation and nitrogen uptake kinetics ofPhragmites australisandGlyceria maxima.Aquat Bot 81:326–342
Walch-Liu P,Neumann G,Bangerth F,Engels C(2000)Rapid effects of nitrogen form on leaf morphogenesis in tobacco.J Exp Bot 51:227–237
Xu GH,Fan XR,Miller AJ(2012)Plant nitrogen assimilation and use ef fi ciency.Annu Rev Plant Biol 63:153–182
Zhang FC,Kang SZ,Li Fusheng ZJ(2007)Growth and major nutrient concentrations inBrassica campestrissupplied with differentratios.J Integr Plant Biol 49:455–462
Zhao DL,Reddy KR,Kakani VG,Read JJ,Carter GA(2003)Corn (Zea maysL.)growth,leaf pigment concentration,photosynthesis and leaf hyperspectral re fl ectance properties as affected by nitrogen supply.Plant Soil 257:205–218
Zhou P,Peng FT,Wei SC,Peng Y(2007)Effects of rhizosphere nitrate and ammonium on the level of cytokinins and leaf growth ofMalus hupenensisRehd.Acta Hortic Sin 34:269–274(in Chinese with English abstract)
31 March 2014/Accepted:8 October 2014/Published online:23 July 2015
Project funding:This work was supported by the Agricultural Comprehensive Development Project of Hebei Province(No. 2012ACDPHP01).
The online version is available at http://www.springerlink.com
Corresponding editor:Zhu Hong
✉Jun-feng Guan junfeng-guan@263.net
1Plant Genetic Engineering Center of Hebei Province,Institute of Genetics and Physiology,Hebei Academy of Agricultural and Forestry Sciences,Shijiazhuang 050051, People’s Republic of China
2School of Food and Bioengineering,Zhengzhou University of Light Industry,Zhengzhou 450002, People’s Republic of China
 Journal of Forestry Research2015年4期
Journal of Forestry Research2015年4期
- Journal of Forestry Research的其它文章
- Drone remote sensing for forestry research and practices
- Life cycle environmental impact assessment of biochar-based bioenergy production and utilization in Northwestern Ontario, Canada
- Growth rates of Eucalyptus and other Australian native tree species derived from seven decades of growth monitoring
- Effect of f i rst thinning and pruning on the individual growth of Pinus patula tree species
- The inf l uence of selective cutting of mixed Korean pine(Pinus koraiensis Sieb.et Zucc.)and broad-leaf forest on rare species distribution patterns and spatial correlation in Northeast China
- Modeling forest f i res in Mazandaran Province,Iran
