Preparation and evaluation of tamsulosin hydrochloride sustained-release pellets modif i ed by two-layered membrane techniques
Jingmin Wang,Yinghua Sun,Bo Li,Rui Fan,Bing Li,Tengrui Yin, Ling Rong,Jin Sun
Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
Preparation and evaluation of tamsulosin hydrochloride sustained-release pellets modif i ed by two-layered membrane techniques
Jingmin Wang,Yinghua Sun,Bo Li,Rui Fan,Bing Li,Tengrui Yin, Ling Rong,Jin Sun*
Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
ARTICLEINFO
Article history:
Received 21 April 2014
Received in revised form
20 July 2014
Accepted 7 August 2014
Available online 28 August 2014
Preparation
In vitro evaluation
Tamsulosin hydrochloride
Sustained-release pellets
Drug release mechanism
Stability study
The aim of the present study was to develop tamsulosin hydrochloride sustained-release pellets using two-layered membrane techniques.Centrifugal granulator and f l uidizedbed coater were employed to prepare drug-loaded pellets and to employ two-layered membrane coating respectively.The prepared pellets were evaluated for physicochemical characterization,subjected to differential scanning calorimetry(DSC)and in vitro release of different pH.Different release models and scanning electron microscopy(SEM) were utilized to analyze the release mechanism of Harnual®and home-made pellets.By comparing the dissolution prof i les,the ratio and coating weight gain of Eudragit®NE30D and Eudragit®L30D55 which constitute the inside membrane were identif i ed as 18:1 and 10%-11%.The coating amount of outside membrane containing Eudragit®L30D55 was determined to be 0.8%.The similarity factors(f2)of home-made capsule and commercially available product(Harnual®)were above 50 in different dissolution media.DSC studies conf i rmed that drug and excipients had good compatibility and SEM photographs showed the similarities and differences of coating surface between Harnual®and self-made pellets before and after dissolution.According to Ritger-Peppas model,the two dosage form had different release mechanism.
©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
1.Introduction
Tamsulosin hydrochloride(TSH),as a model drug,is a highly selective α1A-adrenoreceptor antagonist.It has been developed to treat lower urinary tract symptoms suggestive of benign prostatic hyperplasia(LUPS/BPH)[1].It is reported that TSH is absorbed rapidly and completely in intestinal and eliminated slowly after oral administration,which may bring many adverse effects.Therefore TSH is a perfect candidate drug for modif i ed-release dosage form to modulate the release rate of drug and the absorption in GIT[2].
Pellets,as carrier of active ingredient,have been applied extensively in sustained-release(SR)formulations.Compared with conventional single-unit drug delivery system,pellets are believed to represent many bio-pharmaceutical advantages.For example,pellets can homogeneously distribute in gastrointestinal(GIT),increase the contact area between drug and GIT,decrease local drug concentration and irritation,and provide stable plasma pro fi les and high bioavailability.In addition,pelletsaremorereproducibleandrepeatable because of its small unit,especially in the stomach[3].
The release homogeneity of commercial product Harnual®was not good because of the particle size distribution of its pellets.To overcome the defect,we employed a different preparation craft to prepare uniform drug-loaded pellets and we gained a formulation with nice release homogeneity.The self-made TSH sustained-release(SR)pellets consist of uniform blank pellets,the layer of drug-loading and two-layered coating membranes.So far,there are many approaches to make SR formulations[4-8].In our study,centrifugal granulator was employed to prepare blank pellets and drug-loading pellets while fl uidized-bed coater was used for coating. Consideringthelowertoxicityandlessenvironmental concern,aqueous polymeric dispersions were chosen as coating materials.In general,through varying the type or weight gain of coating material,we could achieve the desired release pro fi le.However,one single type aqueous polymer dispersion is not enough to produce a wonderful drug release behavior sometimes.Binary blends of polymers were proposed to make up the defect.This technique can not only provide broad range of drug release patterns,but also improve fi lm thermo-sensitivity[9].Eudragit®NE30D and Eudragit®L30D55 were utilized as the inside coating material to achieve a particular pH-sensitive release[10,11].Eudragit®L30D55,as the enteric coating material,can prohibit drug release in the simulated gastric fl uid and easily be dissolved in the intestinal environment as pore-forming agents,which is also the reason for selecting Eudragit®L30D55 as the outside coating polymer [3].The model of TSH SR pellets is depicted as Fig.1.
2.Materials and methods
2.1.Materials
TSH(99.8%purity)was purchased from Zhejiang Jinhua pharmaceutical Co.,Ltd(Zhejiang,China),Microcrystalline cellulose(MCC)(Avicel PH101)was purchased from Huzhou Zhanwang Pharmaceutical Co.,Ltd,(Huzhou,China),HPMC was a gift from Shanhe Pharmaceutical Co.,Ltd,(Anhui, China),methacrylic acid copolymers(Eudragit®NE30D and Eudragit®L30D55)were kindly provided by Degussa(Esson, Germany),No.3 hard gelatin capsules were purchased from Suzhou Capsule Co.(Suzhou,China),Commercially available controlled-releaseTSHcapsule(Harnal,0.2mg/capsule, Yamanouchi Pharmaceutical Co.Ltd.,Japan)was chosen for comparison.All organic solvents were of high-performance liquid chromatography(HPLC)grade.All other chemicals were of analytical grade.
2.2.Methods
2.2.1.Drug-excipient interaction studies
Differential scanning calorimetry(DSC)was used to investigate the possibility of drug-excipient interaction.Pure drug, the mixture of excipients and drug-excipient mixtures were separately sealed in aluminum cells at a heating rate of 10°C/ min in a nitrogen atmosphere over a temperature range of 30-300°C.Alumina was utilized as the reference standard.
2.2.2.The preparation of blank pellets
Centrifugal granulator was chosen to prepare blank pellets and subsequent drug spraying.The particle size distribution of blank pellets is 0.3-0.4 mm.Pelletization by centrifugal granulator is an advanced technique.Being a multivariable process,it is of great signif i cance to know and control the process variables and status.Rotational speed of plate,the ratio of spray rate of binding solution and rotating rate of powder feeder and grounding time were found to be critical parameters affecting the characteristics of pellets.Table 1 denotes the optimized process parameters.
400 g microcrystal cellulose(MCC)was loaded into the chamber of centrifugal granulator,then moistened with purif i ed water.Adding MCC via a hopper to the above wet mass after 10 min wetting.In the process,we must keep all the parameters constant.The pellets need to be granulated for another 4 mineven thoughthe particle size distribution meets our requirement.The f i nal products were discharged from the chamber and half-dry in the room temperature.Then,the pellets were placed into oven of 60°C for 2 h.Finally,sieving the pellets through 40-50 mesh screen.
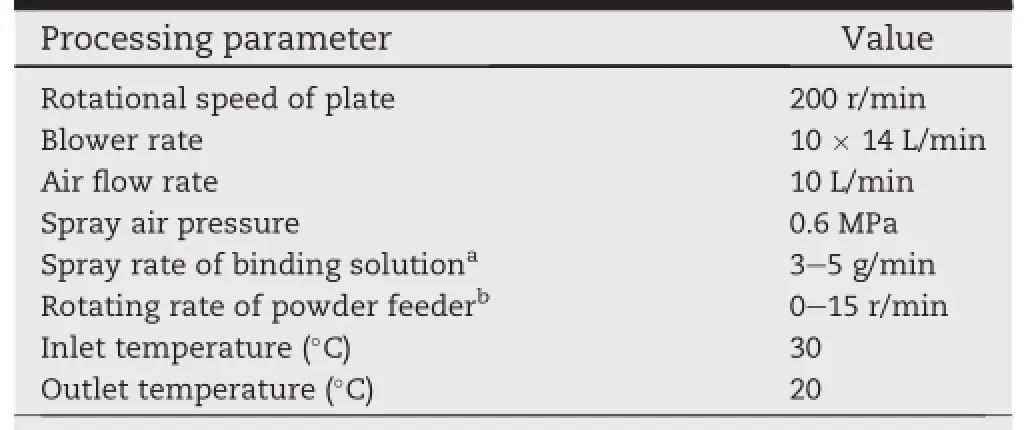
Table 1-The optimized parameters of centrifugal granulator for the preparation of blank pellets.
2.2.3.The preparation of TSH pellets
TSH SR capsule contains 0.2 mg active ingredient which occupies 0.13%to the whole dosage form.TSH was dissolved to solution and sprayed to blank pellets homogeneously to meet the requirement of content uniformity.Firstly,desired tween-80(2.3%,wt/wt,based on the solution)was weighed and put into 1000 ml distilled water,adding TSH to the above solution, then sonicating for 10 min,standby for 5 min,which goes for 3 times again and again until the TSH dissolved entirely. Hydroxypropyl methylcellulose(HPMC)(0.8%,wt/wt,based on the solution)was added to 1000 ml purif i ed water which had been heated to 50°C previously,stirring constantly until it fully dissolved.Pouring the above solution into drug solution when it cools down to room temperature.Mixing for 5 min and laying aside.The amount of drug and excipients must strictly comply with the drug solution formulation.The processing parameters were the same as the preparation of blank pellets except Spray rate of drug solution(3 g/min)and Rotating rate of powder feeder(The rotating rate of powder feeder was 0 r/min for the f i rst 5 min,and subsequently 8 r/ min until the end).Through adjusting the ratio of drug solution and MCC powders,we could avoid the agglomeration of pellets.
2.2.4.Coating of TSH sustained-release pellets
The operating procedure of inside coating material was as follows:Shaking the polymer dispersions every time before using to promise reproducibility.The desired amount of Eudragit®NE30D was stirred by a magnetic stirrer,dispersing micronized talc(55%,wt/wt,based on the dry polymer weight) in distilled water which is the same amount as Eudragit®NE30D polymer dispersion with a high-speed disperser for 5 min.The talc solution was poured into the above Eudragit®NE30D solution,making the total coating suspension with a solid content of 15%.Eudragit®L30D55 and the same amount of purif i ed water were weighed respectively and mixed. Triethyl citrate(TEC)(20%,wt/wt,based on dry polymer weight)was added to the mixture,stirring suff i ciently.Then Eudragit®L30D55 was blended with Eudragit®NE30D under the magnetic stirrer.Mixing for 30 min and laying aside.
Theoutsidecoatingmaterialwasverysimple.As mentioned above,desired Eudragit®L30D55 was weighed and stirred with a magnetic stirrer.Micronized talc(20%,wt/wt, based on dry polymer weight)was dispersed in the distilled water which was also the same amount as Eudragit®L30D55. Adding the talc solution prepared above and desired TEC(20%, wt/wt,based on dry polymer weight)to Eudragit®L30D55 dispersion under stirring.Mixing for 10 min and standby.
In the coating process,the coating dispersion must be stirred continuously.After the inside coating,Talc(1%,wt/wt, based on pellets)was mixed with pelletsto avoid tack problem before moving into the oven and cured for 20 h at 40°C.The outside coating was just need curing for 2 h without blending with anything.
Coating conditions:air source pressure:5.2 bar,atomization pressure:1.8 bar,inlet temperature:25°C,outlet temperature 20°C,material temperature 25°C,air f l ow:60-70 m3/ h,spray rate:6 g/min.
2.2.5.Study of characteristics of prepared pellets
Three batches of qualif i ed products were measured to determine the characteristics of pellets,such as Percentage Yield of pellets,angle of repose,friability.Table 2 summarized the physical parameters of three batches of pellets.The actual Percentage Yields of pellets were calculated by the following formular(1).
The f i xed base cone method was utilized to evaluate the angle of repose of pellets.A funnel was f i xed at 1 cm above the horizontal f l at surface until the apex of the conical pile just touched to the tip of the funnel,allowing the pellets to fall freely.Measuring the height and diameter of the cone and calculating the angle of repose by the following formular(2).
where h,r expresses the Height of pile and the Radius of pile, respectively.
The friability of the pellets were determined as%weight lossafter100revolutionsof10gofpelletsinafriabilator[12,13].
2.2.6.Determination of drug content
The drug content was measured by the method of HPLC.The HPLC system consisted of a chromaster 5110 pump and chromaster 5410 detector(HITACHI chromaster Japan).A Thermo C18reverse-phase column(5 μm,150×4.6 mm)was fi tted in a column oven with the temperature of 40°C.The mobile phase was made up of acetonitrile and perchloric acid (dissolve 8.7 ml of perchloric acid and 3.0 g of sodium hydroxide in 1900 ml distilled water.Adjust with 1N sodium hydroxide to a pH of 2.0,and add suf fi cient water to 2000 ml) with the ratio of 31:69(v/v).The injection volume was 10 μl, the fl ow rate was 1.3 ml/min,and the UV detector wavelength was set at 225 nm.
From each batch of coated pellets, fi ne powders which were ground previous with a mortar and pestle were accurately weighed(equivalent to approximately 0.5 mg TSH)and placed into a 25 ml volumetric fl ask.10 ml NaOH solution (0.05 mol/l)was added,then putting the fl ask into a water bath which had been heated to 50°C for 10 min,shaking well.Themixture was sonicated for 20 min.After cooling to room temperature,the solution was diluted with miscible liquids of HCl solution(0.2 mol/l)and acetonitrile(1:5).Sonicated again, until the drug was dissolved completely.The drug solution was centrifuged at 3500 r/min for 5 min,passing the supernatant liquid through a 0.45-μm membrane f i lter.The f i ltrate was then analyzed by HPLC[14].
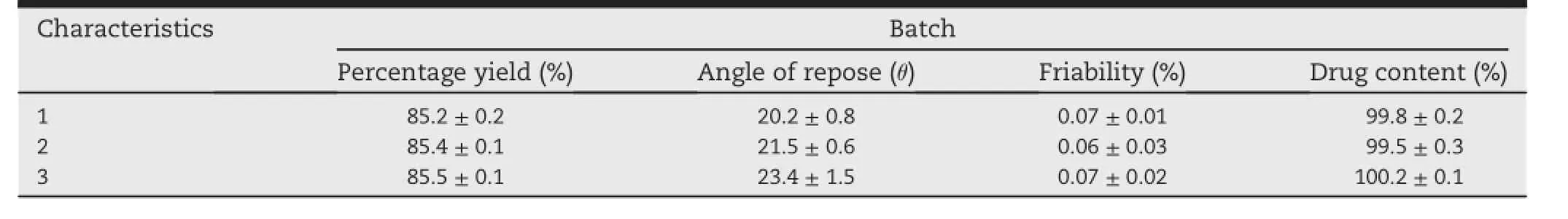
Table 2-The physical parameters of three batches of pellets.
2.2.7.Drug release measurements and comparisons
In vitro drug dissolution test was performed using USP XXVII Type 2 dissolution apparatus(paddles method).Before the test,500 ml simulated gastric f l uid(SGF)without pepsin (withdraw 5.7 ml HCl to 1000 ml purif i ed water,and add 2 g NaCl)containing polysorbate 80(0.003%,wt/wt)was put into dissolution containers of a ZRS-8G Test Dissolution Tester (Tianjin University Radio Factory,Tianjin,China)which could keep the solution at 37±0.1°C.Then the TSH SR capsule equivalent to 0.2 mg TSH with a sinker was placed into it, meanwhile,rotating speed of paddles was setted at 100 r/min. At 2 h after the start of the test,withdrawing 10.0 ml of the solution under test.Draining the SGF with the help of a 100-mesh screen and rinsing the screen while adding the simulated intestinal f l uid(pH 7.2,phosphate buffer)previous warmed,then continue the test.At each predetermined time point,10.0 ml of the solution was withdrawn and replaced by the same volume of fresh solution until 4 h(in the simulated intestinal f l uid).All the sample solutions were f i ltered through 0.22 μm f i ltration membrane and analyzed by HPLC as the assay of drug content,with the injection volume of 20 μl[14].
In vitro dissolution test could ref l ect the drug release kinetics in vivo.In order to better evaluate the similarity between home-made capsule and Harnual®,different pH of phosphate bufferas dissolution mediumwereemployed,such as 5.0,5.5,6.2,6.8 and water.
As the tool of similarity evaluation,the similarity factor(f2) plays an important role in the comparison of dissolution prof i les.f2is a logarithmic transformation of the sum-squared error of differences between the test and reference product over all time points[15]:
where log indicates logarithm based on 10.It is suggest that two dissolution prof i les can be concluded to be similarity when f2value is more than 50.Generally,only one time point can be used to calculate f2with the release amount reaching 85%.
2.2.8.Scanning electron microscopy
To evaluate the coating surface of coating polymer membrane,scanning electron microscopy(SEM,ZEISS,Carl Zeiss Jena)images were achieved from the pellets of Harnual®and self-made before and after dissolution.For further analyzing the drug release mechanisms,the pictures were taken at magnif i cation of 400×.
2.2.9.Drug release mechanism
It was concluded that there were three possible pathways for drug release from the inside of polymeric membrane to outside medium:(1)the drug distributed inside the membrane and migrated into the consistent polymeric network,then redistributed in the framework and dispersed to the outside medium;(2)the drug dispersed to outside medium through tiny holes or cracks on the membrane;(3)the coalesces of the above two[16-18].All kinds of mathematical models were put forward to study the dissolution behavior in vitro including zero-order[19],f i rst-order[20],Higuich model[21],Hixson--Crowell model and Baker-Lonsdale model[22].By linear or nonlinear least-squares f i tting methods,the optimum values of the parameters expressed in each equation were obtained. Regression analysis was carried out and best f i ts were calculated on the basis of correlation coeff i cient as r2.
In addition,for further analysis of drug release mechanism,Ritger-Peppas model[23]was used.The equation of Ritger-Peppas model was the following formular(3).Qtexpressed the accumulation release at time t,K denotes the release rate constant.n is the special parameter describing the release mechanism.If“n”≤0.45,the mechanism of drug release was Fick-diffusional.If 0.45<“n”<0.89,the drug release mechanism was non-Fick dispersion including Fickdiffusional and relaxation.If the“n”≥0.89,only the relaxation played the main role in drug release.
2.2.10.Stability study
Put the home-made pellets into the condition of high temperature(40°C,60°C),high humidity(RH 75%,RH 92.5%)and light(4500±500)Lx for 10 d.Take out the sample at 5 d and 10 d respectively.Inspect the contents,related substances and dissolution release of samples.
3.Results and discussion
3.1.Drug-excipient interaction by differential scanning calorimetry(DSC)
DSC curves obtained for TSH,mixture of excipients and drugexcipients mixture are shown in Fig.2.The DSC thermogram for TSH showed a sharp melting endothermic peak at 233.2°C. The mixture of excipients did not show any characteristic peaks.There was no shift in the endothermic peak of TSH in the drug-excipients mixture,indicating good compatibility of the drug with all the excipients.
3.2.Effect of processing parameters of centrifugal granulator on the preparation of blank pellets
As stated above,rotational speed of plate/the ratio of spray rate of binding solution and rotating rate of powder feeder/ grounding time were processing parameters,which have potential in fl uence on the morphologic characteristic and rheological parameters of pellets.In our study,a 33full factorial design was used to optimize the processing variables [16].The responses studied were the yield of 0.3-0.4 μm pellets,angle of repose,friability,surface roundness.In general, fi xing other variable quantities,the particle size of pellets decreased with the increase of rotational speed of plate.The ratio of spray rate of binding solution and rotating rate of powder feeder was of great importance to the yield of pellets. The particle size of pellets and the agglomeration condition of pellets would increase when the ratio was much higher.In contrast,the pellets would not grow up and MCC powders became superf l uous.With the increase of grounding time,the particle size distribution tended to be wider and shift to large size.In addition,the bulk intensity and sphericity were increased,meanwhile,the friability was decreased.The grounding time had few inf l uence on the angles of repose.The optimizedprocessing parameters were determined as follows: rotational speed of plate:200 r/min,the ratio of spray rate of binding solution and rotating rate of powder feeder is 1:1, grounding time:4 min.
3.3.Inspect of the amount of tween-80 and HPMC in TSH solution
TSH was slightly soluble in water,thus,tween-80,as solubilizing agent,was added to the drug solution.Separating out crystals or not was chosen as the evaluation standard to determine the formulation amount of tween-80.2.0%,2.3%, 2.5%tween-80 was added to 2000 ml TSH solution respectively,and sonicated for 10 min in parallel,laid aside for 10 min,which was performed in triplicate.The result suggested that some drug crystals were still undissolved when 2.0%tween-80 was added.However,2.3%and 2.5%tween-80 both made the TSH solution to be transparent.Finally,2.3% tween-80 was conf i rmed to the last formulation quantity.
In the process of drug spraying,HPMC played the role of adhesion,so it could cohere the MCC powders drying the drug pellets in time to blank pellets.Different concentrations of HPMC solution including 0.5%,0.8%,1.0%were prepared to evaluate their inf l uence on the procedure.MCC powders became small particles by itself under the spraying of 0.5% HPMC solution resulting in low drug-loading capacity.The viscosity of 1.0%HPMC solution was high enough to agglomerate the blank pellets on the bottom of centrifugal granulator so that the spraying process could not go on smoothly.However,0.8%HPMC solution could avoid the above problems and made the industrial manufacture into reality.
3.4.The temperature and relative humidity of environment for coating
The coating temperature not only inf l uences the coating process,but also determines the quality of membrane.In general,10-20°Cwasdemandedovertheminimummembrane formation temperature.Too high or low temperature could affect the intact of the membrane and change the drug release behavior consequently.Eudragit®NE30D requires coating temperature at 22-25°C,and the highest temperature was 28°C which made the pellets agglomeration during coating.In our experiment,we controlled the inlet temperature at 25°C and the outlet temperature at 20°C.
Relative humidity was another signif i cant factor affecting coating procedure.Static electricity would be produced between pellets and chamber wall of f l uidized-bed coater when the relative humidity was too low,resulting in nonuniform coating.Pellets would be agglomerated under the high relative humidity,changing the pellets'condition of circulation.The coating process will be performed successfully under the 30%-50%relative humidity of the environment around according to experience.
3.5.Effect of concentration of aqueous polymer dispersions on drug release and coating process
Generally,aqueous polymer dispersions contain 30%solid polymers.The dispersions need to be diluted to appropriate concentration before coating.In our study,10%,15%,20% aqueous dispersion were prepared to explore the effect of concentration of coating solution on the drug release and coating process.10%and 15%coating solution both could complete the coating successfully,nevertheless,the concentration of 10%consumes much more time to gain the identical coating level.The pellets appeared to conglutinate under the spraying of 20%aqueous dispersion,which could not meet the requirement of production in industrial-scale.Therefore,we usually dilute the aqueous polymer dispersion to 15%.Under the same coating parameters,the pellets with the equal coating level represent the consistent release kinetics even through the concentration of coating dispersion is different. Fig.3A expressed the dissolution prof i les of TSH SR pellets coated by different concentrations of coating solutions.
3.6.Effect of the ratio of Eudragit®NE30D and Eudragit®L30D55 on the release of TSH SR pellets

Table 3-The f2of dissolution prof i les of home-made capsule and marketed one in different dissolution medium.
Different ratios of Eudragit®NE30D and Eudragit®L30D55 were prepared containing 15:1,18:1 and 20:1.A batch drugload pellets which was divided into triplicate were coated by the different coating materials with the same coating level. The differences of drug release prof i les were evaluated among three baches of TSH SR pellets.The experimental result manifested that few difference was appeared for the f i rst 2 h ofdissolution release.However,the last4 h ofdrugdissolution in the simulated intestinal f l uid presented huge differences. With increase of the amount of Eudragit®NE30D,the accumulative release decreased.The release amount of drug is much lower than Harnual®with the ratio of 20:1.Finally,the ratio of 18:1 was chosen by comprehensive analysis.Fig.3B denoted the release prof i les of TSH SR pellets inf l uenced by the ratio of Eudragit®NE30D and Eudragit®L30D55.
3.7.Effect of f i lm thickness on drug release
The ratio of Eudragit®NE30D and Eudragit®L30D55 was controlled at 18:1 and some coated pellets were taken out from discharge hole of f l uidized-bed coater respectively when the coating weight gain reached to 7%,10%,12%.As can be seen,the accumulative release was signif i cantly decreased with the increase of coating amount because of the increased diffusion path length.The drug was released entirely at 3 h with 7%coating amount,thus,the membrane did not play the role of prolonging drug release.When the coating weight gain reached to 12%,the accumulative release was near to Harunal®except to the f i rst 2 h.After coating the outside membrane,the release rate would decrease overall compared with the commercially available product.Therefore,we controlled the coating weight gain at the range of 10%-11%.Fig.3C depicted the effect of the coating thickness on drug release.
3.8.In vitro dissolution testing
3.8.1.The release prof i les of uncoated pellets,one-layer coated pellets,two-layer coated pellets and Harnual®
Fig.4A represented the dissolution prof i le of uncoated pellets. We could conclude that the drug was dissolved completely at about 30 min and the dissolution would not be inf l uenced by the solubility of drug.The release amount of one-layer coated pellets was a little higher than Harnual®and the two-layer membranes coatedpelletshad thesame releasebehaviorwith the marketed products,as shown in Fig.4B.The reason was that pellets were agglomerated together by Eudragit®L30D55 at the f i rst 2 h of the dissolution test,reducing the contacting area between pellets and dissolution medium.Therefore,the release amount was decreased notably in the SGF.
3.8.2.Drug release comparison
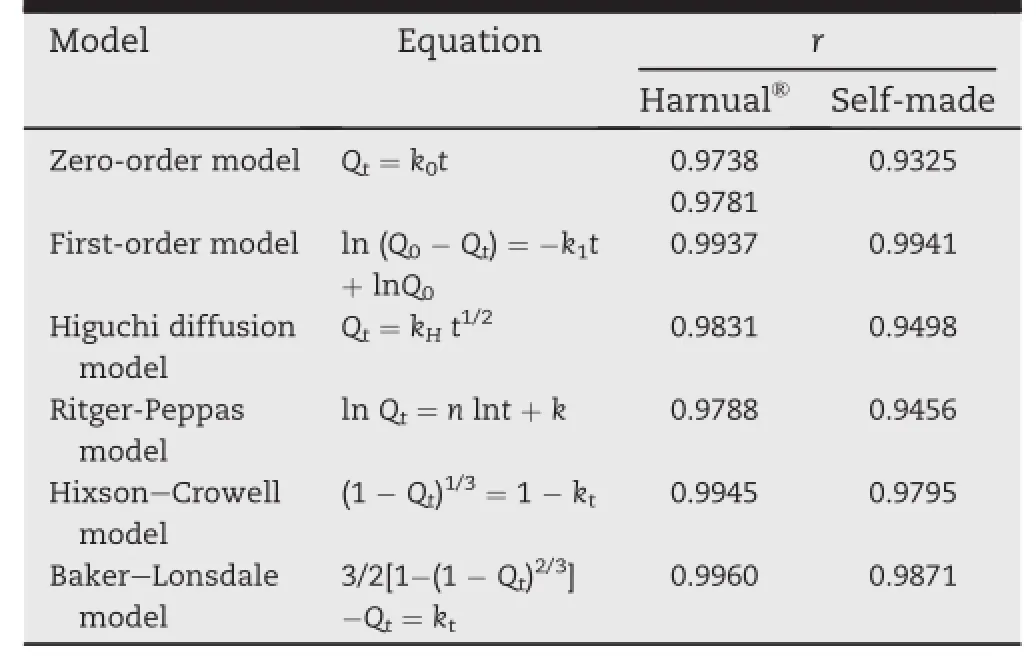
Table 4-Models of drug release and correlation coeff i cients.
The release prof i les of self-made capsule and Harnual®were compared in different dissolution medium.From the dissolution result,we could reach a conclusion that the home-made capsules were similar to the marketed product in 7.2,6.8 and water.While,the accumulation release of self-made capsule was a little higher than Harnual®in the dissolution medium of 5.0,5.5,6.2.The two dosage forms were similar through comparing the f2of dissolution prof i les in different pH (expressedintheTable3).Thedissolutionprof i leswereshown in Fig.5.
3.9.SEM experiments
SEM images of dry pellets before and after dissolution are shown in Fig.6.From A1,B1 photos we can conclude that the market product and self-made pellets are both have smooth coating surface with a few pores before dissolution.Many pores appeared after dissolution which can be seen from A2.Compared with market product,the appearance of self-made pellets which can be seen from B2 was rough and some cracks appeared.This may contribute to different preparation graft in the two dosage forms.
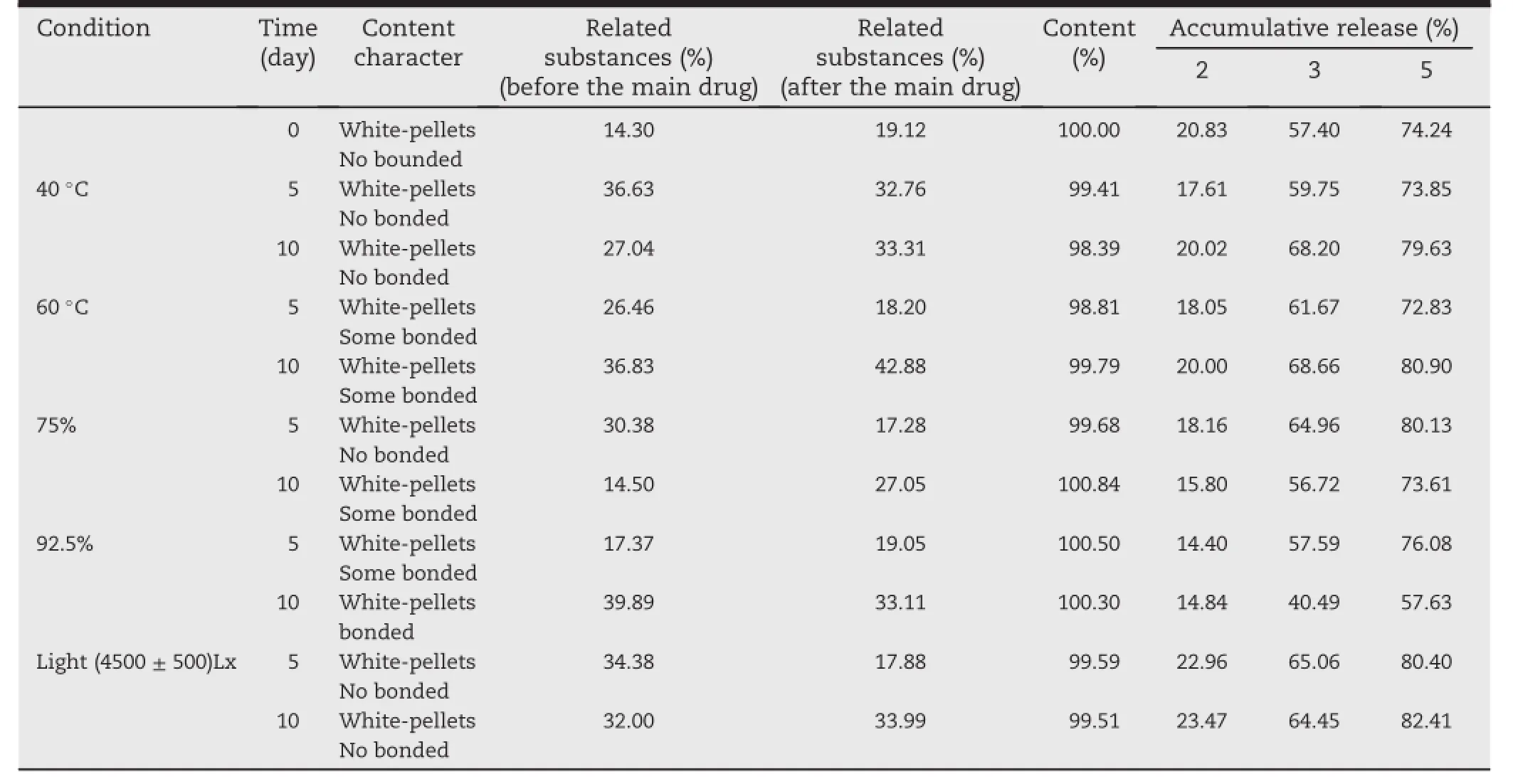
Table 5-exposed to different conditions. Stability of f1
3.10.The mechanism of drug release
As shown in Table 4,First-order model,Hixson-Crowell model and Baker-Lonsdale model were all f i tted for Harnual®, and Baker-Lonsdale model was the best f i tted one.The result demonstrated that both free diffusion and relaxation played important role in the drug release of marketed product.Firstorder model,Baker-Lonsdale model were conformed to home-made capsule.This result expressed that free diffusion was the main drug release mechanism.The“n”of Ritger-Peppas model were 0.8327and 0.7386 of home-made and Harnual®respectively.From the result,we can conclude that both free diffusion and relaxation plays the role in the drug release of Harnual®and self-made pellets.This result is identical with the above conclusion.
3.11.The results of stability study
As shown in Table 5,the contents,related substances of samples with high temperature,high humidity and light at 5 d and 10 d are both unchanged.However,the dissolution release of samples with high humidity(RH 92.5%)at 10 d was decreased signif i cantly.This indicated that the permeability of the coating membrane was inf l uenced seriously.Therefore, the pellets must be kept away from moisture during the package and transportation and stored in a cool and dark place away from moisture.
4.Conclusions
TSH SR pellets capsule was successfully manufactured in a laboratory-scale.During the preparation process,the parameters of equipments must be well controlled to promise the quality of pellets.The drug release behavior of two dosage forms were different through the analysis of release models and SEM experiment.To testify the fungibility of home-made capsules,the experiment of bioequivalence may be needed to carry out.
REFERENCES
[1]O'Leary MP.Tamsulosin:current clinical experience.Urology 2001;58:42-48.
[2]Chapple C,Andersson KE.Tamsulosin:an overview.World J.Urol 2002;19:397-404.
[3]Marvola M,Nyk¨anen P,Rautio S,et al.Enteric polymers as binders and coating materials in multiple-unit site specif i c drug delivery systems.Eur J Pharm Sci 1999;7:259-267.
[4]Kim MS,Jun SW,Lee S,et al.The inf l uence of surelease and sodium alginate on the in-vitro release of tamsulosin hydrochloride in pellet dosage form.J Pharm Pharmacol 2005;57:735-742.
[5]Kim MS,Park GD,Jun SW,et al.Controlled release tamsulosin hydrochloride from alginate beads with waxy materials.J Pharm Pharmacol 2005;57:1521-1528.
[6]Maeda A,Shinoda T,Ito N,et al.Evaluating tamsulosin hydrochloride-released microparticles prepared using single-step matrix coating.Int J Pharm 2011;408:84-90.
[7]Zhang X,Tang X,Yang R.Development of a tamsulosin hydrochloride controlled-release capsule consisting of two different coated pellets.Drug Dev Ind Pharm 2009;35:26-33.
[8]Kim JS,Kim MS,Park HJ,et al.Statistical optimization of tamsulosin hydrochloride controlled release pellets coated with the blend of HPMCP and HPMC.Chem Pharm Bull 2007;55:936-939.
[9]Siepmann F,Siepmann J,Walther M,et al.Polymer blends for controlled release coatings.J Control Release 2008;125:1-15.
[10]Lecomtea F,Siepmanna J,Waltherb M,et al.Blends of enteric and GIT-insoluble polymers used for f i lm coating: physicochemical characterization and drug release patterns. J Control Release 2003;89:457-471.
[11]Cuppok Y,Muschert S,Marucci M,et al.Drug release mechanisms from Kollicoat SR:Eudragit NE coated pellets. Int J Pharm 2011;409:30-37.
[12]Raval MK,Ramani RV,Sheth NR,et al.Formulation and evaluation of sustained-release enteric-coated pellets of budesonide for intestinal delivery.Int J Pharm Investig 2013;3:203-211.
[13]Prasad MB,Vidyadhara S,Sasidhar RLC,et al.Development and evaluation of diltiazem hydrychloride controlled-release pellets by f l uid bed coating process.J Adv Pharm Technol Res 2013;4:101-107.
[14]USP 23:Tamsulosin hydrochloride sustained-release capsule.
[15]Moore JW,Flanner HH.Mathematical comparison of dissolution prof i les.Pharma Tech 1996;20:64-74.
[16]Siepmann F,Wahle C,Leclercq B,et al.pH-sensitive f i lm coatings:towards a better understanding and facilitated optimization.Eur J Pharm Biopharm 2008;68:2-10.
[17]Nakamura K,Nara E,Akiyama Y.Development of an oral sustained release drug delivery system utilizing pH-dependent swelling of carboxyvinyl polymer.J Control Release 2006;111:309-315.
[18]Yang QW,Flament MP,Siepmann F,et al.Curing of aqueous polymeric f i lm coatings:importance of the coating level and type of plasticizer.Eur J Pharm Biopharm 2010;74:362-370.
[19]Samuelov Y,Donbrow M,Friedman M.Sustained release of drugs from ethylcellulose polyethylene glycol f i lms and kinetics of drug release.J Pharm Sci 1979;68:325-329.
[20]Lapidus H,Lordi NG.Some factors affecting the release of a water-soluble drug from a compressed hydrophilic matrix. J Pharm Sci 1966;55:840-843.
[21]Higuchi T.Rate of release of medicaments from ointment bases containing drugs in suspension.J Pharm Sci 1961;50:874-875.
[22]Baker RW,Lonsdale HK.Controlled release of biologically active agents.In:Tanquary AC,Lacey RE,editors.New York: Plenum;1974.p.15-71.
[23]Ritger PL,Peppas NA.A simple equation for description of solute release II.Fickian and anomalous release from swellable devices.J Control Release 1987;5:37-42.
*Corresponding author.Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China.Tel.:+86 24 23986325; fax:+86 24 23986320.
E-mail address:sunjin0529@aliyun.com(J.Sun).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.08.009
1818-0876/©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
 Asian Journal of Pharmacentical Sciences2015年1期
Asian Journal of Pharmacentical Sciences2015年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- Preparation and evaluation of taste masked oral suspension of arbidol hydrochloride
- Targeted delivery of docetaxel to the metastatic lymph nodes:A comparison study between nanoliposomes and activated carbon nanoparticles
- Degradation kinetic study of lysine in lysine hydrochloride solutions for injection by determining its main degradation product
- Enhancement of solubility and therapeutic potential of poorly soluble lovastatin by SMEDDS formulation adsorbed on directly compressed spray dried magnesium aluminometasilicate liquid loadable tablets:A study in diet induced hyperlipidemic rabbits
- Evaluation of chitosan-anionic polymers based tablets for extended-release of highly watersoluble drugs
