Targeted delivery of docetaxel to the metastatic lymph nodes:A comparison study between nanoliposomes and activated carbon nanoparticles
Tintin Ye,Wen Xu,Tinyu Shi,Rui Yng,Xinggng Yng, Shujun Wng,Weisn Pn,*
aDepartment of Pharmaceutics,School of Pharmacy,Shenyang Pharmaceutical University,103 Wenhua Road, Shenyang 110016,China
bDepartment of R&D,Shenyang Green Pharmaceutical CO.,LTD,176 Shenbei Road,110164,China
Targeted delivery of docetaxel to the metastatic lymph nodes:A comparison study between nanoliposomes and activated carbon nanoparticles
Tiantian Yea,Wen Xub,Tianyu Shia,Rui Yanga,Xinggang Yanga, Shujun Wanga,Weisan Pana,*
aDepartment of Pharmaceutics,School of Pharmacy,Shenyang Pharmaceutical University,103 Wenhua Road, Shenyang 110016,China
bDepartment of R&D,Shenyang Green Pharmaceutical CO.,LTD,176 Shenbei Road,110164,China
ARTICLEINFO
Article history:
Received 27 June 2014
Received in revised form
28 July 2014
Accepted 9 August 2014
Available online 27 August 2014
Activated carbon nanoparticle
Nanoliposome
Docetaxel
Metastatic lymph node
Lymph node targeting
The objective of this study is to compare the targeting ability of activated carbon nanoparticles and nanoliposomes,which are used as carriers for delivering docetaxel(DTX)to the metastatic lymph nodes.In this study,we f i rst prepared the DTX-loaded activated carbon nanoparticles(DTX-AC-NPs)by modifying the activated carbon with nitric acid oxidation and absorbing DTX in the concentrated nitro-oxide nanocarbon.We then prepared DTX-loaded nanoliposomes(DTX-LPs)by the proliposome method.The physiochemical properties of DTX-AC-NPs and DTX-LPs were carefully evaluated in vitro.The metastatic lymph node uptake and the injection site retention were investigated by analyzing the DTX concentration in metastatic lymph nodes and injection sites.The result showed that DTX-AC-NPs and DTX-LPs with suitable and stable physicochemical properties could be used for in vivo lymph node targeting studies.DTX-AC-NPs signif i cantly increasedDTX-AUC(0-24)andprolongedDTX-retentioninmetastaticlymphnodes compared to DTX-LPs and non-modif i ed activate carbon in vivo.This study demonstrated activated carbon nanoparticles may be potential intralymphatic drug delivery system to preferentially target regional metastatic lymph nodes.
©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
1.Introduction
Lymph node metastasis plays an active role in cancer metastasis and may occur at an early stage of many types of cancer.A signif i cant number of efforts have been made to improve the outcome of treating cancer lymph node metastasis,including surgical resection,chemotherapy,aggressive surgery and radiotherapy.However,the effectiveness ofcurrently available treatment options remains limited.In fact, conventional systemic chemotherapy cannot be effectively delivered to the lymphatic system without dose-limiting toxicities[1].Moreover,clinical evidences have shown the minimal benef i ts of chemotherapy to treat lymph node metastasis compared to its risks.Therefore,it is necessary to investigate novel strategies to deliver chemotherapeutic drugs specifically to the regional metastatic lymph nodes.Colloidal particles,such as liposomes and activated carbon particles, which are known to be highly taken up by the lymphatic,can be used as potential carriers for lymphatic chemotherapeutic drug delivery[2,3].
The utilities of liposomes[4,5]and activated carbon particles[6,7]as carriers in lymphatic delivery have been widely investigated in the past decade.Because liposomes can be selectively absorbed by the lymphatics after local administration[8],researchers have taken advantage of this property to deliver liposomal contrast agents[9]and chemotherapeutic drugs[10]to tumor metastatic lymphatic.The activated carbon particles quickly gather in the lymph nodes and dye them black after macrophage phagocytosis due to their high aff i nity to the lymphatic system.In recent years,this unique selective distribution has been studied in many applications,such as sentinel lymph node staining,drug carriers and thermotherapy[11-13].CNSI®(Carbon nanoparticles suspension injection)is already commercialized as an activated carbon lymphatic tracer to track lymph nodes in the region of gastric carcinoma.However,theactivatedcarbonhaspoor dispersion stability.Certain suspending compounds can serve as stabilizing agents to remain their stability.In particular,it has been reported that the nitric acid oxidation of the activated carbon is able to improve the stability of the activated carbon,and the modif i ed activated carbon shows a good lymphatic targeting [14].
Docetaxel is an antineoplastic agent derived from the taxane class.It is one of the most important chemotherapeutic agents for a broad range of human cancer malignancies [15-17].Due to the directly binding to the tubulin,docetaxel disrupts microtubule,and then leads to mitotic arrest and eventually causes apoptosis[18].In clinical practice,docetaxel has been successfully used,mostly for the treatment of nonsmall cell lung cancer,locally advanced and metastatic breast cancer,head and neck cancer,prostate cancer among others[19-21].Commercial formulation of docetaxel(i.e. Taxotere®)by intravenous injection(i.v.)is often associated with acute hypersensitive reactions,owing to the impact of the drug itself and the presence of polysorbate-80 and ethanol in the formulation,thus hindering its wide medical utility for intravenous applications[22-25].
As two most commonly intralymphatic-targeted nanocarriers,there is no previous study focusing on a systematic comparison of activated carbon particles and liposomes in terms of targeting delivery ability of docetaxel to metastatic lymph node.To f i ll this gap,we prepared docetaxel-loaded activated carbon nanoparticles(DTX-AC-NPs)and docetaxelloaded nanoliposomes(DTX-LPs).The DTX-AC-NPs were produced by the nitric acid oxidation method.And DTX-LPs were prepared by the proliposome method previously reported[24].The physicochemical properties of DTX-AC-NPs and DTX-LPs were evaluated in vitro.Most importantly,the lymph node targeting ability of DTX-AC-NPs and DTX-LPs were compared with the aspects of metastatic lymph node uptake and the injection site retention after subcutaneous administration.
2.Materials and methods
2.1.Materials and animalsDocetaxel,the purity of which was over 99%,was purchased from Lianyungang Gabriel Biochemical Technology Co.,Ltd., China.Taxotere®was supplied by Sanof i-Aventis Group, France.LIPOID S100 soybean phospholipids(SPC)were purchased from Germany Lipoid GmbH Co.Ltd.,Germany. Cholesterol was purchased from Tianjin Bodi Chemical Holding Co.,Ltd.,China.Activated carbon was purchased from Taiji Ring Nano-Products Co.,Ltd.,China.All other chemicalswereofreagentgradeandobtained commercially.
BalB/c nude mice with 26-28 g of weight and 6-8 weeks of age were supplied by the Experimental Animal Center of Shenyang Pharmaceutical University.All animal experiments were carried out according to the guidelines listed in“Principles of'Laboratory Animal Care”(NIH publication#85-23, revised in 1985)and approved by the Animal Ethics Committee of Shenyang Pharmaceutical University.
2.2.Nanoliposome preparation
DTX-loadedliposomes(DTX-LPs)werepreparedbythe carrier-deposition method according to our previous study [26].Brief l y,a mixture of SPC/cholesterol/DTX(35/26/4,by mM)were dissolved in anhydrous ethanol,then the resultant solution and 1.50 g of 100 μm dry dextrose powder were mixed and dried to freely f l ow by a rotary evaporator(RE52CS, Shanghai Yarong Bio-chem Instruments,China).The dry powderofDTX-LPsobtainedwassievedby a150μm sifter.The dry powder of DTX-LPs was reconstituted to form the liposomes solution by the following method:the dry powder of DTX-LPs was placed into 10 ml pH7.4 PBS with magnetic stirring at 50rpm.The reconstituted DTX-LPs solution was sonicated(400 W,5 min),followed by using an Avanti Mini Extruder(Avanti Polar Lipids)to extrude through polycarbonate membranes with 0.08 μm pore sizes.A gel chromatography column(1.0 cm×10 cm)was f i lled with SephadexG-50(GE Healthcare,US)which was applied to separate the carrier systems and free drug.
2.3.Preparation of DTX-AC-NPs
2.3.1.Preparation of nitro-oxide nanocarbon
To obtain nitro-modif i ed nanocarbon,the nitric acid oxidation method was utilized.Brief l y,4 g activated carbons were added into 35 ml ethyl acetate,followed with heating ref l ux under 60°C for 2 h and drying for 24 h.The acquired activated carbons were treated with 30 ml 10%nitric acid(V/V)and 0.1 mol/ l NaOH for 1 h respectively,and then washed with distilled water and dried for 24 h under 60°C.The dry activated carbon was added into 30%nitric acid(V/V)with constant stirring.After 48 h reaction under 100°C,nitro-modif i ed activated carbons were obtained.Nitro-modif i ed activated carbons were centrifuged for 30 min at 8000 rpm.Black supernatant was dried in a vacuum dryer,then resuspended with distilled water,andf i nallydriedtoobtainthenitro-modif i ed nanocarbon.
2.3.2.Preparation of DTX-AC-NPs
The DTX-loaded activated carbon nanoparticles(DTX-ACNPs)were prepared as follows.The nitro-modif i ed nanocarbon was added into 1 mg/ml DTX solution by sonicating and then stirring for 45 min under 37°C,with a weight ratio of nanocarbon to DTX ranging from 1:1 to 10:1(W/W).DTX-ACNPs were f i ltered through a 0.22 μm f i lter membrane.The DTX concentration of the f i ltrate was determined by the HPLC method and isothermal adsorption curves were plotted to determine an optimal weight ratio of nanocarbon to DTX.The DTX-loaded activated carbon(DTX-ACs)was prepared by the above method which adsorbed DTX in the original activated carbon.
2.4.Characterization of nitro-oxide nanocarbon
The nitration nanocarbon was conf i rmed by FT-IR.To perform the FT-IR spectrum analysis,the nitro-oxide nanocarbon which was pressed into a sheet was placed in the sample hole and scanned.The surface morphology of the nitro-oxide nanocarbon powder and the activated carbon powder was examined by a scanning electron microscope(SEM)(F1I-F, Philips,Dutch).The particle size and the particle distribution of the nitration nanocarbon and the activated carbon were determined by using a laser particle size analyzer(LS-230, Beckman Coulter,USA).
Dispersion stability of the nitration nanocarbon was determined in the sedimentation experiment.The nitro-oxide nanocarbon and activated carbon were dispersed in distilled water(1:100,W/V)by ultrasound for 30 min and then centrifuged(3500 r/min,15 min).The transmittance of supernatant was measured at a wavelength of 720 nm by UV spectrophotometer(UV-2000,Unico,China).The stability index(D%)was calculated using the following equations to evaluate dispersion stability:D=(T0-T1)/T0×100%,in which T1was the light transmittance of the sample;T0was the transmittance of the dispersion medium.
2.5.Characterization of DTX-AC-NPs and DTX-LPs
The morphology of DTX-AC-NPs and DTX-LPs was investigated using transmission electron microscopy(TEM)(JEM-1200EX,Japan).The particle size and zeta potential of DTXACs,DTX-AC-NPs and DTX-LPs were measured by laser light scattering using a Zetasizer(Nano-ZS90,Malvern,England)at 25°C.
The drug entrapment eff i ciency(EE),drug loading eff iciency(LE)and drug loading(LD)of DTX-ACs,DTX-AC-NPs and DTX-LPs were analyzed by the mini-column centrifugation method according to our previous study[27].DTX was determined by the HPLC method.Each measurement was carried out in triplicate.The EE,LE and LD were calculated by the following equations according to mass balance:
WTotalwas the total weight of DTX added;WEnvelopedwas the weight of the loaded DTX in nanoliposome system; Wunloadingwas the weight of the unloaded DTX in nanoparticle system;WLipids/Carbonswas the total weight of carriers.
2.6.In vivo evaluation
2.6.1.Chromatography
DTX concentrations of the in-vivo samples were analyzed by the HPLC instrument(Shimadzu,Japan)which was composed of a SPD-20A UV detector,a SIL-10AF auto-sampler,and a LC-10AT quaternary pump.Chromatographic conditions were set to:Century SIL C18-BDS column(200×4.6 mm,5 μm)(Bischoff,Germany)was used at 25°C.The mobile phase consisted of acetonitrile and water(55:45,V/V).The f l ow rate was 1.0 ml/min.The detection wavelength was 230 nm.
2.6.2.Nude mice bearing tumor metastatic lymph node model BalB/c nude mice with 26-28 g of weight and 6-8 weeks of age were used for in-vivo evaluation.Tumor metastatic lymph node model was established according to previously reported procedure[28].The suspended Hela cells(2×106 cells in 0.2 ml of cell culture medium)were injected subcutaneously via left hind foot pad in the BalB/c nude mice.The lymphatic metastatic tumors were observed to form in the left(inoculated side)popliteal and iliac metastatic lymph nodes after 8 weeks.
2.6.3.Animal experimental procedure
The lymphatic drainage and lymph node uptake ability of DTX-ACs,DTX-AC-NPs and DTX-LPs were investigated in nude mice bearing tumor lymph node metastasis.Using the tumor metastatic lymph node model demonstrated in“Section2.6.2”,thenudemicebearingmetastatic lymphnodewere randomly divided into 3 groups(DTX-ACs,DTX-AC-NPs and DTX-LPs group,n=6/group).DTX-ACs,DTX-AC-NPs and DTXLPs were subcutaneously(s.c.)injected via left foot pad with the dose of 1 mg/kg DTX.At predetermined time postinjection,the mice were sacrif i ced.The metastatic lymph nodes(metastatic popliteal lymph nodes and metastatic iliac lymph nodes)and foot(injection site)were immediately harvested.An aliquot(50 ul)of tissue homogenates was loaded into an Eppendorf tube followed by the addition of 10 ul paclitaxel solution(20 μg/ml)as the internal standard and 250 μl acetonitrile.The mixture was vortexed for 5 min and centrifuged at 10,000×g for 10 min.Supernatants were obtained through this procedure,and it was dried under a stream of nitrogen and redissolved in 100 ul mobile phase, vortexed for 3 min,and centrifuged at 10,000×g for 5 min,and then DTX concentration of the supernatant was analyzed using HPLC as mentioned in“Section 2.6.1”.
2.6.4.Pharmacokinetics and statistical analysis
Statisticswereexpressedas meanvalues±standarddeviation (SD).Non-compartmental Pharmacokinetic data analysis was carried out by using DAS 2.0 software(Mathematical Pharmacology Professional Committee of China,Shanghai,China). Statistical analysis of this study was performed with ANOVA using SPSS 11.5 software.Values of P<0.05 were considered to be statistically signif i cant.
3.Results and discussion
3.1.Preparation of DTX-AC-NPs and DTX-LPsProliposomes were def i ned as dry and free-f l owing particles that immediately form a liposomal dispersion on contact with water[29,30].Based on this concept,proliposomes were prepared with a solution of drugs and lipids which were distributed into the micro-porous structure of carrier particles by volatilizing the organic solvents.The DTX-LPs,prepared by the proliposome method with dextrose powder as carriers, hadsatisfactoryphysicochemicalcharacteristicsduring storage.
The DTX-AC-NPs were prepared by absorption method. The result showed that when the ratio of nanocarbons to DTX was more than 8:1,the DTX-adsorption was close to 99%, which implied a nearly complete adsorption.According to the adsorption equilibrium,Freundlich equation was f i t and could be expressed as follows:M=69.88×0.1237(r=0.9917).
3.2.Characterization of nitro-oxide nanocarbonThe FT-IR spectra for the original activated carbon and the nitro-oxide nanocarbon were shown in Fig.1.The spectrum of original activated carbon exhibited a broad,strong-OH stretch band at 3413.3 cm-1,which indicated that numerous -OH groups were present in original active carbon.In the spectrum of the nitro-oxide nanocarbon,the intensity of the band at 3428.2 cm-1increased,which could be attributed to the phenolic-OH groups generated after oxidation.Typical features of nitro-oxide nanocarbon compared with the original activated carbon showed four new peaks of the characteristicpeakposition:astrong-NO2symmetricalstretchband at 1383.9 cm-1,a strong-NO2asymmetrical stretch band at 1591.5 cm-1,a C-NO stretch band at 618.6 cm-1and a C-O bend band at 1124.6 cm-1,which demonstrated the successful preparation of the nitro-oxide nanocarbon.
Fig.2 illustratedthat thesurfacemorphology ofthe original activatedcarbonandthenitro-oxidenanocarbonwere respectively detected by SEM at×100 and×1000 magnif i cations,respectively.There was a clear difference between Fig.2A(original activated carbon)and Fig.2B(oxidized nanocarbons).It showed that the particle diameter of the nitrooxide nanocarbon were signif i cantly decreased and also became more uniform than the original activated carbon.
The particle size distribution analysis of the original activated carbon and oxidized nanocarbons were shown in Fig.3. It revealed that the average particle size of original activated carbon was 1.353±0.643 μm and appeared double peaks which implied uneven particle size in Fig.3A.But the average particle size of oxidized nanocarbons was 80.5±19 nm and particle size was uniform in Fig.3B.
Dispersion stability of the original and oxide activated carbon was determined by the sedimentation experiment.In conditions of un-centrifugation,stability factor of the original activated carbon in aqueous systems was 95.4%.When original activated carbons were centrifuged for 30 min,it had been completely precipitated.While the stability factor of the nitrooxide nanocarbon was still 82.5%after 60 min centrifugation. It implied that nitric acid oxidation greatly enhanced the dispersion stability of the activated carbon in aqueous systems.
3.3.Characterization of DTX-AC-NPs and DTX-LPs
The morphology of reconstituted DTX-LPs by TEM was shown in Fig.4.It could be seen that the DTX-LPs after being reconstituted became the monolayer liposomes solution,and the typical bilayer structure of liposome.DTX-AC-NPs were in circular shape and well dispersed.The EE/LE,DL,particle size and zetapotentialof DTX-AC-NPs and DTX-LPswereshownin the Table 1.The results showed that they both had a high EE/ LE and DL,which were suitable for loading DTX.Particle size was the key factor for subcutaneous administrating for targeting lymphatic system.The formation of too large particles intheinterstitialsurroundingscouldimpairlymphatic drainage[31].A similarity of particle sizes between DTX-ACNPs and DTX-LPs was shown in Table 1 which could minimize the inf l uence of the particle size on lymphatic targeting.
3.4.Method validation tests
Calibration curves for DTX in plasma,lymph nodes and foot were obtained.Calibration equations,linear range and correlation coeff i cients were presented in Table 2.The results showed that there was a good linear relationship.The limit of quantif i cation(LOQ)of plasma,lymph nodes,and foot were 10 ng/ml,10 ng/mg,and 250 ng/mg respectively.The intra-day and inter-day precisions of plasma,lymph nodes and foot of high,medium,and low concentrations were within 15%, which indicated that the method was reproducible.The extraction recoveries of DTX in plasma,lymph nodes,and foot were 88.32%,87.15%and 85.81%,respectively.Therefore,this method for the analysis of biological samples exhibited a good precision and a high recovery.
3.5.Uptake and pharmacokinetics in metastatic popliteal lymph nodes
The DTX concentration in metastatic popliteal lymph nodes and the non-compartmental pharmacokinetic parameters were shown in Fig.5B and Table 3.As shown in Fig.5A,the popliteal and iliac lymph nodes of nude mice bearing tumor lymph node metastasis appeared lymphadenectasis.It proved that the animal model was successfully established.The max concentrations of three formulations were obtained at 30 min. When the time continued to increase,the DTX-loaded carriers had transferred to the next lymph node via the lymphatic circulation.Therefore,the concentration of DTX reduced. Comparing with DTX-ACs group,the DTX concentration-time curves of DTX-AC-NPs and DTX-LPs groups revealed a slow downward trend.DTX concentrations of DTX-AC-NPs and DTX-LPs groups at any time-point were clearly higher than DTX-ACs group in metastatic lymph node.Before the 24 h time-point,the drug retentions in DTX-AC-NPs and DTX-LPs group were similar,but were unable to be detected in DTXACs group after 12 h post-injection.After the 24 h timepoint,the drug retention of DTX-AC-NPs group appeared to be more than that of DTX-LPs group.
Thenon-compartmentalpharmacokineticparameters were shown in Table 3.The Cmaxof three formulations could be observed at 30 min(Tmax).AUC(0-48h)values indicated a higher amount of DTX in DTX-AC-NPs group disposed in metastatic popliteal lymph nodes than DTX-ACs and DTX-LPs (5.273-fold(P≤0.05)and 1.341-fold increase).The results showed that DTX-AC-NPs showed more metastatic lymph node uptake compared with DTX-ACs and DTX-LPs.Mean residence time(MRT)of DTX was signif i cantly prolonged (P≤0.05)by loading in DTX-AC-NPs and DTX-LPs compared to load in DTX-ACs.And MRT was further prolonged by DTX-ACNPs group.It showed that DTX in DTX-AC-NPs group resided in metastatic lymph nodes for a longer time than DTX-AC-NPs and DTX-LPs groups.The clearance(CL)was signif i cantly decreased in DTX-AC-NPs compared with DTX-AC-NPs andDTX-LPs(P≤0.01),so was the distribution volume(V) (P≤0.01).
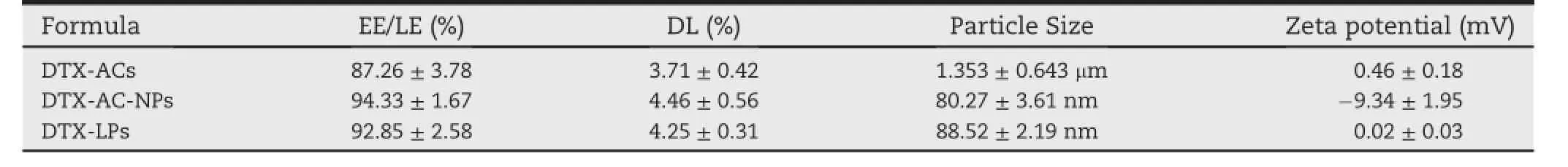
Table 1-3).Physicochemical properties of DTX-ACs,DTX-LPs and DTX-AC-NPs(Mean±SD,n=
3.6.Uptake and pharmacokinetics in metastatic iliac lymph nodes
The DTX concentrations in metastatic iliac lymph nodes were shown in the Fig.5C and the non-compartmental pharmacokinetic parameters were in the Table 2.The DTX concentration curve of metastatic iliac lymph nodes was similar to that of metastatic popliteal lymph nodes.The max concentrations of three formulations were also obtained at 30 min.The AUC(0-48h)values indicated a higher amount of DTX in DTX-AC-NPs group disposed in iliac metastatic lymph nodes than DTX-ACs and DTX-LPs(33.828-fold(P≤0.01)and 1.530-fold increase).And DTX-ACs had the worst lymphatic drainage ability compared with DTX-LPs and DTX-AC-NPs. Comparingwithmetastaticpopliteallymphnodes,the AUC(0-48h)values of three formulations decreased in metastatic iliac lymph nodes,by 9.257-fold decrease in DTX-ACs group,1.646-fold decrease in DTX-LPs group and 1.443-fold decrease in DTX-AC-NPs group,respectively.It implied that one portion of administration dose,which was not taken in metastatic popliteal lymph nodes,was transferred to the iliac lymph nodes and then taken in them.And another portion wastransferredbymicrocirculationandtrans-cellular pathway.
3.7.Injection site retention
The DTX concentration of injection site was shown in Fig.6. From Fig.6,we could see that drug retention in DTX-ACs group was larger than that in DTX-LPs and DTX-AC-NPs groups.83.45±8.71%and 87.14±5.53%of DTX in DTX-LPs and DTX-AC-NPs group was cleared from the injection site at 30 min time-point,but which in DTX-ACs group was 95.39±3.96%.DTX in three formulations at the injection site gradually eliminated post-injection.DTX in DTX-LPs group had the highest clearance compared with DTX-ACs and DTX-AC-NPs groups.But the difference of clearance wasinsignif i cantbetweenDTX-LPsandDTX-AC-NPsgroup (P>0.05).At 48 h after administration,DTX-ACs group still had 85.41±6.01%of the residues at the injection site, however,42.55±1.96%and 48.45±5.12%of the remaining DTX in DTX-LPs and DTX-AC-NPs group at the injection site. For the injection site,the clearance of three formulations was in descending order of DTX-LPs,DTX-AC-NPs and DTXACs.

Table 2-Calibration curves of DTX for plasma,lymph nodes and foot by HPLC method.
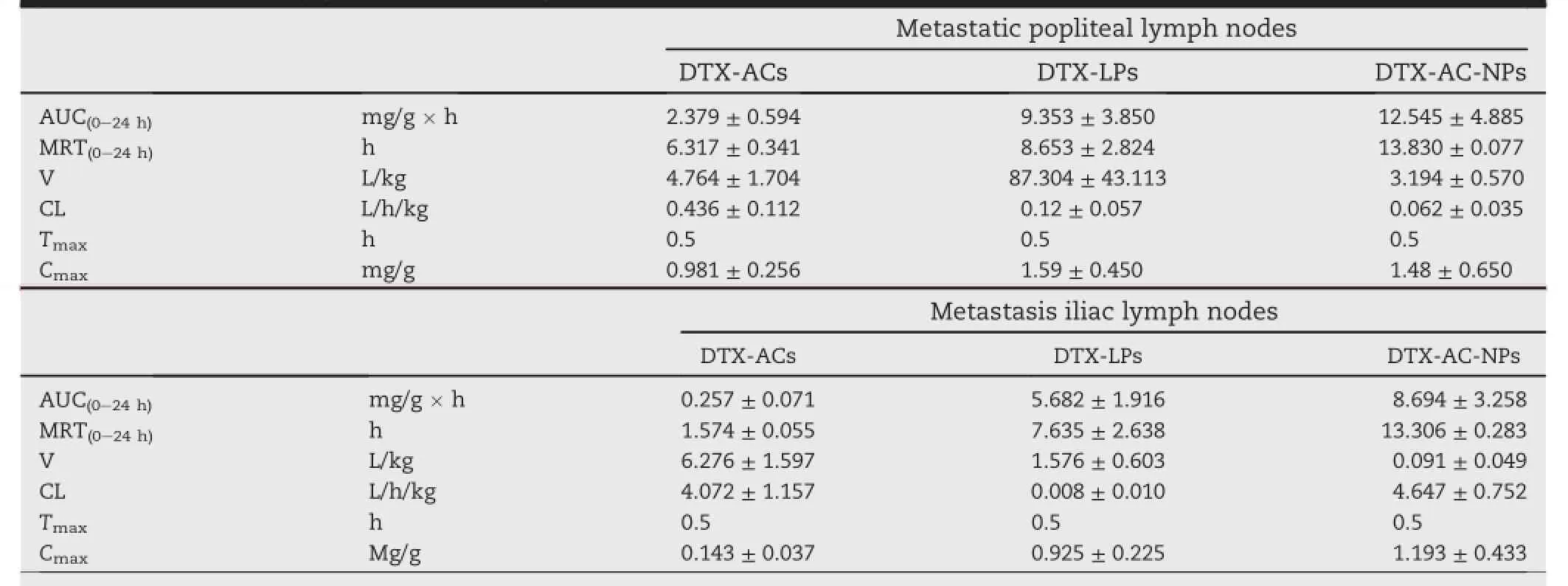
Table 3-Pharmacokinetic parameters in metastatic popliteal and iliac lymph nodes after subcutaneously administration at the dose of 1 mg/kg(Mean±SD,n=6).
4.Conclusion
In this study,we prepared DTX-loaded nanoliposomes by the proliposomemethod,andDTX-loadedactivatedcarbon nanoparticles by modifying nanocarbon.The activated carbon was modif i ed by nitric acid oxidation,and then by centrifuged to obtain modif i ed nanocarbon.We found that the modif i ednanocarbon had smaller particle size and higher adsorption capacity than the original activated carbon.Thus DTX-AC-NPs exhibited properties of uniform particle sizes,high drugloading and adequate dispersion stability.
The substances injected interstitially must traverse the interstitium,which was the f i rst obstacle of the intralymphatic drug delivery system administered interstitially. Particle size was one of the most important factors to determine the extent of absorption from the injection site after s.c. injection[32-34].Largeparticles(roughlylarger than 0.1 μm in diameter)had more diff i culties to pass through the complex structures of the interstitium and tendto be trapped at the site of injection.Thus,in this study,DTX-LPs and DTX-AC-NPs withunder0.1μmparticlesizehadmorelymphatic drainage than DTX-ACs.The interstitial space consisted of the mucopolysaccharides with low isoelectric pointsresulting in a negative charge within a physiological pH range and the presence of narrow aqueous tissue channels[35]allowed the passage of nanocarriers by diffusion[36].The nitric acid oxidation equipped DTX-AC-NPs with more negative charge and stronger hydrophilicity,which were benef i ted for the DTX-AC-NPs to diffuse through these tissue channels.But DTX-AC-NPs remained more drugs at the injection site than DTX-LPs.It might be due to the slow destabilization and degradation over time of the liposomes remaining at the injection site,while losing their drug content.
The lymph node uptake was the other obstacle.Macrophage phagocytosis and reticular cells f i ltration affected in lymph node uptake.It was known that the nanocarriers were transported by the lymphatic and then were blocked by macrophages phagocytosis and f i ltration effect in lymph nodes.The metastatic lymph node localization was also dependent on liposome size.In this study,the DTX-AC-NPs and DTX-LPs were supposed to have similar metastasis lymph node uptake due to similar particle diameter.But because of the functional sustained release of activated carbon nanoparticles,there was a dynamic equilibrium between the chemotherapy drugs loaded in nanocarbons with the free drug around nanocarbons.As the consequence of this phenomenon,DTX-AC-NPs extended DTX residence time in the lymph node and had a larger AUC(0-48h)which thus exhibited better metastatic lymph node uptake.
Acknowledgment
We wish to acknowledge the support of Pharmacy Laboratory Centre and Animal Centre of Shenyang Pharmaceutical University.And this work was supported by the State Key Laboratory(Long-acting and Targeting Drug Delivery System)and the Special Construction Project(Taishan Scholar-Pharmacy Specially Recruited Experts).
REFERENCES
[1]O'Hagan DT,Christy NM,Davis SS.Particulates and lymphatic drug delivery.In:Charman WN,editor.Lymphatic transport of drugs.CRC Press;1992.p.282.
[2]Charman WN,Stella VJ.Lymphatic transport after parenteral drug administration.Lymphatic transport of drugs.CRC Press;1992.p.279-315.
[3]Yang F,Jin C,Jiang Y,et al.Liposome based delivery system in pancreatic cancer treatment:from bench to bedside. Cancer Treat Rev 2011;37:633-642.
[4]Moghimi SM,Moghimi M.Enhanced lymph node retention of subcutaneously injected IgG1-PEG2000-liposomes through pentameric IgM antibody-mediated vesicular aggregation. BBA-Biomembranes 2008;1778:51-55.
[5]Rabac¸a Roque Botelho MF,Tavares Marques MA,Freitas Gomes CM,et al.Nanoradioliposomes molecularly modulated to study the lung deep lymphatic drainage.Rev Port Pneumol 2009;15:261-293.
[6]Okamoto K,Sawai K,Minato H,et al.Number and anatomical extent of lymph node metastases in gastric cancer:analysis using intra-lymph node injection of activated carbon particles(CH40).Jpn J Clin Oncol 1999;29:74-77.
[7]Ito T,Hagiwara A,Takagi T,et al.Local administration of methotrexate bound to activated carbon particles(MTX-CH) for treating cancers in mice.Anticancer Res 2002;23:1401-1404.
[8]Oussoren C,Storm G.Liposomes to target the lymphatics by subcutaneous administration.Adv Drug Deliv Rev 2001;50:143-156.
[9]Gu B,Xie C,Zhu J,et al.Folate-PEG-CKK2-DTPA,a potential carrier for lymph-metastasized tumor targeting.Pharm Res 2010;27:933-942.
[10]Yang F,Hu J,Yang D,et al.Pilot study of targeting magnetic carbon nanotubes to lymph nodes.Nanomedicine 2009;4:317-330.
[11]Madru R,Kjellman P,Olsson F,et al.99mTc-labeled superparamagnetic iron oxide nanoparticles for multimodality SPECT/MRI of sentinel lymph nodes.J Nucl Med 2012;53:459-463.
[12]Lian HY,Hu M,Liu CH,et al.Highly biocompatible,hollow coordination polymer nanoparticles as cisplatin carriers for eff i cient intracellular drug delivery.Chem Commun 2012;48:5151-5153.
[13]Asin L,Ibarra MR,Tres A,et al.Controlled cell death by magnetic hyperthermia:effects of exposure time,f i eldamplitude,and nanoparticle concentration.Pharm Res 2012;29:1319-1327.
[14]Xie P,Xie YM,Song X,et al.Preparation and study of lymphatic targeted hydrophilic carbon black.West China J Pharm Sci 2006;21:337-339[in Chinese].
[15]Eric K,Rowinsky MD.The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med 1997;48:353-374.
[16]Gelmon K.The taxoids:paclitaxel and docetaxel.Lancet 1996;8932:1267-1272.
[17]Fulton B,Spencer CM.Docetaxel,a review of its pharmacodynamic and pharmacokinetic properties and therapeutic eff i cacy in the management of metastatic breast cancer.Docetaxel Drugs 1996;51:1075-1092.
[18]Morikawa Y,Koike H,Sekine Y,et al.Rapamycin enhances docetaxel-induced cytotoxicity in a androgen-independent prostate cancer xenograft model by surviving down regulation.Biochem Biophys Res Commun 2012;419:584-589. [19]Kintzel PE,Michaud LB,Lange MK.Docetaxel-associated Epiphora.Pharmacotherapy 2006;26:853-867.
[20]Ian CS,Steven DH,Andrew WH,et al.Neoadjuvant chemotherapy in breast cancer:signif i cantly enhanced response with docetaxel.J Clin Oncol 2002;20:1456-1466.
[21]Ridwelski K,Gebauer T,Fahlke J,et al.Combination chemotherapy with docetaxel and cisplatin for locally advanced and metastatic gastric cancer.Ann Oncol 2001;12:47-51.
[22]Earhart RH.Docetaxel(Taxotere):preclinical and general clinical information.Semin Oncol 1999;26:8-13.
[23]Verweij J.Docetaxel(Taxotere):a new anti-cancer drug with promising potential?Br J Cancer 1994;70:183.
[24]Piccart MJ,Di LA.Future perspectives of docetaxel(Taxotere) in front-line therapy.Semin Oncol 1997;24:1027-1033.
[25]Baker J,Ajani J,Scottˊe F,et al.Docetaxel-related side effects and their management.Eur J Oncol Nurs 2009;13:49-59.
[26]Wang SJ,Ye TT,Yang BY,et al.7-Ethyl-10-hydroxy camptothecin proliposomes with a novel preparation method:optimized formulation,characterization and invivo evaluation.Drug Dev Ind Pharm 2013;39:393-401.
[27]Song SS,Chen F,Qi H,et al.Multifunctional tumor-targeting nanocarriers based on hyaluronic acid-mediated and pH-sensitive properties for eff i cient delivery of docetaxel.Pharm Res 2014;31:1032-1045.
[28]Yan ZQ,Wang F,Wen ZY,et al.LyP-1-conjugated PEGylated liposomes:a carrier system for targeted therapy of lymphatic metastatic tumor.J Control Release 2012;157:118-125.
[29]Payne NI,Timmins P,Ambrose CV,et al.Proliposomes:a novel solution to an old problem.J Pharm Sci 1986;75:325-329.
[30]Katare OP,Vyas SP,Dixit VK.Proliposomes of indomethacin for oral administration.J Microencapsul 1991;8:1-7.
[31]Oussoren C,Zuidema J,Crommelin DJA,et al.Lymphatic uptake and biodistribution of liposomes after subcutaneous injection.II.Inf l uence of liposomal size,lipid composition and lipid dose.Biochim Biophys Acta 1997;1328:261-272.
[32]Allen TM,Hansen CB,Guo LSS.Subcutaneous administration of liposomes:a comparison with the intravenous and intraperitoneal routes of injection.BBA-Biomembranes 1993;1150:9-16.
[33]Patel HM.Fate of liposomes in the lymphatics.In: Gregoriadis G,editor.Liposomes as drug carriers.New York: Wiley;1988.p.51-61.
[34]Tümer A,Kirby C,Senior J,et al.Fate of cholesterol-rich liposomes after subcutaneous injection into rats.BBA-Gen Subj 1983;760:119-125.
[35]Casley-Smith JR.The f i ne structure and functioning of tissue channels and lymphatics.Lymphology 1980;13:177.
[36]Hawley AE,Davis SS,Illum L.Targeting of colloids to lymph nodes:inf l uence of lymphatic physiology and colloidal characteristics.Adv Drug Deliv Rev 1995;17:129-148.
*Corresponding author.Department of Pharmaceutics,School of Pharmacy,Shenyang Pharmaceutical University,103 Wenhua Road, Shenyang 110016,China.Tel./fax:+86 24 23986313.
E-mail address:pppwwwsss@163.com(W.Pan).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.08.004
1818-0876/©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
 Asian Journal of Pharmacentical Sciences2015年1期
Asian Journal of Pharmacentical Sciences2015年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- Preparation and evaluation of taste masked oral suspension of arbidol hydrochloride
- Degradation kinetic study of lysine in lysine hydrochloride solutions for injection by determining its main degradation product
- Enhancement of solubility and therapeutic potential of poorly soluble lovastatin by SMEDDS formulation adsorbed on directly compressed spray dried magnesium aluminometasilicate liquid loadable tablets:A study in diet induced hyperlipidemic rabbits
- Preparation and evaluation of tamsulosin hydrochloride sustained-release pellets modif i ed by two-layered membrane techniques
- Evaluation of chitosan-anionic polymers based tablets for extended-release of highly watersoluble drugs
