硅胶的表面硫醇化改性及对水溶液中罗丹明B的固载
杨汉培 俞咪虹 傅小飞 吴俊明,2
(1河海大学浅水湖泊综合治理与资源开发教育部重点实验室,河海大学环境学院,南京210098)
(2河海大学淮安研究院,淮安223001)
硅胶的表面硫醇化改性及对水溶液中罗丹明B的固载
杨汉培*,1,2俞咪虹1傅小飞1吴俊明1,2
(1河海大学浅水湖泊综合治理与资源开发教育部重点实验室,河海大学环境学院,南京210098)
(2河海大学淮安研究院,淮安223001)
运用γ-巯丙基三乙氧基硅烷对硅胶表面硫醇化修饰改性,红外及拉曼光谱分析表明硅胶的表面硫醇化借助硅胶表面羟基与γ-巯丙基二乙氧基硅烷间的化学键联实现。实验条件下改性硅胶对水体罗丹明B的吸附性能明显提升,改性硅胶表面罗丹明B的吸附动力学符合准二级动力学模型,吸附等温线服从Langmuir等温线规律,由等温吸附计算得到的热力学函数变化表明吸附系自发、放热过程。吸附动力学、热力学及光谱表征均表明改性硅胶上罗丹明B的吸附主要为其在改性硅胶表面借助电价和共价键联的化学吸附。
罗丹明B;硅胶;硫醇化;吸附;机制
At present,more than 100 000 types of dyes are used in plastics,paper and pulp,cosmetics,leather, textiles and food industries[1-2].Dyes can be noticed at concentration even as low as 1 mg·L-1,which makesit unfit for human consumption[3].The complex aromatic molecular structures owned by some dyes make them toxicorcarcinogenicandalsolimittheir biodegradation[4-5].
Many techniques and methods for removing dyes from water have been developed,including advanced oxidation[6],photocatalytic oxidation[7],coagulation and flocculation[8],membraneseparation[9].Fromthe economic and efficiency point of view,adsorption is regarded as the most promising and widely used method to treat dye polluted effluents.Adsorption of dyesonawidevarietyofmaterialshasbeen comprehensively summarized[10].Silica gel has a large surface area,mechanical stability,chemical inert and is easily to be modified[11].Silicious materials have been widely applied to treat heavy metals in water[12-16]. At present,their application to adsorption of dyes molecules has aroused more and more attentions[17-19]. Monash et al.[20]synthesized a sulfated mesoporous MCM-41 with a large surface area as a promising adsorbent to treat polluted water containing dyestuff. Arabinda et al.[21]synthesized hollow silica nanoparticles using anionic surfactant templating method,and the fluorescence spectroscopy results show that the hollow nanoparticlesentrapthedyeintraceamounts effectively.However,among the most of studies,the adsorption mechanisms are less involved[20].
In this study,a thiolated silica gel treated by coupling of as-prepared pristine silica gel with γmercaptopropyltriethoxysilane was synthesized,and thesurfacenatureofmodifiedsilicagelwas investigated.The removal of aqueous Rhodamine B (RhB)by the adsorption of the modified silica gel and the effects of factors such as pH,initial concentration of RhB,adsorbent dosage,and adsorption temperature were evaluated.The adsorption kinetics,isotherms, thermodynamics werestudiedandtheadsorption mechanism was proposed and discussed.
1 Experimental
1.1 Materials and methods
The tetraethylorthosilicate(TEOS,Tetraethoxysilane CAS No.562-90-3)of analytical grade(AR) used as the precursor of pristine silica gel was purchased from Sinopharm Chemical Reagent Co., Ltd.γ-Mercaptopropyltriethoxysilane(MPTS,AR)was supplied by Capatue Chemical Co.,Ltd.(Nanjing, China).The dye RhB was received from Sinopharm Chemical Reagent Co.,Ltd.The other chemicals used were all of commercial analytical grade,and were used without further purification.Deionized water was used throughout.
The pH value of the solution was determined withaPB-10pHmeter(Sartorius,Germany). Concentration of RhB was analyzed on a V-5100 visiblespectrophotometer(Yuanxi,China).SEM images were obtained by a field emission S-4800 SEM (Hitachi,Japan).Infrared spectra of KBr powderpressedpelletswererecordedonaTensor37 spectrometer(Bruker,Germany).Raman spectra were obtained on an Invia laser Raman spectrophotometer (Renishaw,England)with a laser resource used for excitation at wavelength of 633 nm.The nitrogen adsorption/desorption isotherms were measured at 77 K with an automatic physisorption analyzer ASAP 2020(Micromertics,USA).The surface area was determinedbytheBrunauer-Emmett-Teller(BET) method and the pore size was obtained by the Barrett-Joyner-Halenda(BJH)calculation.The surface zeta potentials of the adsorbents were measured using a Zetasizer(Malvern,England).
1.2 Preparation of pristine silica gel
Pristine silica gel(pSiO2)was prepared through a sol-gel route according to Pitoniak et al.[22].In a typical synthesis,10 mL deionized water,20 mL ethanol and 10 mL TEOS were mixed under vigorous stirring.After further stirring for 10 min,nitric acid(1 mol·L-1,2 mL)and hydrofluoric acid(3wt%,2 mL), whichwereusedascatalyststoincreasethe hydrolysis rate of TEOS and to reduce the gelation time,were added to this mixture solution dropwise under magnetic stirring.The obtained sol was covered and aged at room temperature for 24 h and dried in an oven at 60℃overnight,the resultant gel was washed with deionized water repeatedly until the supernatant was neutral,it was then ground and driedin an oven at 100℃for 24 h,the obtained material was finally calcined at 550℃in a muffle furnace for 4 h.
1.3 Preparation of modified silica gel
Modified silica gel(mSiO2)was prepared by an impregnation method as follows:25 g pSiO2was mixed with 250 mL N,N-dimethylformamide(DMF)solution containing 25 mL MPTS into a three-necked flask. The mixture was stirred and heated in reflux for 5 h under nitrogen atmosphere.The resultant particles were washed with ethanol several times to remove the solvent or unconverted MPTS.The collected particles were dried in an oven at 60℃for 4 h.
1.4 Adsorption tests
DesiredamountsofpSiO2ormSiO2were introduced into conical flasks with plug,to which 50 mL of RhB solution with various concentrations at adjusted pH value was added.The solutions were agitated in an orbital shaker at a constant speed of 150 r·min-1at different temperatures(25,35 and 45℃).The two phases were separated by centrifugation at 4 000 r·min-1.The final RhB concentration in solution was analyzed on visible spectrophotometer by monitoring their absorbance at the optimal wavelength of 554 nm.The equilibrium RhB adsorption capacity (qe)and removal rate(R)were calculated for each samplebyusing the expressionreported[6].All experiments were conducted by batch technique and the experimental data were the average of duplicate determinations.
1.5 Modeling of adsorption data and thermodynamics calculation
The adsorption kinetic data of RhB measured on pSiO2and mSiO2,respectively,were analyzed in terms of pseudo-first-order and pseudo-second-order adsorption equations.The two most common isotherms, Freundlich and Langmuir models were employed for modeling equilibrium adsorption of RhB obtained on pSiO2or mSiO2.The changes in Gibbs free energies were calculated based on the equation reported[23],the changes in enthalpies and entropies of adsorption process were obtained from each intercept and slope of linear form of Van′t Hoff equations.
2 Results and discussion
2.1 FTIR and Raman spectroscopy
Fig.1 presents the IR spectra acquired on pSiO2and mSiO2.The bands at wave number around 3 425 cm-1and 1 630 cm-1correspond to the stretching and bending vibrations of-OH(preferentially from the adsorption of H2O),respectively,the peaks of-OH in aggregative silanol groups(Si-OH)are overlapped with the peak from H2O[24].The broad peak at about 1 105 cm-1corresponds to the asymmetric stretching vibration while the peaks around 805 and 470 cm-1are attributed to the symmetric and deformation stretching vibration of Si-O-Si,respectively[16].The band centered at 970 cm-1represents the stretching vibration of Si-O in silanol groups[14].
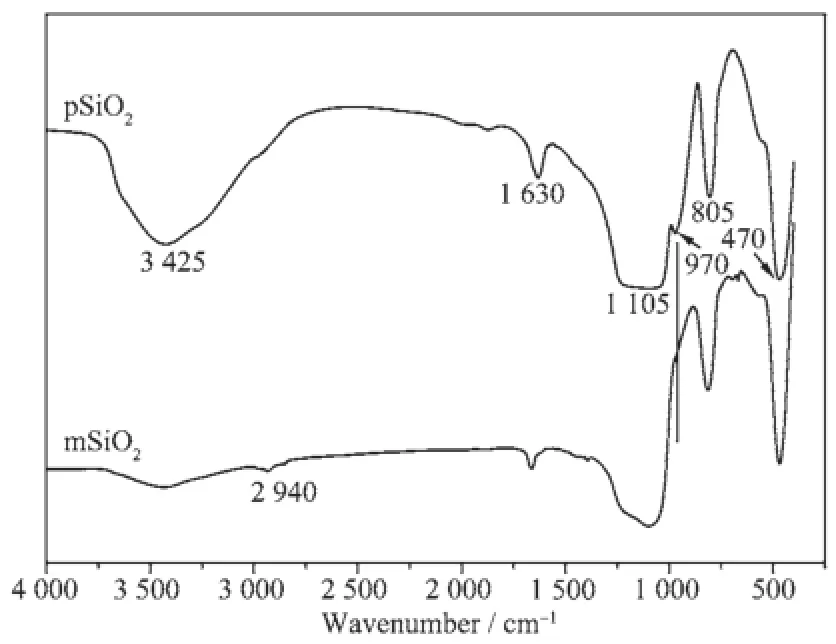
Fig.1 FTIR spectra for pSiO2and mSiO2
Compared to the spectrum on pSiO2,the peak at around 970 cm-1,which represents the Si-O in silanol groups,is not observed on the spectrum of mSiO2, accompanying a significant decreasing in intensity of peaks from-OH,this may be an evidence for the reactionbetweensilanolgroupsandcoupling molecules,and may imply a mechanism in scheme 1.
The spectrum on mSiO2shows a new weak band at 2 935 cm-1which represents the asymmetric stretching vibration from-CH in-CH2or-CH3groups. However,it is different from the results reported[25], the absorption peak corresponds to mercapto groups is neither observed on mSiO2,probably because of the limitedsensitivityoftheinstrumentunderour measuring conditions.
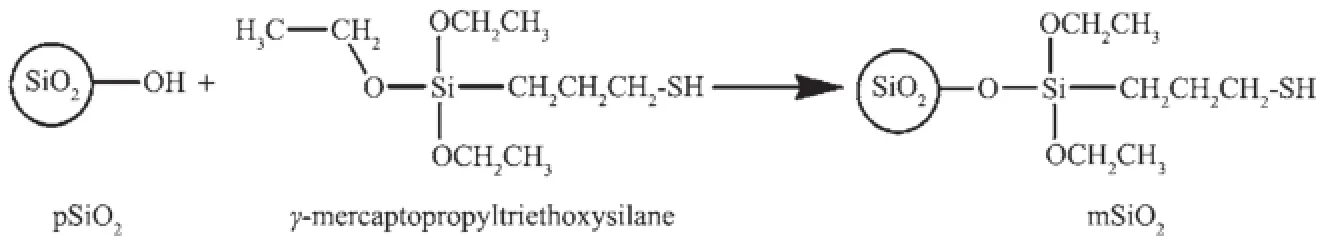
Scheme 1Possible route for silica gel modification
The mercapto groups show a strong intensity on Raman spectra at the wave number of 2 600~2 500 cm-1[26].As shown in Fig.2 ,an evident peak appears at around 2 580 cm-1on the spectrum of mSiO2, confirming the modification process in Scheme 1.
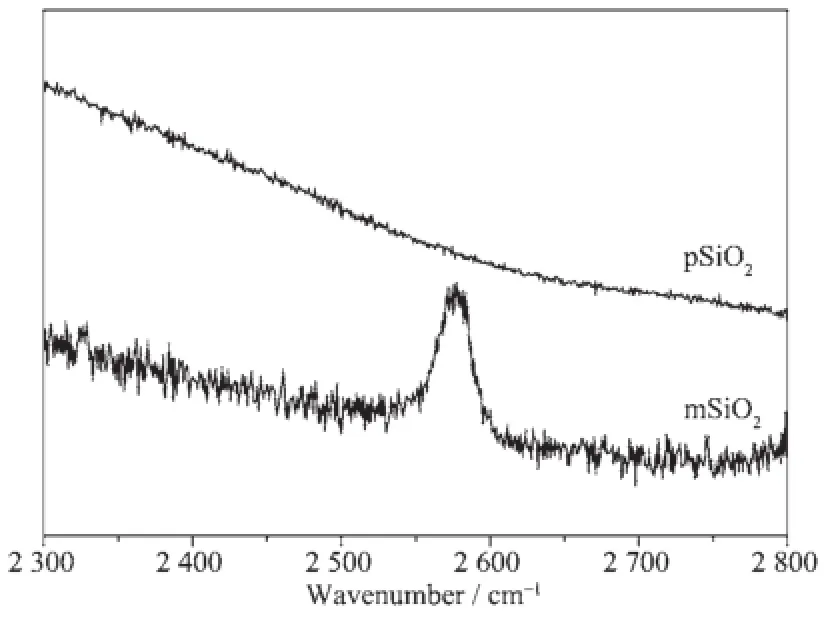
Fig.2 Raman spectra acquired on pSiO2and mSiO2
2.2 Surface analysis
Fig.3 shows the SEM images of pSiO2and mSiO2. The surface feature of modified silica gel and that of the pristine are different,a rough and heterogeneous surfaceisobservedonpSiO2,whileauniform aggregation of sphere-type particles are obtained on mSiO2as shown in Fig.3 (b).
As listed in Table 1,the mSiO2shows an overall decrease in total pore volume,specific surface area and the average pore diameter compared to that of pSiO2, which could be attributed to the chemical grafting of MPTS molecules on the mSiO2that partially precludes the adsorbing of nitrogen molecules[27].The modification process mainly changes the surface properties of the material rather than enlarging the surface area or changing the pore properties.
ζ potential for pSiO2and mSiO2is shown in Fig. 4.The pH value at zero point for charge(pHZPC)of pSiO2is about 2.3,which is in accord with literature report[28].The pHZPCof mSiO2shifts to a lower position at approximately 1.5 because of the chemical linking of acidic mercapto molecules.
2.3 Adsorption of RhB towards the as-prepared adsorbents
2.3.1 Effect of contact time

Fig.3 SEM images of pSiO2(a)and mSiO2(b)

Table1 Surface characteristics from the N2adsorption/desorption measurement
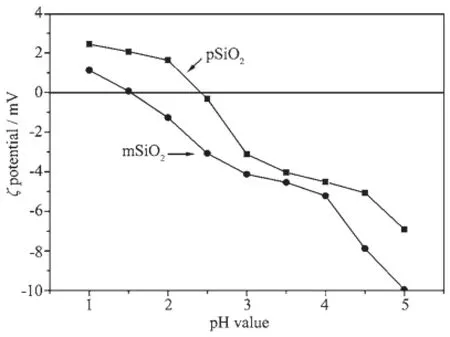
Fig.4 ζ potential on pSiO2and mSiO2
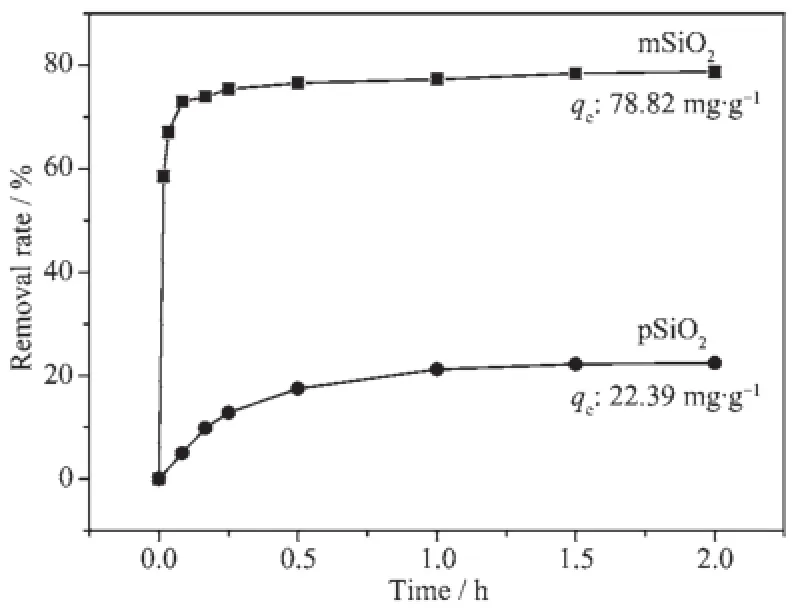
Fig.5 Removal of RhB from solution onto pSiO2and mSiO2as a function of contact time at 100 mg· L-1initial concentration of RhB,1 g·L-1adsorbents dosage,pH=6 and T=25℃
Fig.5 presents the plots of removal percentage versus contact time of RhB on pSiO2and mSiO2, respectively,underthesamebatchadsorption conditions.TheadsorptionpropertyofmSiO2is significantlyimprovedasevidencedfromthe comparison between the two curves in Fig.5 ,and the mSiO2reaches the equilibrium stage within the first 5 minutes,which is very important for dye removal in practical applications.The mSiO2saturated by RhB can be quickly reproduced by stirring it in alcohol solution(5 mol·L-1),followed by washing it with 1 mol·L-1HCl and deionized water repeatedly,the reproducedmSiO2maintains90%ofadsorption capacity on fresh mSiO2after 10 consecutive cycles.
2.3.2 Effect of mSiO2dosage
Fig.6 shows the adsorption of RhB as a function of mSiO2dosage.It is observed that with the raise of adsorbent dosage,the adsorption capacity decreases while the removal rate increases.The increase in the removal rate of RhB is mainly caused by the increase in free active sites of mSiO2with the raise of mSiO2dosage.The decrease in the adsorption capacity of RhB on mSiO2is probably resulted from the aggregation of mSiO2under its higher dosage.In this study,we choose 1 g·L-1as the adsorbent dosage for high removal rate and acceptable adsorption capacity it gives.

Fig.6 Removal of aqueous RhB as a function of mSiO2dosage at 100 mg·L-1initial concentration of RhB,pH=6,T=25℃and contact time=1h
2.3.3 Effect of pH value
Fig.7 shows the adsorption of RhB as a function of pH value on the modified silica gel.With the increase in pH value,the removal percentage of RhB increases significantly between pH values of 3~6 and faintly at pH value of 6~7,the removal rate decreases when pH value exceeds 7.
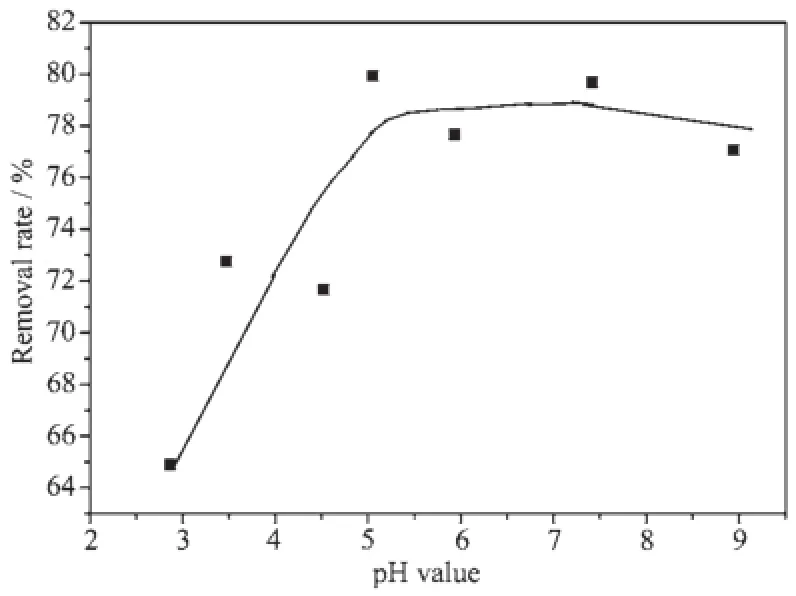
Fig.7 Removal of aqueous RhB as a function of pH values on mSiO2at 100 mg·L-1initial RhB concentration,1 g·L-1mSiO2content,T=25℃and contact time=1 h
The dependence of removal quantity on pH value of the RhB solution correlates mainly to the charge state of mSiO2particles and RhB molecules.The mSiO2is totally negatively charged within our pHvalue for the pHzpc of mSiO2as shown in Fig.4 , favoring the adsorption of cationic RhB.The negativecharge density on the surface of mSiO2increases with the increase of pH value,thereby increasing the adsorption quantity of RhB.On the other hand,RhB is an amphoteric dye with the coexistence of amine and carboxyl groups,and the pKa of-COOH in RhB is about 4.0[28],it will dissociate and reduce the its positive-charge density with pH value above 4,thus making it get on the state of adverse adsorption.If the electrostatic attraction is the main interaction between mSiO2and RhB,the maximum removal capacity should be near the pH value of 4.But this is not the case as illustrated in Fig.7 ,suggesting that other interactionsbetweenmSiO2andRhBthrough hydrogen[24]or covalent bonding[28-29]must be involved.
2.3.4 Effect of RhB initial concentration

Fig.8 Removal of RhB as a function of RhB initial concentrations at 1g·L-1mSiO2content,pH=6, T=25℃,contact time=1 h
Adsorption results of RhB on mSiO2as a function of RhB initial concentration are shown in Fig.8 . Expected decrease is observed for removal percentage of RhB along with the increase of initial RhB concentration.The available sites on the surfaces of mSiO2are constant,the competition among the RhB ionsincreaseswithRhBconcentration,andthe competitionresultsinthedecreaseofremoval percentage because of the the electronic repulsion. However,more than 50%of RhB are absorbed even the concentration of RhB reaches 300 mg·L-1.
2.4 Adsorption kinetics

Fig.9 Adsorption kinetics of RhB on pSiO2and mSiO2
The kinetic adsorption data of RhB on mSiO2(shown in Fig.5 )are simulated with two simplified models:pseudo-first-order and pseudo-second-order models,the results are shown in Fig.9 .
Both the pseudo-first and second-order model fit the experimental data well with high coefficient of determination(R2)for adsorption on pSiO2,however, thepseudo-first-ordermodelfitsbetterwithan adsorption capacity calculated(21.17 mg·g-1)and matches closer to the experimental data(22.39 mg·g-1) than that from the pseude-second-order model(25.56 mg·g-1).For the adsorption of RhB on mSiO2,the pseudo-second-order model adequately describes the kinetics of sorption of RhB with high R2,and the calculated qeis in good agreement with the experimental one(78.99 and 78.82 mg·g-1,respectively), indicatingthatchemisorptionsmayinvolvein adsorption of RhB on mSiO2[30].
2.5 Adsorption isotherms
In order to further determine the mechanism of RhB adsorption and evaluate the effect of temperature on the adsorption capacity,the experimental data (shown in Fig.1 0)are fitted to Langmuir[31]and Freundlich[32]liner equations,the results are plotted in Fig.1 1.As shown in Fig.1 0,a distinct diminution of adsorption capacity is observed with the increase of temperature.The obtained results shown in Fig.1 1 indicate that the adsorption isotherm of RhB on mSiO2is better predicted by Langmuir isotherm.
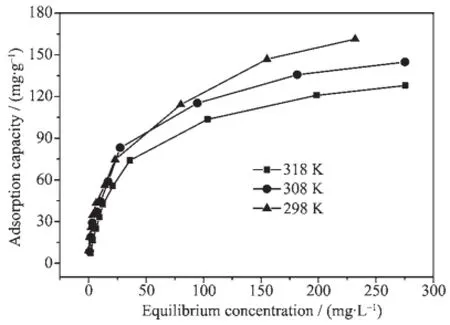
Fig.1 0Adsorption isotherm of RhB on mSiO2
The Langmuir adsorption model assumes that the adsorbed layer is one molecule thick and the sites are homogeneous.As shown in Fig.1 1,the Langmuir isotherm hasa higher R2for the adsorption of RhB, suggesting that the adsorption process is dominated by a monolayer formation rather than a multilayer,and more importantly,implying a predominant status of the chemical adsorption[33].The maximum adsorption capacity of RhB on mSiO2reaches 166 mg·g-1.

Fig.1 1Langmiur(left)and Freundlich(right)isotherms fitted from the experimental data
2.6 Adsorption thermodynamics
The thermodynamic parameters of the adsorption process are obtained from adsorption results at various temperatures(298,308 and 318 K).Plot of lnb versus 1/T is shown in Fig.1 2.The negative of values of all ΔG⊖(-8.25,-7.87 and-7.19 kJ·mol-1at 298,308 and 318 K,respectively)indicate that the adsorption process has a feasible and spontaneous nature,and the absolute value of ΔG⊖decreases with the increase in temperature,suggesting that the spontaneous natureof adsorption is inversely proportional to temperature. The negative value of ΔS⊖(-52.0 J·mol-1·K-1)indicates the randomness decreasing during the adsorption of RhB molecules onto mSiO2.The negative value of ΔH⊖(-23.9 kJ·mol-1)indicates the exothermic nature of adsorption and thenumericsizereflectsthe adsorptionprocessisprobablycontrolled chemically[34].

Fig.1 2lnb versus T-1plot for RhB adsorption on mSiO2
2.7 Adsorption mechanism
Intheprecedingdiscussion,achemical adsorption mechanism involved in the adsorption process between RhB and mSiO2are proposed.To verifythisassumption,theFTIRandRaman spectroscopy analysis are performed and the results are displayed in Fig.1 3.

Fig.1 3FTIR and Raman spectra:FTIR spectra for pure and immobilized RhB(left); Raman spectra for fresh and RhB adsorbed mSiO2(right)
The following principal bands in the FTIR spectra of pure RhB are assigned according to literature[35]:the peak at about 1 693 cm-1is assigned to the stretching vibration of the carboxylic group,the peak around 1 644 cm-1is due to the C-N bond, peaks in the region of 1 450~1 600 cm-1are from the heterocycleandaromaticringvibrations,and absorbance at around 1 341 cm-1is attributed to the C-aryl bond vibrations.
Differen cesareo bservedin the FTIRon immobilized RhB(RhB-mSiO2).The peak assigns to stretching vibration of carboxylic group which is shifted to a higher frequency centered at 1 717 cm-1, suggesting that RhB are bonded to the surface of mSiO2through ester-like linkage.The peak from C-N vibration is overlapped in that of silanol Si-OH (bending vibration)locates near 1 648 cm-1.A new peak appears around 1 627 cm-1,this might be due to the vibration of C-N in a structure which is established through the electrostatic interaction between RhB and mSiO2[35].
The ester-like linkage between mercapto groups on the surface of mSiO2and carboxylic groups in RhB during the adsorption process are further verified by Raman spectra acquired on fresh and RhB loaded mSiO2(mSiO2-RhB).The band of mercapto group locates in 2 578 cm-1vanishes from the spectrum on mSiO2-RhB,suggestinganadsorptionmechanism through the covalent linking by esterification between -SH and-COOH.Thetwoprobablyinteractions betweenRhBandmSiO2areillustratedinthe following schematic diagram.
3 Conclusions
This study has shown a successful modification of pristine silica gel achieved by grafting mercapto groups onto silica gel through a covalent linking.The modified silica gel can be a good candidate for remediation of dyes in industrial effluent due to its high adsorption capacity.The adsorption capacity of modified SiO2is superior to pristine one due to the attaching of-SH groupsonmodifiedSiO2.The adsorption process is dependent on factors such as solution pH value, contact time, initial RhB concentration, adsorbents dosage and temperature.The adsorption kinetics, equilibrium and thermodynamics studies suggest that the chemisorptions dominate the uptaking of RhB by thiolated silica gel,and the FTIR and Raman spectroscopy analyses indicates that both electrovalent and covalent interaction are involved in the adsorption hanisms.
[1]SONG Xiao-Cui(宋晓翠),GU JING-Hua(谷景华),YAO Hong -Ying(姚红英),et al.Chinese J.Inorg.Chem.(无机化学学报),2012,28(6):1239-1244
[2]Ahmed S A,Soliman E M.Appl.Surf.Sci.,2013,284:23-32
[3]Malik R,Ramteke D S,Wate S R.Waste Manage.,2007,27 (9):1129-1138
[4]WANG Qiao-Qiao(王巧巧),NI Zhe-Ming(倪哲明),ZHANG Feng(张峰),et al.Chinese J.Inorg.Chem.(无机化学学报), 2009,25(12):2156-2162
[5]Barragán B E,Costa C,Márquez M C.Dyes Pigm.,2007,75 (1):73-81
[6]Nawi M A,Sheilatina S S.J.Colloid Interface Sci.,2012, 372(1):80-87
[7]De Mendonca V R,Lopes O F,Fregonesi R P,et al.Appl. Surf.Sci.,2014,298:182-191
[8]Yang Z,Yang H,Jiang Z W,et al.J.Hazard Mater.,2013, 254-255:36-45
[9]Zheng Y P,Yao G H,Cheng Q B,et al.Desalination,2013, 328:42-50
[10]Crini G.Bioresour.Technol.,2006,97(9):1061-1085
[11]Aguado J,Van Grieken R,López-Muoz M J,et al.Appl. Catal.A,2006,312:202-212
[12]Gao B J,GaoY C,Li Y B.Chem.Eng.J.,2010,158:542-549
[13]Otsuka R,Yoshitake H.J.Colloid Interface Sci.,2014,415: 143-150
[14]Chen J,Qu R J,Zhang Y,et al.Chem.Eng.J.,2012,209: 235-244
[15]Xu L,LiuY Q,Hu H P,et al.Desalination,2012,294:1-7
[16]TianY,Yin P,Qu R J,et al.Chem.Eng.J.,2010,162(2): 573-579
[17]Moritz M.Appl.Surf.Sci.,2013,283:537-545
[18]Ayad M M,El-Nasr A A.J.Nanostruct.Chem.,2013,3:3-11
[19]Santos D O,Santos M L N,Costa J A S,et al.Environ.Sci. Pollut.Res.,2013,20(12):5028-5035
[20]Monash P,Pugazhenthi G.Adsorption,2009,15:390-405
[21]Baruah A,Kumar S,Vaidya S,et al.J.Fluorescence,2013, 23(5):1287-1292
[22]Pitoniak E,Wu C Y,Londeree D,et al.J.Nanopart.Res., 2003,5(3/4):281-292
[23]Neghlani P K,Rafizadeh M,Taromi F A.J.Hazard Mater., 2011,186(1):182-189
[24]Jung K Y,Park S B.Appl.Catal.B,2000,25:249-256
[25]Xu R,Jia M,Li F T,et al.Appl.Phys.A,2012,106(3):747-755
[26]Xaviera R J,Gobinath E.Spectrochim.Acta A,2012,86: 242-251
[27]Xue X M,Li F T.Microporous Mesoporous Mater.,2008, 116:116-122
[28]Gupta V K,Mohan D,Sharma S,et al.Sep.Sci.Technol., 2000,35(13):2097-2113
[29]Li L,Liu S X,Zhu T.J.Environ.Sci.,2010,22(8):1273-1280
[30]Zeng X W,Fan Y G,Wu G L,et al.J.Hazard Mater.,2009, 169(1/2/3):1022-1028
[31]Bayramogˇlu G,Bektas S,Arica M Y.J.Hazard Mater.,2003, 101:285-300
[32]Chegrouche S,Mellah A,Telmoune S.Water Res.,1997,31: 1733-1737
[33]Yu Q,Deng S B,Yu G.Water Res.,2008,42:3089-3097
[34]Zhang Z Y,Zhang Z B,Fernández Y.et al.Appl.Surf.Sci., 2010,256(8):2569-2576
[35]Wang Q,Chen C C,Zhao D,et al.Langmuir,2008,24:7338 -7345
Thiolated Silica Gel:Synthesis and Immobilization of Rhodamine B from Aqueous Solution
YANG Han-Pei*,1,2YU Mi-Hong1FU Xiao-Fei1WU Jun-Ming1,2
(1Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes, Ministry of Education,College of Environment,Hohai University,Nanjing 210098,China)
(2Huaian Research Institute,Hohai University,Huaian,Jiangsu 223002,China)
A thiolated silica gel was obtained by treating the as-prepared pristine silica gel with γmercaptopropyltriethoxysilane through an impregnation route.The FTIR and Raman spectroscopy results indicate that the thiolation of silica gel is achieved by attaching γ-mercaptopropyldiethoxysilane onto the surface of silica gel via a chemical linking.The results for batch removal of Rhodamine B(RhB)from aqueous solution by the thiolated silica gel demonstrate that the thiolated silica gel can effectively adsorb RhB under a wide range of conditions.The data of adsorption kinetics are fitted well with the pseudo-second-order model,and the equilibrium data are well described by the typical Langmuir adsorption isotherm.The thermodynamic derivation shows an exothermic and spontaneous nature of adsorption process with a high value of enthalpy change.The adsorption kinetics,isotherm and thermodynamics suggest a mechanism involving chemisorptions of RhB on thiolated silica gel as confirmed by FTIR and Raman spectroscopy.
Rhodamine B;silica gel;thiolation;adsorption;mechanism
O613.71
A
1001-4861(2015)03-0571-09
10.11862/CJIC.2015.064
2014-10-04。收修改稿日期:2014-12-09。
河海大学淮安研究院开放基金(No.1061-51402012),江苏省高校优势学科建设工程资助项目。*
。E-mail:yanghanpei@hhu.edu.cn

