马铃薯三糖五环三萜类化合物体外抗H5N1流感病毒的活性评价
宋高鹏,申新田,李素梅,黎奕斌,范继鸿,梁倩倩,刘叔文
(1.华南农业大学资源环境学院,广东 广州 510642;2.南方医科大学药学院,广东 广州 510515;3.暨南大学医学院,广东 广州 510632)
马铃薯三糖五环三萜类化合物体外抗H5N1流感病毒的活性评价
宋高鹏1,申新田2,李素梅3,黎奕斌1,范继鸿1,梁倩倩1,刘叔文2
(1.华南农业大学资源环境学院,广东 广州 510642;2.南方医科大学药学院,广东 广州 510515;3.暨南大学医学院,广东 广州 510632)
H5N1;流感病毒进入抑制剂;合成;血凝素蛋白;构效关系;五环三萜
自1997年香港首次爆发了人感染高致病性H5N1禽流感事件以来,禽流感疫情已经在世界范围内常态化,各地先后出现了人感染H5N1禽流感病例[1]。人感染H5N1禽流感死亡率达到60%,远远高于SARS等突发性传染病[2]。最新研究表明,H5N1型禽流感病毒已演化出两个能感染人类的毒株,而且两个毒株都有引发严重人传人流感疫情的危险[3-5]。这表明,所有的H5N1型禽流感病毒毒株都应被视为“公共卫生的潜在威胁”。
目前市场上的两大类抗流感病毒药物(以金刚烷胺为代表的M2蛋白抑制剂和以达菲为代表的神经氨酸酶NA抑制剂)的广泛应用已导致H5N1流感病毒对其产生了耐药性[6-8]。病毒感染宿主细胞的第一个过程是病毒表面的血凝素蛋白HA与宿主细胞表面的唾液酸受体识别结合[9]。因此,抑制HA介导的病毒融合或抑制病毒进入宿主细胞对抑制流感病毒的传染至关重要[10-11]。本课题组此前采用H5N1假病毒实验方法筛选皂苷化合物库,发现了一个新系列的H5N1进入抑制剂[12]。研究发现,马铃薯三糖薯蓣皂苷衍生物(1)对两种假H5N1亚型禽流感病毒H5N1 (A/Viet Nam/1203/2004)和H5N1 (Goose/Qinghai/59/05)的血凝素蛋白在体外均具有较强的抑制作用,其IC50分别为7.8 及7.2 μmol·L-1[12]。初步构效关系研究发现,先导化合物1结构中的马铃薯三糖为活性必需片段,用其它三糖替换或简化成二糖片段均导致抗病毒活性的下降[12-13]。五环三萜主要包括齐墩果烷型、乌苏烷型及羽扇豆烷型等三种类型,其对多种病毒均具有较强的抑制活性,为开发抗病毒药物提供了新的结构骨架[14-17]。本论文基于“拼合原理”,将先导化合物1结构中的活性片段“马铃薯三糖”与三种不同类型的五环三萜甘草次酸乙酯、熊果酸乙酯及桦木酸乙酯等通过“氧苷键”分别进行偶联设计,合成目标化合物1a-1c,结构式见Fig 1。
1 材料与方法
1.1 仪器与试药Genios Pro型Tecan酶标仪(Tecan),Nikon倒置显微镜(Nikon),CO2恒温培养箱(Thermo),-80 ℃超低温保存箱(Thermo),GS-15R型台式低温高速离心机(Beckman),生物安全柜class Ⅱ (ESCO),U-3031型紫外分光光度仪,BP211D十万分之一电子天平(Sartorius),Milli-Q超纯水器(Millipore),自动高压消毒锅(Sanyo),Eppendorf 离心机5810(Eppendorf),Thermo Mixmate(Thermo),NMR 用600 Mz核磁共振仪(美国 Varian)测定;MS用Waters Quattro Premier XE 质谱仪测定。
DMEM细胞培养基、胎牛血清、胰蛋白酶、青霉素及链霉素购自中国Invitrogen 公司;感受态细胞DH5α购自TaKaRa 公司。荧光素酶检测试剂盒购自Promega公司。聚乙稀亚胺(PEI)转染试剂购自上海起福生物公司。反应试剂购自阿拉丁或者北京偶合科技有限公司,其它所有试剂均为市售分析纯,柱层析硅胶使用青岛海洋化工厂的柱层析硅胶(200~300 目)。
1.2 化合物的合成目标化合物1a-1c的合成路线见Fig 2。在碳酸钾催化作用下,商品化的原料甘草次酸、熊果酸及桦木酸分别与溴乙烷进行酯化反应得到用乙基(Et)保护的中间体2a-2c。中间体2a-2c作为糖基受体与糖基给体即全苯甲酰基(Bz)保护的D-葡萄糖三氯亚胺脂[18],在TMSOTf催化作用下进行糖苷化反应得到糖苷化产物3a-3c。用甲醇钠将中间体3a-3c结构中的Bz脱除得到中间体4a-4c,接着用1-BBTZ区域选择性保护其D-葡萄糖的C-3与C-6羟基,得到中间体5a-5c。利用“反加法”,糖基受体5a-5c与全乙酰基(Ac)保护的L-鼠李糖糖基给体[19]在TMSOTf作用下进行双糖糖苷化,在D-葡萄糖的C-2与C-4羟基同时引入全Ac保护的L-鼠李糖得到三糖中间体,最后用甲醇钠/甲醇将三糖中间体的Ac与Bz同时脱除得到目标化合物1a-1c。
1.2.1 化合物2a-2c的制备 将商品化的原料甘草次酸1.00 g (2.2 mmol)溶于无水DMF(30 mL)中,然后加入碳酸钾0.61 g (4.4 mmol),室温下搅拌4 h后加入对溴乙烷0.66 mL (8.8 mmol)。该反应液在室温下继续搅拌反应12 h,减压蒸去溶剂,将剩余物溶解于150 mL乙酸乙酯中,然后依次用水(50 mL×3)、饱和碳酸氢钠溶液(50 mL×3)和饱和NaCl溶液(80 mL)洗涤,有机相用无水Na2SO4干燥后浓缩,经无水乙醇重结晶得1.18 g白色固体2a (92.5%)。化合物2b与2c的制备可参照化合物2a的制备。
1.2.2 化合物3a-3c的制备 将化合物2a 1.50 g (3.1 mmol)、2,3,4,6-四-O-苯甲酰基-α-D-葡萄糖三氯亚胺酯[18]3.44 g (4.80 mmol) 、活化后的4℃分子筛粉末和干燥的CH2Cl2(60 mL) 混合,氮气保护下室温下搅拌0.5 h后降温至-10℃,加入TMSOTf 56 μL (0.31 mmol)。0℃反应0.5 h后,室温继续反应0.5 h,然后用三乙胺终止反应。滤除分子筛粉末,滤液浓缩后经硅胶柱纯化(乙酸乙酯-石油醚=1 ∶6) 得3.05 g白色固体3a (92.7%)。按上述方法可制备化合物3b与3c。

Fig 1 Structures of the target compounds
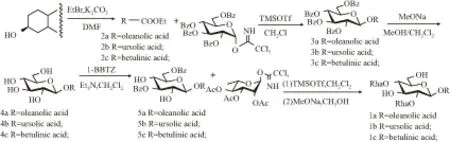
Fig 2 Synthesis route of the target compounds 1a-1c
1.2.3 化合物4a-4c的制备 将化合物3a 2.55 g (2.4 mmol)溶于CH3OH-CH2Cl2(1 ∶1, 100 mL),加入催化量的甲醇钠调pH=10。室温搅拌8 h后,用阳离子树脂 (H+)中和反应液至中性,滤除固体颗粒,浓缩后经硅胶柱纯化(二氯甲烷-甲醇=10 ∶1) 得1.48 g白色固体4a (95.5%)。化合物4b与4c的制备如上述所示。
1.2.4 化合物5a-5c的制备 将化合物4a 1.20 g(1.9 mmol)和1-(苯甲酰基)苯并三唑1.80 g (10.4 mmol)溶于干燥的CH2Cl2(60 mL)中,加入三乙胺1.60 mL(18.0 mmol)后室温反应36 h。TLC显示原料完全消失,减压浓缩后经硅胶柱纯化(乙酸乙酯-石油醚=1 ∶3) 得1.17 g白色固体5a (73.6%)。按上述方法可制备化合物5b与5c。
为了调查结果能充分反映西安市售水产品中甲醛本底值,试验扩大采样范围和采样数量,以保证取样的全面性。所采集样品涉及鲜活水产品和水产加工品,其中鲜活水产品包括鱼类、贝类、虾类、蟹类等,存在状态主要以鲜活为主,部分以冰冻状态存在;水产加工品主要为鱿鱼丝和冻虾仁。采集样品具体包括32种52个鱼类样品、6种31个虾类样品、3种7个虾类样品、2种7个鱿鱼类样品及其他3个水产样品,共计100个水产样品中。所有样品购买于西安市各商场或市场,均经过严格监控,排除了使用甲醛保鲜和被甲醛污染的可能。
1.2.5 化合物1a的制备 将化合物5a 0.50 g (0.59 mmol)、活化后的适量4Å分子筛粉末、干燥的二氯甲烷(10 mL) 混合液在氮气保护下室温搅拌20 min后,降温至-78℃搅拌10 min,加入TMSOTf 21μL (0.12 mmol),10 min后,加入1.26 g糖基给体(2,3,4-三-O-乙酰基-α-L-鼠李糖三氯乙酰亚胺酯,2.93 mmol)的干燥的二氯甲烷(5 mL)溶液。-30℃反应1 h,室温搅拌0.5 h后,用三乙胺终止反应。滤除分子筛粉末,滤液减压浓缩后经硅胶柱纯化(乙酸乙酯-石油醚=1 ∶4)得三糖糖苷化产物粗品(含有糖基给体的分解产物)。将上述三糖糖苷化产物粗品溶于CH3OH-CH2Cl2(1 ∶1, 20 mL),加入催化量的甲醇钠调pH=10。室温搅拌24 h后,用阳离子树脂 (H+)中和反应液至中性,滤除固体颗粒,浓缩后经硅胶柱纯化(二氯甲烷-甲醇=6 ∶1) 得0.42 g白色固体1a (76.4%,两步反应)。按上述方法可制备化合物1b与1c。
1.3 化合物的体外抗H5N1流感活性测试
1.3.1 H5N1假病毒的制备 参照我们前期所建立的H5N1假病毒模型[10]制备了源自A/Thailand/Kan353/2004的H5N1假病毒毒株。
1.3.2 待测化合物体外抗H5N1活性 以每孔1×104个细胞密度接种MDCK细胞于96孔细胞培养板,培养24 h;2倍稀释化合物到不同浓度,50 μL 化合物与假病毒(1 ng p24每孔)在37℃孵育30 min;往96孔板中加入化合物和假病毒的混合物,在37℃的细胞培养箱继续培养48 h;吸去培养上清,用PBS洗2次细胞,每孔加50 μL 裂解液,轻轻摇晃,继续静置30 min,待细胞裂解完毕,吸取40 μL裂解物到白板,加入荧光素酶显色底物,在多功能酶标仪上检测化学发光值,判断药物抑制病毒进入的活性。化合物抑制率/%=[1-(E-N)/(P-N)]×100,其中E代表实验组的化学发光值,P代表阳性即不加药物只加病毒的化学发光值,N代表阴性对照组的化学发光值。化合物的半数抑制浓度(IC50)作为化合物抗病毒活性的指标,通过Calccusyn软件计算得出。
1.3.3 化合物细胞毒性的测定 采用标准的MTT[10]法测试化合物对MDCK细胞的细胞毒性并进一步计算半数细胞死亡浓度(CC50)。
2 结果
2.1 化合物的合成表征
2.1.1 glycyrrhetinic acid ethyl ester (2a)1H NMR (DMSO-d6): δ 5.61 (s, 1H, H-12), 4.17 (dq, 1H, J=10.8, 7.2 Hz OCH2CH3-1), 4.10 (dq, 1H, J=10.8, 6.8 Hz OCH2CH3-2), 3.20 (dd, 1H, J=11.1, 5.2 Hz, H-3), 2.78 (ddd, 1H, J=13.5, 4.6, 3.6 Hz, H-1), 2.30 (s, 1H, H-9), 2.06-2.09 (m, 1H, H-18), 1.98-2.01 (m, 1H, H-15), 1.95-1.97 (m, 1H, H-21), 1.88 (ddd, 1H, J=13.6, 4.2, 2.9 Hz, H-19), 1.23 (t, 3H, J=7.2 Hz, CH2CH3), 1.12, 1.11, 1.10, 0.98, 0.78, 0.77 (each s, each 3H, CH3), 0.68 (dd, 1H, J = 11.5, 1.7 Hz, H-5); ESIMS calcd for C32H50NaO4521.4; found 521.4;
2.1.2 ursolic acid ethyl ester (2b)1H NMR (CDCl3):δ 5.27 (t, 1H, J=3.6 Hz, H-12), 3.65 (q, 2H, J=7.2 Hz, OCH2CH3), 3.25 (dd, 1H, J=11.3, 4.7, H-3), 2.25 (d, 1H, J=11.4 Hz, H-18), 0.97 (d, 3H, J=6.2 Hz, CH3), 0.96 (t, 3H, J=7.0 Hz, CH2CH3), 0.89 (d, 3H, J=6.2 Hz, CH3), 1.10, 1.01, 0.95, 0.82, 0.78 (each s, each 3H, CH3); ESIMS calcd for C32H52NaO3507.4; found 507.4;
2.1.3 betulinic acid ethyl ester (2c)1H NMR (CDCl3):δ 4.75 (brs, 1H, H-29), 4.61 (brs, 1H, H-29), 3.68 (d, J=9.7 Hz, 1H, H-28), 3.65 (q, 2H, J=7.0 Hz, OCH2CH3), 3.33 (d, J=9.8 Hz, 1H, H-28), 3.16 (dd, J=11.0, 4.4 Hz, 1H, H-3), 3.00-3.03 (m, 1H, H-19), 2.20-2.26 (m, 2H), 1.90-1.91 (m, 2H), 1.82-1.85 (m, 1H), 1.12 (t, 3H, J=7.2 Hz, CH2CH3), 1.18, 0.99, 0.97, 0.93, 0.84, 0.79 (each s, each 3H, each CH3); ESIMS calcd for C32H52NaO3507.3; found 507.4;
2.1.4 Ethyl 3β-O-(2,3,4,6-Tetra-O-benzoyl-β-D-glucopyranosyl)-11-oxo-olean-12-en-30-oate (3a)1H NMR (CDCl3): δ 7.35-8.02 (m, 20H, Ar-H), 5.68 (s, 1H, H-12), 5.65 (t, 1H, J=9.6 Hz, H-3′), 5.53 (t, 1H, J=9.6 Hz, H-4′), 4.63 (d, 1H, J=8.1 Hz, H-1′), 4.63 (dd, 1H, J=11.8, 3.2 Hz, H-6′-1), 4.48 (dd, 1H, J=11.9, 6.7 Hz, H-6′-2), 4.18 (dq, 1H, J=10.8, 7.2 Hz OCH2CH3-1), 4.09-4.12 (m, 1H, OCH2CH3-2), 4.02-4.08 (m, 1H, H-2′), 3.88-3.92 (m, 1H, H-5′), 3.25 (dd, 1H, J=11.8, 4.7 Hz, H-3), 2.79 (dt, 1H, J=13.8, 3.5 Hz, H-1), 2.55 (d, 1H, J=3.2 Hz, H-9), 2.31 (s, 1H), 2.12(dd, 1H, J=13.6, 3.6 Hz, H-18), 2.10 (dd, 1H, J=13.6, 4.5 Hz, H-15), 2.01-2.03 (m, 1H, H-21), 1.96-1.99 (m, 1H, H-19), 1.90-1.93 (m, 1H, H-2), 1.23 (t, 3H, J=7.2 Hz, CH2CH3), 1.18, 1.16, 1.15, 1.07, 0.89, 0.85 (each s, each 3H, each CH3); ESIMS calcd for C66H76KO131115.5; found 1115.5;
2.1.5 3β-O-(2,3,4,6-Tetra-O-benzoyl-β-D-glucopyranosyl)-ursolic-28-ethyl ester (3b)1H NMR (CDCl3): δ 7.28-8.06 (m, 20H, Ar-H), 5.93 (t, 1H, J=9.7 Hz, H-3′), 5.57-5.60 (m, 2H, H-2′, H-4′), 5.26 (t, 1H, J=3.6 Hz, H-12), 4.85 (d, 1H, J=8.2 Hz, H-1′), 4.56-4.58 (m, 2H, H-6′×2), 4.14-4.17 (m, 1H, H-5′), 3.66 (q, 2H, J=7.1 Hz, OCH2CH3), 3.09 (dd, 1H, J=11.8, 4.3 Hz, H-3), 2.25 (d, 1H, J=11.5 Hz, H-18), 1.05, 0.85, 0.70, 0.69, 0.62 (each s, each 3H, each CH3), 0.99 (t, 3H, J=7.1 Hz, CH2CH3), 0.95 (d, 3H, J=6.2 Hz, CH3), 0.90 (d, 3H, J=6.3 Hz, CH3);ESIMS calcd for C66H78NaO121085.6; found 1085.6;
2.1.6 Ethyl betulinate 3β-O-2,3,4,6-Tetra-O-benzoyl-β-D-glucopyranoside (3c)1H NMR (CDCl3): δ 7.28-8.05 (m, 20H, Ar-H), 5.91 (t, 1H, J=9.6 Hz, H-3′), 5.60 (t, 1H, J=9.6 Hz, H-4′), 5.55 (dd, 1H, J=9.6, 8.0 Hz, H-2′), 4.85 (d, 1H, J=8.0 Hz, H-1′), 4.76 (d, 1H, J=2.2 Hz, H-29-1), 4.65-4.66 (m, 1H, H-29-2), 4.61 (dd, 1H, J=11.8, 3.4 Hz, H-6′-1), 4.53 (dd, 1H, J=11.9, 6.7 Hz, H-6′-2), 4.13-4.16 (m, 1H, H-5′), 3.66 (q, 2H, J=7.0 Hz, OCH2CH3), 3.06 (dd, 1H, J=11.8, 4.5 Hz, H-3), 2.98-3.02 (m, 1H, H-19), 2.15-2.22 (m, 2H), 1.86-1.93 (m, 2H), 1.80-1.84 (m, 1H), 1.10 (t, 3H, J=7.2 Hz, CH2CH3), 0.98, 0.88, 0.76, 0.69, 0.63 (each s, each 3H, each CH3); ESIMS calcd for C66H78NaO121085.5; found 1085.5;
2.1.7 Ethyl 3β-O-(D-glucopyranosyl)-11-oxo-olean-12-en-30-oate (4a)1H NMR (CDCl3): δ 5.65 (s, 1H, H-12), 4.36 (d, 1H, J=7.8 Hz, H-1′), 4.08-4.15 (m, 2H, OCH2CH3), 3.80-3.88 (m, 2H, H-6′), 3.63 (t, 1H, J=9.8 Hz, H-3′), 3.60 (t, 1H, J=9.7 Hz, H-4′), 3.45 (t, 1H, J=9.7 Hz, H-2′), 3.30-3.33 (m, 1H, H-5′), 3.20 (t-like, 1H, J=10.2 Hz, H-3), 2.77 (d, 1H, J=13.2 Hz, H-1), 2.31 (s, 1H, H-9), 2.07-2.11 (m, 1H, H-18), 2.00-2.02 (m, 1H, H-15), 1.23 (t, 3H, J=7.0 Hz, CH2CH3), 1.25, 1.16, 1.14, 1.12, 1.04, 0.86, 0.82 (each s, each 3H); ESIMS calcd for C38H60NaO9683.4; found 683.4;
2.1.8 3β-O-(D-glucopyranosyl)-ursolic-28-ethyl ester (4b)1H NMR (CDCl3): δ 5.16 (t, 1H, J=3.2 Hz, H-12), 4.15 (d, 1H, J=7.6 Hz, H-1′), 3.68 (q, 2H, J=7.0 Hz, OCH2CH3), 3.63-3.65 (m, 1H, H-6′-1), 3.41-3.45 (m, 1H, H-6′-2), 3.10-3.13 (m, 1H, H-3′), 3.01-3.05 (m, 3H, H-3, H-4′, H-5′), 2.94-2.98 (m, 1H, H-2′), 2.15 (d, 1H, J=11.2 Hz, H-18), 1.01 (t, 3H, J=7.1 Hz, CH2CH3), 0.96 (d, 3H, J=6.2 Hz, CH3), 0.91 (d, 3H, J=6.2 Hz, CH3), 1.04, 0.84, 0.70, 0.69, 0.63 (each s, each 3H, each CH3); ESIMS calcd for C38H62NaO8669.5; found 669.4;
2.1.9 Ethyl betulinate 3β-O-D-glucopyranoside (4c)1H NMR (CDCl3): δ 4.78 (s, 1H, H-29-1), 4.64 (s, 1H, H-29-2), 4.34 (d, 1H, J=7.6 Hz, H-1′), 3.79-3.87 (m, 2H, H-6′), 3.69 (q, 2H, J=7.2 Hz, OCH2CH3), 3.63 (t, 1H, J=8.8 Hz), 3.56-3.59 (m, 1H), 3.45 (t, 1H, J=8.5 Hz), 3.29-3.31 (m, 1H, H-5′), 3.12 (dd, 1H, J=10.5, 4.2 Hz, H-3), 2.99-3.04 (m, 1H, H-19), 2.21-2.27 (m, 2H), 1.89-1.90 (m, 2H), 1.82-1.85 (m, 1H), 1.11 (t, 3H, J=7.0 Hz, CH2CH3), 1.18, 0.99, 0.97, 0.92, 0.83, 0.79 (each s, each 3H, each CH3); ESIMS calcd for C38H62NaO8669.4; found 669.4;
2.1.10 Ethyl 3β-O-(3,6-Di-O-benzoyl-β-D-glucopyranosyl)-11-oxo-olean-12-en-30-oate (5a)1H NMR (CDCl3): δ 7.46-8.12 (m, 10H, Ar-H), 5.68 (s, 1H, H-12), 5.26 (t, 1H, J=9.1 Hz, H-3′), 4.72 (dd, 1H, J=11.8, 1.2 Hz, H-6-1′), 4.63 (dd, 1H, J=11.8, 6.0 Hz, H-6-2′), 4.51 (d, 1H, J=7.8 Hz, H-1′), 4.06-4.11 (m, 2H, OCH2CH3), 3.76-3.80 (m, 3H, H-2′, H-4′ H-5′), 3.23 (dd, 1H, J=11.8, 4.4 Hz, H-3), 2.78 (dt, 1H, J=13.6, 3.0 Hz, H-1), 2.31 (s, 3H, H-9), 2.10-2.13 (m, 1H, H-18), 2.03-2.05 (m, 1H, H-15), 1.21 (t, 3H, J=7.2 Hz, CH2CH3), 1.34, 1.19, 1.14, 1.13, 1.02, 0.87, 0.82 (s, 3H, CH3);ESIMS calcd for C52H69O11869.5; found 869.5;
2.1.11 3β-O-(3, 6-Di-O-benzoyl-β-D-glucopyranosyl)-ursolic-28-ethyl ester (5b)1H NMR (CDCl3): δ 7.41-8.07 (m, 10H, Ar-H), 5.27 (t, 1H, J=3.2 Hz, H-12), 5.20 (t, 1H, J=9.6 Hz, H-3′), 4.62-4.65 (m, 2H, H-6′×2), 4.46 (d, 1H, J=7.8 Hz, H-1′), 3.70-3.75 (m, 3H, H-5′, H-4′, H-2′), 3.72 (q, 2H, J=7.2 Hz, OCH2CH3), 2.94 (dd, 1H, J=9.8, 4.4 Hz, H-3), 2.84 (dd, 1H, J=13.8, 3.7 Hz), 1.00 (t, 3H, J=7.2 Hz, CH2CH3), 0.96 (d, 3H, J=6.2 Hz, CH3), 0.92 (d, 3H, J=6.2 Hz, CH3), 1.05, 0.84, 0.71, 0.69, 0.62 (each s, each 3H, each CH3); ESIMS calcd for C52H70NaO10877.5; found 877.5;
2.1.12 Ethyl betulinate 3β-O-(3, 6-Di-O-benzoyl)-β-D-glucopyranoside (5c)1H NMR (CDCl3): δ 8.07 (td, 4H, J=8.2, 1.3 Hz, Ar-H), 7.60 (t, 2H, J=7.8 Hz, Ar-H), 7.47 (td, 4H, J=8.2, 1.4 Hz, Ar-H), 5.24 (t, 1H, J=8.8 Hz, H-3′), 4.79 (d, 1H, J=1.6 Hz, H-29-1), 4.70 (dd, 1H, J=11.8, 2.2 Hz, H-6′-1), 4.62-4.66 (m, 1 H, H-6′-2), 4.64 (s, 1H, H-29-2), 4.51 (d, 1H, J=7.8 Hz, H-1′), 3.74-3.78 (m, 3H, H-2′, H-4′, H-5′), 3.72 (q, 2H, J=7.2 Hz, OCH2CH3), 3.15 (dd, 1H, J=11.8, 4.5 Hz, H-3), 3.01-3.05 (m, 1H, H-19), 2.25-2.27 (m, 1H), 2.19-2.22 (m, 1H), 1.14 (t, 3H, J=7.2 Hz, CH2CH3), 1.72, 0.99, 0.96, 0.92, 0.80, 0.79 (each s, each 3H, each CH3); ESIMS calcd for C52H70NaO10877.5; found 877.5;
2.1.13 Methyl 3β- O-[2,4-Di-O-(α-L-rhamnopyranosyl)-β-D-Glucopyranosyl]-11-oxo-olean-12-en-30-oate (1a)1H NMR (CD3OD): δ 5.56 (s, 1H, H-12), 5.37 (d, 1H, J=1.7 Hz, Rha-H-1), 4.60 (brs, 1H, Rha-H-1), 4.42 (d, 1H, J=7.7 Hz, H-1′), 4.04-4.10 (m, 2H, OCH2CH3), 3.96-4.00 (m, 2H), 3.90-3.93 (m, 1H), 3.85 (dd, 1H, J=3.2, 1.8 Hz, Rha-H-2), 3.81 (dd, 1H, J=12.0, 1.7 Hz, H-6′-1), 3.75 (dd, 1H, J=9.5, 3.2 Hz, Rha-H-3), 3.66 (dd, 1H, J=12.1, 4.0 Hz, H-6′-2), 3.62 (dd, 1H, J=9.4, 3.2 Hz, Rha-H-3), 3.60 (t, 1H, J=8.9 Hz), 3.54 (t, 1H, J=8.8 Hz), 3.37-3.48 (m, 2H), 3.20 (dd, 1H, J=11.8, 4.4 Hz, H-3), 3.10-3.13 (m, 1H, H-5′), 2.72 (dt, 1H, J=13.4, 2.8 Hz, H-1), 2.45 (s, 1H, H-19), 1.27 (d, 3H, J=6.2 Hz, Rha-H-6), 1.24 (d, 3H, J=6.2 Hz, Rha-H-6), 1.43, 1.17, 1.15, 1.12, 1.08, 0.91, 0.85 (each s, each 3H, CH3), 0.98 (d, 3H, J=6.4 Hz, CH3), 0.90 (d, 3H, J=6.4 Hz, CH3);13C NMR (CD3OD): δ 201.1 (CO), 171.1 (C=OO), 171.0 (C=), 121.5 (C=), 104.1 (C-1′), 101.6 (Rha-C-1), 100.5 (Rha-C-1), 88.0, 79.1, 78.1, 77.8, 76.8, 75.2, 72.6, 72.3, 71.0, 70.8, 70.6, 69.4, 68.8, 61.8, 60.6, 55.5, 50.9, 48.5, 45.4, 43.9, 43.2, 41.1, 39.2, 39.1, 37.6, 36.7, 32.5, 31.5, 30.6, 27.8, 27.1, 27.0, 26.2, 25.9, 25.9, 22.4, 17.9, 17.0, 16.7, 16.5, 15.8, 15.7, 14.4; HRESIMS calcd for C50H80O17Na 975.5278; found 975.5293;
2.1.14 3β-O-[2,4-Di-O-(α-L-rhamnopyranosyl)-β-D-Glucopyranosyl]-ursolic-28-ethyl ester (1b)1H NMR (CD3OD): δ 5.36 (d, 1H, J=1.7 Hz, Rha-H-1), 5.23 (t, 1H, J=3.5 Hz, H-12), 4.85 (d, 1H, J=1.8 Hz, Rha-H-1), 4.44 (d, 1H, J=8.0 Hz, H-1′), 3.96-3.99 (m, 2H), 3.89-3.93 (m, 1H), 3.82 (dd, 1H, J=3.2, 1.8 Hz, Rha-H-2), 3.80 (dd, 1H, J=12.0, 1.9 Hz, H-6′-1), 3.74 (dd, 1H, J=9.6, 3.2 Hz, Rha-H-3), 3.70 (q, 2H, J=7.2 Hz, OCH2CH3), 3.66 (dd, 1H, J=12.0, 4.0 Hz, H-6′-2), 3.61 (dd, 1H, J=9.5, 3.2 Hz, Rha-H-3), 3.58 (t, 1H, J=8.5 Hz), 3.54 (t, 1H, J=9.5 Hz), 3.44 (t, 1H, J=8.6 Hz), 3.41 (t, 1H, J=9.5 Hz), 3.39 (t, 1H, J=9.6 Hz), 3.18 (dd, 1H, J=11.8, 4.4 Hz, H-3), 2.22 (d, 1H, J=11.0 Hz, H-18), 1.26 (d, 3H, J=6.2 Hz), 1.21 (d, 3H, J=6.2 Hz), 1.02 (t, 3H, J=7.0 Hz, CH2CH3), 1.10, 1.05, 0.86, 0.76 (each s, each 3H), 0.93 (d, 3H, J=6.2 Hz), 0.87 (d, 3H, J=6.2 Hz);13C NMR (CD3OD): δ 179.9, 139.7, 127.2, 105.5, 103.2, 102.0, 90.4, 80.5, 79.2, 78.1, 76.5, 74.1, 73.7, 72.5, 72.3, 72.0, 70.8, 70.1, 62.0, 57.4, 54.5, 52.1, 43.4, 40.8, 40.5, 40.3, 40.1, 37.9, 37.8, 34.3, 31.6, 29.1, 28.6, 27.2, 25.5, 24.4, 24.3, 21.5, 19.4, 18.0, 17.9, 17.8, 17.6, 17.2, 16.3, 14.5; HRESIMS calcd for C50H82O16Na 961.5532; found 961.5501;
2.1.15 Ethyl betulinate 3β-O-2,4-Di-O-(α-L-rhamnopyranosyl)-β-D- glucopyranoside (1c)1H NMR (CD3OD): δ 5.35 (d, 1H, J=1.1 Hz, Rha-H-1), 4.85 (brs, 1H, Rha-H-1), 4.73 (s, 1H, H-29-1), 4.63 (s, 1H, H-29-2), 4.42 (d, 1H, J=7.8 Hz, H-1′), 3.95-3.99 (m, 2H), 3.90-3.93 (m, 1H), 3.82 (dd, 1H, J=3.2, 1.8 Hz, Rha-H-2), 3.80 (d, 1H, J=11.0 Hz, H-6′-1), 3.75 (dd, 1H, J=9.5, 3.2 Hz, Rha-H-3), 3.71 (q, 2H, J=7.2 Hz, OCH2CH3), 3.64-3.67 (m, 2H), 3.63 (dd, 1H, J=9.5, 3.1 Hz, Rha-H-3), 3.58 (t, 1H, J=8.3 Hz), 3.54 (t, 1H, J=9.4 Hz), 3.37-3.46 (m, 3H), 3.15 (dd, 1H, J=11.5, 3.3 Hz, H-3), 2.98-3.03 (m, 1H, H-19), 2.20-2.23 (m, 2H), 1.93-1.95 (m, 1H), 1.83-1.88 (m, 2H), 1.27 (d, 3H, J=6.2 Hz, Rha-H-6), 1.22 (d, 3H, J=6.2 Hz, Rha-H-6), 1.12 (t, 3H, J=7.2 Hz, CH2CH3), 1.70, 1.02, 1.00, 0.95, 0.87, 0.83 (each s, each 3H, each CH3), 0.96 (d, 3H, J=6.4 Hz, CH3), 0.90 (d, 3H, J=6.2 Hz, CH3);13C NMR (CD3OD): δ 176.7 (C=OO), 150.2 (C=), 108.8 (C=), 104.0 (C-1′), 101.5 (Rha-C-1), 100.4 (Rha-C-1), 89.0, 79.0, 78.1, 77.8, 76.6, 75.0, 72.5, 72.2, 71.0, 70.7, 70.5, 69.3, 68.6, 60.6, 56.9, 56.0, 50.6, 50.3, 49.3, 42.0, 40.5, 38.9 (two), 38.2, 36.7, 36.5, 34.2, 31.6, 30.2, 29.5, 27.0, 26.1, 25.5, 20.7, 18.0, 17.9, 16.6, 16.5, 15.6, 15.5, 15.2, 14.3, 13.8; HRESIMS calcd for C50H82O16Na 961.5544; found 961.5501。
2.2 化合物的抗流感活性我们前期的研究发现,3-三氟甲基苯甲酰胺类衍生物CL-385319对多种H5N1活病毒有抑制作用,并且初步确认了其作用靶点是血凝素的HA2 亚基[10]。本文以先导化合物1及CL-385319作阳性对照药,采用细胞水平的H5N1假病毒活性检测方法测试了所合成的3个目标化合物1a-1c对H5N1流感假病毒A/Thailand/Kan353/2004的抑制活性,其IC50分别为(6.19±0.68)、(1.25±0.22)和(3.83±0.42) μmol·L-1(Tab 1)。上述3个化合物对犬肾MDCK细胞表现出不同强度的细胞毒性,其半数细胞死亡浓度CC50分别为(24.36±0.70)、(29.02±0.12)和(28.40±0.50) μmol·L-1(Tab 1)。实验结果表明,化合物1a-1c比先导化合物1具有更强的体外抗H5N1流感病毒活性,提示五环三萜苷元可作为开发新型抗流感病毒药物的结构骨架。

Tab 1 Inhibitory activity of the compounds against H5N1 in vitro
选择系数(SIa)=CC50/IC50
2.3 目标化合物对VSV-G假病毒及神经氨酸酶NA的抑制目标化合物1a-1c对源自A/Thailand/Kan353/2004的H5N1假病毒毒株有明显的抑制作用,但在20 μmol·L-1浓度下对VSV-G假病毒没有抑制活性(Fig 3A)。由于两种假病毒仅是包膜蛋白不同,表明这3个化合物可特异性作用于H5N1流感病毒的包膜蛋白,即血凝素蛋白HA或神经氨酸酶NA。为进一步研究目标化合物1a-1c可能的作用机制,我们测试了3个目标化合物1a-1c对神经氨酸酶NA的抑制活性。实验中我们发现在20 μmol·L-1浓度下,上述3个化合物1a-1c对神经氨酸酶NA没有抑制活性(Fig 3B)。
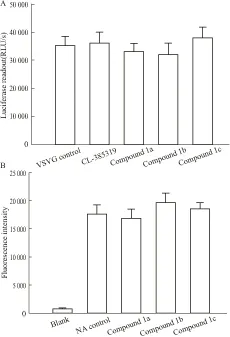
Fig 3 Target compounds do not inhibit VSVG pseudo virus and neuraminidase activity
3 讨论
皂苷对艾滋病、流感等多种病毒均具有较强的抑制作用,可作为开发新型抗病毒药物的先导化合物。本课题组前期的研究发现,马铃薯三糖薯蓣皂苷衍生物1作为H5N1进入抑制剂对两种H5N1假病毒A/Viet Nam/1203/2004和A/Goose/Qinghai/59/05均具有较强的抑制作用,并且确认了其作用靶点是血凝素蛋白。以化合物1为先导化合物合成了系列衍生物并评价了其抗H5N1禽流感活性,初步确立了其构效关系[12-13]。本研究基于前期的工作基础,将先导化合物1结构中的活性必需片段“马铃薯三糖”与常见的三种不同类型的五环三萜苷元进行偶联设计,合成了三个目标化合物1a-1c,通过测试其抗H5N1假病毒活性进一步研究苷元类型的改变对抗流感活性的影响。
3种不同结构类型的马铃薯三糖五环三萜即目标化合物1a-1c对源自A/Thailand/Kan353/2004的H5N1假病毒毒株有明显的抑制作用,但对VSV-G假病毒和神经氨酸酶NA没有抑制活性。因此,马铃薯三糖五环三萜作为新型的H5N1禽流感小分子进入抑制剂,可专一作用于病毒表面的血凝素蛋白。初步构效关系表明:在保留活性必需片段“马铃薯三糖”的基础上将薯蓣皂苷元替换成五环三萜苷元后可保持或提高其抗病毒活性,同时有助于降低对MDCK细胞的毒性,提高化合物的选择指数和成药性;以熊果酸为代表的乌苏烷型五环三萜较齐墩果烷型或羽扇豆烷型的五环三萜作为母体苷元抗病毒活性更佳。
化合物1a-1c对H5N1流感假病毒A/Thailand/Kan353/2004的抑制活性均比先导化合物1更强,且选择系数更高。其中马铃薯三糖熊果酸乙酯1b的抗病毒活性最强,选择性系数最高,比化合物1更适合作为先导化合物。化合物1b体外对真病毒H5N1的抑制活性及体内抑制实验正在研究中。化合物1a-1c由水溶性的马铃薯三糖糖链及脂溶性的五环三萜苷元组成,具有较好的溶解度;其脂水分配系数logP在1~2之间,满足新药设计的“类药规则”。皂苷往往具有一定的溶血活性,化合物1a-1c是否具有较强的溶血活性或其它毒性需要进一步的研究。总之,马铃薯三糖熊果酸乙酯1b抗病毒H5N1活性较强,选择性系数较高,具有潜在的临床应用价值,这为进一步设计高效、低毒的新型H5N1进入抑制剂提供了启示和基础。
[1] Morens D M, Fauci A S. The 1918 influenza pandemic: insights for the 21st century [J].JInfectDis, 2007, 195 (7): 1018-28.
[2] 李湘敛,刘叔文,杨 洁. 表没食子儿茶素没食子酸酯抗甲型流感病毒的作用研究[J].中国药理学通报, 2013, 29(5): 622-5.
[2] Li X L, Liu S W, Yang J. Anti influenza A virus activity of epigallocatechin gallate [J].ChinPharmacolBull, 2013, 29(5): 622-5.
[3] An J, Lee D, Law A, et al. A novel small-molecule inhibitor of the avian influenza H5N1 virus determined through computational screening against the neuraminidase [J].JMedChem, 2009, 52 (9): 2667-72.
[4] Kobasa D, Jones S M, Shinya K, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus [J].Nature, 2007, 445 (7125): 319-23.
[5] Liu K, Fang J, Jan J, et al. Enhanced anti-influenza agents conjugated with anti-inflammatory activity [J].JMedChem, 2012, 55 (19): 8493-501.
[6] Pinto L H, Holsinger L J, Lamb R A. Influenza virus M2 protein has ion channel activity [J].Cell, 1992, 69 (3): 517-28.
[7] Gupta R K, Nguyen-Van-Tam J S. Oseltamivir resistance in influenza A (H5N1) infection [J].NEnglJMed, 2006, 354 (18): 1423-4.
[8] Kiso M, Mitamura K, Sakai-Tagawa Y, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study [J].Lancet, 2004, 364 (9436): 759-65
[9] Cianci C, Yu K L, Dischino D D, et al. PH-dependent changes in photoaffinity labeling patterns of the H1 influenza virus hemagglutinin by using an inhibitor of viral fusion [J].JVirol, 1999, 73(3):1785-94.
[10] Zhu Z B, Li R M, Xiao G K, et al. Design, synthesis and structure-activity relationship of novel inhibitors against H5N1 hemagglutinin-mediated membrane fusion [J].EurJMedChem, 2012, 57(9): 211-6.
[11] Skehel J J, Wiley D C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin [J].AnnuRevBiochem, 2000, 69(7): 531-69.
[12] Song G P, Yang S, Zhang W, et al. Discovery of the first series of small molecule H5N1 entry inhibitors [J].JMedChem, 2009, 52 (23): 7368-71.
[13] Ding N, Chen Q, Zhang W, et al. Structure-activity relationships of saponin derivatives: a series of entry inhibitors for highly pathogenic H5N1 influenza virus [J].EurJMedChem, 2012, 53(4):316-26.
[14] Cassels B K, Asencio M. Anti-HIV activity of natural triterpenoids and hemisynthetic derivatives 2004-2009 [J].PhytochemRev, 2011, 10(4): 545-64.
[15] Yuan T, Zhang C R, Yang S P, Yue J M. Limonoids and triterpenoids from khaya senegalensis [J].JNatProd, 2010,73(4): 669-74.
[16] Wen C C, Kuo Y H, Jan J T, et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus [J].JMedChem, 2007, 50(17):4087-95.
[17] Yu F, Wang Q, Zhang Z, et al. Development of oleanane-type triterpenes as a new class of HCV entry inhibitors [J].JMedChem, 2013, 56(11):4300-19.
[18] Li Y X, Zhang Y C, Guo T T, et al. Synthesis of the cytotoxic gitogenin 3β-O-[2-O-(a-L-Rhamnopyranosyl)- β-D-galactopyranoside]and its congeners [J].Synthesis, 2006, 5:775-82.
[19] Song G P, Liu H C, Zhang W, et al. Synthesis and biological evaluation on antitumor activitie of novel anthracene L-rhamnopyranosides [J].BioorgMedChem, 2010, 18(14):5183-93.
Inhibitory activities of 3-O-β-chacotriosyl pentacyclic triterpenoids against the entry of H5N1 influenza virusesinvitro
SONG Gao-peng1,SHEN Xin-tian2,LI Su-mei3, LI Yi-bin1,FAN Ji-hong1,LIANG Qian-qian1,LIU Shu-wen2
(1.CollegeofResourcesandEnvironment,SouthChinaAgriculturalUniversity,Guangzhou510642,China;2.SchoolofPharmaceuticalSciences,SouthernMedicalUniversity,Guangzhou510515,China;3.SchoolofMedicine,JinanUniversity,Guangzhou510632,China)
Aim To study the inhibitory activities of potential new anti-influenza virus agents, 3-O-β-chacotriosyl pentacyclic triterpenoids against the entry of H5N1influenza viruses. Methods Three target compounds were designed and synthesized structurally related to the lead compound 3-O-β-chacotriosyl dioscin derivative (1) with inhibitory activities against H5N1 influenza viruses. The inhibitory activities of these target compounds were tested at a cellular level pseudo virus system targeting H5N1 influenza viruse entry. Results All the compounds 1a, 1b and 1c showed potent inhibitory activities against the entry of A/Thailand/Kan353/2004 pseudo virus into the target cells, of which compound 1b showed the best inhibitory activity with an IC50value of (1.25±0.22) μmol·L-1. Conclusion The SARs analysis of these compounds indicated that replacement of the aglycone moiety of compound 1 with pentacyclic triterpenoids could increase antiviral activity. Different types of pentacyclic triterpen as aglycone residue had the significant influence on the inhibitory activity (1b > 1c > 1a), suggesting ursane type of triterpenes was superior to the two other kinds of triterpenes as aglycone residue.
H5N1 avian influenza virus; influenze virus entry inhibitor; synthesis; hemagglutinin; structure-activity relationships; pentacyclic triterpenoids
时间:2015-4-15 15:44 网络出版地址:http://www.cnki.net/kcms/detail/34.1086.R.20150415.1545.013.html
2014-12-04,
2015-01-07
国家自然科学基金资助项目(No 21202047, U1301224);广东省自然科学基金博士启动项目(No S2012040007711);广东高校优秀青年创新人才培养计划项目(No LYM10037);高等学校博士学科点专项科研基金(新教师类)(No 20114404120016);广州市科技计划重点项目(No 11C32100704)
宋高鹏(1980-),男,博士,讲师,研究方向:药物化学, E-mail: vinsin1021@126.com; 刘叔文(1972-),男,博士,教授,博士生导师,研究方向:药理学,通讯作者,E-mail: liusw@smu.edu.cn
10.3969/j.issn.1001-1978.2015.05.012
A
1001-1978(2015)05-0647-08
R282.71;R284.1;R373.13;R511.7;R978.7摘要:目的 研究3种不同类型的马铃薯三糖五环三萜能否通过抑制H5N1流感病毒进入靶细胞,作为潜在的新型抗流感药物进行研发。方法 以马铃薯三糖薯蓣皂苷衍生物1为先导化合物,设计并合成3个目标化合物,利用建立的H5N1假病毒活性检测方法,测试化合物的抑制活性。结果 目标化合物1a、1b和1c对源自A/Thailand/Kan353/2004的H5N1假病毒毒株均具有明显的抑制作用,且化合物1b的活性最好,其IC50达到(1.25±0.22) μmol·L-1。结论 初步构效关系研究表明,将先导化合物1结构中的薯蓣皂苷苷元替换成五环三萜苷元后可提高其抗病毒活性;五环三萜的苷元类型对抗病毒活性有重要影响,乌苏烷型的五环三萜为苷元其抗病毒活性最强。

