Design and comparative in-vitro and in-vivo evaluation of starch-acrylate graft copolymer based salbutamol sulphate sustained release tablets
Pnkj Kumr,Ashok Lxmnro Gnure,Bhrt Bhushn Suuhi, Shuhnjli Shukl,Pooj Uphyy
aSchool of Pharmaceutical Sciences,Siksha O Anusandhan University,Khandagiri Square,Bhubaneswar, Orissa 751030,India
bSahyadri College of Pharmacy,Sangola,Solapur,M.S.413307,India
cDepartment of Pharmaceutics,Indian Institute of Technology(Banaras Hindu University),Varanasi 221005,IndiadDepartment of Advanced Pharmaceutical Science,Manipal University,Karnataka 576104,India
Design and comparative in-vitro and in-vivo evaluation of starch-acrylate graft copolymer based salbutamol sulphate sustained release tablets
Pankaj Kumara,*,Ashok Laxmanrao Ganureb,Bharat Bhushan Subudhia, Shubhanjali Shuklac,Pooja Upadhyayd
aSchool of Pharmaceutical Sciences,Siksha O Anusandhan University,Khandagiri Square,Bhubaneswar, Orissa 751030,India
bSahyadri College of Pharmacy,Sangola,Solapur,M.S.413307,India
cDepartment of Pharmaceutics,Indian Institute of Technology(Banaras Hindu University),Varanasi 221005,IndiadDepartment of Advanced Pharmaceutical Science,Manipal University,Karnataka 576104,India
ARTICLEINFO
Article history:
Received 6 May 2014
Received in revised form
9 November 2014
Accepted 4 December 2014
Available online 22 December 2014
Salbutamol sulphate
Methyl methacrylate
Graft copolymers
Acetylated starch
Korsmeyer's model
In vitro and in vivo
The present work deals with the development of controlled release tablets of salbutamol sulphate(SS)using graft copolymers of methyl methacrylate(St-g-PMMA and Ast-g-PMMA) on starch and acetylated starch.Formulations were evaluated for physical characteristics like hardness,friability,drug release,drug content and weight variations,which fulf i lled all the off i cial requirements of tablet dosage form.The release rates from formulated matrix tablets were studied at SGF(pH 1.2)followed by SIF(pH 6.8).Drug release from the graft copolymer based tablets was found to be sustained upto the 14 h with>75%drug release. The in-vitro release study showed that the graft copolymer based matrix formulations(F3& F4)exhibited highest correlation value(r2)for higuchi kinetic model and Korsmeyer's model with n values between 0.61 and 0.67 proved that release mechanisms were governed by both diffusion and erosion mechanism.There was no signif i cant difference in the pharmacokinetic parameters(tmax,Cmax,AUC,Ke,and t1/2)of the graft copolymers matrices and HPMC K100M matrix tablets,indicating their comparable sustained release effect.The potential of graft copolymers to sustain the drug release is well supported by in-vivo pharmacokinetic studies and their adequate physicochemical properties make them promising excipients for controlled drug delivery system.
©2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1.Introduction
Controlled release technology has rapidly emerged over the past few decades as a new interdisciplinary science that offers novel approaches for delivery of bioactive agents into systemic circulation at a predetermined rate,achievement of optimumtherapeuticresponses,prolongedeff i cacyand decreased toxicity[1].Salbutamol Sulphate(SS),a directly acting sympathomimetic drug,is a good candidate for controlled release formulations since its short half life(2-4 h) which necessitates frequent administration to maintain constant therapeutic drug levels,but it is challenging because of its high water solubility[2].In recent years,use of natural polymers such as starch,cellulose,chitosan etc.as carriers in controlled drug delivery applications has attracted the attention of investigators because of their inherent biocompatibility,biodegradabilityandbiosafety[3-5].Butnatural polymers share some common disadvantages like poor f l ow properties,inadequate compression behavior,thermal liability and enormous swelling owing to their hydrophilic nature. Hydrophilicity results in premature release of drug in the stomach/upper intestine,and therefore they should be protected while gaining entry into stomach and small intestine. This can be achieved by the modif i cation of polysaccharides, such as cross linking,addition of protective coating,or grafting using acrylic monomers[6-8].Among the currently available graft copolymers,starch-based graft copolymers has drawn considerable attention due to their potential value as directlycompressibleexcipientsforcontrolledrelease matrices and methyl methacrylate was chosen for grafting becauseofitsknownbiocompatibilityandnon-toxicbehavior, together with its hydrophobicity and ease of polymerization [9,10].In terms of controlled release formulations,reservoir and matrix type tablets are most commonly used for modif i ed release formulation.Especially matrix tablets,in which drug particles are embedded in the matrix core of the retardant polymeric material,formulated with direct compression technique,one of the best method to sustain the release rate effectively over a period of 10 h[11].The afore mentioned facts,directed our interest to design oral controlled release matrix tablets of SS using starch-based graft copolymers as hydrophobic inert matrix.Evaluation of tablets for various physical characteristics,in-vitro release study and in-vivo bioavailability studies in rabbits upon oral administration were performed.Release kinetics are studied in depth by fi tting dissolution pro fi le in various kinetic models(viz.zero order, fi rstorder,Higuchi,Hixon-CrowellandKorsmeyer-Peppasmodels)and in-vitro-in-vivo correlation(IVIVC)is established by using the Wagner-Nelson method.
2.Materials and methods
2.1.Materials
Maize starch(St)was obtained from Universal Starch Chem Allied Ltd.,(Mumbai India).Solvents of analytical grade were obtainedfrom MerckLtd.,Germany.Gift sampleof salbutamol sulphate was received from Micro Labs Limited Ltd.,(Mumbai, India).Sprayed dried lactose and magnesium sulphate were obtained from Micro Labs Limited Ltd.,(Mumbai,India). Hydroxypropylmethyl cellulose(HPMC K 100)was obtained from Micro Labs Limited Ltd.,(Mumbai,India).
2.2.Method
2.2.1.Synthesis of acetylated starch and graft copolymers [starch grafted poly(methyl methacrylate)(St-g-PMMA)& acetylated starch grafted poly(methyl methacrylate) (Ast-g-PMMA)]
Acetylated starch(Ast)and graft copolymers were prepared in laboratory,based on information provided in our recent published research article[12].Here on starch back bone, methyl methacrylate was grafted via redox reaction.Wherein, Ce(IV)ion was reduced to Ce(III)ion and made an active siteon starchbackboneforgraftingofmethylmethacrylate. Synthesized samples were used for further study.
2.2.2.Tabletting
2.2.2.1.Blend preparation.HPMC K100M was chosen for comparison with graft copolymers for its controlled release properties.Various combinations were selected and blend was prepared for salbutamol sulphate using starch/acetylated starch(Ast)/St-g-PMMA/Ast-g-PMMA/HPMCK100M,spay dried lactose,and magnesium stearate.These were physically blended until a homogenous mixture was obtained(composition as shown in Table 1).No more additives were included in order to get intrinsic information of the polymeric material itself.

Table 1-Composition of salbutamol sulphate controlled release tablets.
2.2.2.2.Angle of repose.Angle of repose was derived for the powder blend as an indicator for f l owability characteristics. This was determined by a f i xed funnel and free standing cone method[13].The blend was poured through a funnel that can be raised vertically until a maximum cone height(h)was obtained.Radiusofthe heap(r)was measuredand theangleof repose(θ)was calculated by using the formula:2.2.2.3.Formulation of tablets.Tablets were prepared by direct compression method.The homogenous mixtures of different blends with required f l ow properties were shifted through#60 sieve.This was manually fed into the die and compressed in hydraulic press using an 8 mm f l at faced punch,with the crushing force of 70-80 N.The compressed tablets were f l at and round shaped,with average weight of 200 mg.Physicochemical properties for various parameters were assessed on 50 tablets.
2.2.3.Evaluation of tablets
2.2.3.1.Physical testing.The physical testing on tablets of each batch was performed after a relaxation period of at least 24 h.Weight variation test was performed on 20 individually weighed(Citizen CY204 Analytical Balance,Minnesota,USA) tablets according to the off i cial method of United State Pharmacopoeia.The thickness and diameter of ten tablets were measured individually using vernier caliper(Edutek Instrumentation,Ambala,India)and average value of thickness was calculated.The crushing strength(Newton)of prepared tablets was determined using Monsanto hardness tester(MHT-20,Campbell Electronics,Mumbai,India).Tablet friability was calculated as the percentage of weight loss occurred due to attrition during revolution of plastic chamber in 4 min span at the rate of 25 rpm,performed on 20 tablets using Roche friabilator(C-FT-20,Pharma Chem Machineries,Mumbai,India).
2.2.3.2.Drug content.Ten tablets were weighed individually and crushed into a fi ne powder by mortar and pestle.Crushed powder was weighed with quantity equivalent to 10 mg of the drug;this was transferred in fi ve volumetric fl asks separately. Distilled water was poured into each conical fl ask and these fl asks were further subjected to sonication.Drug was extracted from 50 ml solution prepared previously using bath sonicator,with sonication time of 2 min.The actual drug content was determined using high performance liquid chromatography system of Adept series CECIL CE 4201 with UV/Visible detector at 277 nm and the drug concentration was determined using the standard calibration curve,covering the drug concentration from 5.0 to 50.0 μg/ml.
2.2.4.In vitro and in vivo studies
2.2.4.1.In vitro release studies.In-vitro release study was carried out using USP dissolution type II apparatus,with rotation speed of 50 rpm to provide information regarding invitro drug release information.This study was performed in simulated gastric f l uid(0.1N HCl)for 2 h followed by same volume of simulated intestinal f l uid(PBS,pH 6.8)for next 12 h. The dissolution media(900 ml)was maintained at 37±0.5°C throughout the study.At predetermined time intervals of 0,1, 2,3,4,5,6,8,10,12 and 14 h,5 ml sample was withdrawn with syringe using 0.45 μm syringe f i lter and the media was replaced with the fresh media to maintain an ideal sink condition.The percentage content was calculated by validated RP-HPLC method and used to calculate the percentage release oneach timeofdissolutionprof i le.Thecumulativepercentage ofdrugreleasedwasplottedagainsttimeinorder toobtain the release prof i le[14].
The column used for separation was octadecyl silane (C18)with length 250 mm and internal diameter 4.6 mm (Phenomenex).Mobile phase used for analysis was composed of acetonitrile,methanol and water in the ratio of 60:20:20(v/ v),with pH adjusted to 2.8 using orthophosphoric acid,with fl ow rate of 0.5 ml/min.Sample of 20 μl was injected manually and peaks were monitored at 277 nm.
Tostudythemechanismofdrugreleasefromtheoptimized formulation of matrix tablets,in vitro release pro fi le were plotted and correlated with various kinetic models like zero order(i.e.cumulative amount of drug released vs time), fi rst order(log cumulative percentage of drug remaining vs time), Higuchi model(cumulative percentage of drug released vs square root of time)Korsmeyer-Peppas(log cumulative percentageofdrugreleasedvslogtime)andHixson-Crowell(cube root of concentration of drug remaining vs time)equations.
2.2.4.2.In vivo studies.Tablets prepared from graft copolymers(F3 and F4)with acceptable physical characteristics and optimum drug release behavior,were chosen for the invivo study.Pharmacokinetic parameters of graft copolymer formulations were compared against tablets prepared by native starch(F1),HPMC K 100M(F5)and commercial sustained release tablets(Asthalin SA-8 mg).
Male albino rabbits weighing 2.5-3.0 kg were randomly selected for the bioavailability studies.The animals were divided into f i ve groups and each group comprised of six rabbits.Each group received one of the tested formulas namely F1,F3,F4,F5 and Asthalin SA-8 mg.The animals were fasted over night(before administering tablets)and during the course of experiment,animals under trial had free access to water.Tablets were kept behind the tongue to avoid its destruction due to biting followed by suff i cient amount of water for easy swallowing.Blood samples(about 1 ml from each animal)were collected from orbital sinus,before dosing (zero time)and afterwards at different intervals post dosing viz.at 1,2,3,4,5,6,8,10,12,14,16,18,20,22,and 24 h.Samples were collected in microcentrifuge tubes containing 50 μl of 10%w/w disodium EDTA as an anticoagulant.The collected samples were immediately centrifuged at 10000 rpm for 10 min and plasma was separated and stored at-20°C until analysis.
A high performance liquid chromatography system of Adept series CECIL CE 4201 with UV/Visible detector was used for analysis.The data was recorded by using the software“power stream”.The column used for separation was octadecyl silane(C18)with length 250 mm,internal diameter 4.6 mm (Phenomenex)and particle size 5 μ.Chloramphenicol was used as an internal standard.In 0.5 ml of plasma sample,20 μl of internal standard(100 μg/ml)was added,drug was extracted from plasma using 5 ml methanol.Mobile phase used for analysis was composed of acetonitrile,methanol and water in the ratio of 60:20:20(v/v),with pH adjusted to 2.8 using orthophosphoric acid,with f l ow rate of 0.5 ml/min.Sample of 20 μl was injected manually and peaks were monitored at 277 nm.Quantif i cation of salbutamol sulphate was obtained by plotting SS to the internal standard peak area ratio as a function of its concentration.
2.2.5.Assay method validation
The developed method was validated following bioanalytical guidelines[15].Bioanalytical method validation required thedetermination of selectivity,linearity,LOD,LOQ,accuracy, recovery and precision respectively.

Table 2-Angle of repose as an indicator of powder f l ow property(All values are expressed as mean±SD,n=3).
2.2.6.In-vivo data analysis
The plasma kinetic data were assessed with Kinetica®software(version 5).The maximum SS concentration in serum (Cmax)and corresponding peak time(tmax)were determined by theinspectionoftheindividualserumdrugconcentration-time prof i les.The elimination rate constants(Ke) were obtained from least square f i tted terminal log-linear portion of the serum concentration-time prof i le.The elimination half life(t1/2)was calculated as 0.693/Ke.The area under the plasma concentration-time curve[AUC]0-24was determined by linear trapezoidal rule until last measurement point.
2.2.7.In-vitro-in-vivo correlation(IVIVC)study
In order to establish a level A IVIVC,the Wagner-Nelson method[16,17]was used to calculate the percentage of the drug absorbed:
where F(t)is the amount absorbed.The fraction absorbed is determined by dividing the amount absorbed at any time by the plateau value,keAUC(0-∞):
In-vitro fraction of drug released at each time points was subjected to Weibul model f i t options in IVIVC tool kit of WinNonlin v 5.3.Level A IVIVC was developed by drawing a plot between the percentage drug absorbed(along y-axis)of a formulation and its percentage drug dissolved(along x-axis) followed by the regression analysis of each curve to evaluate the strength of correlation determining whether the curve is linear or non-linear[18,19].The closer the value of determination coeff i cient to 1,the stronger is the correlation and linear is the curve.The correlation coeff i cient,prediction error of Cmaxand AUC were calculated to validate the predictability of IVIVC.

Table 3-Physical properties of formulated salbutamol matrix tablets using graft copolymers and HPMC K 100 M as release retardants.(All values are expressed as mean±SD,n=3).
2.2.8.Statistical analysis
Data were subjected to analysis of variance(ANOVA)(Graph Pad Instat software v 3.06,CA,USA).Signif i cant differences between formulations were analyzed using student newmann keuls multiple comparison test and obtained p values of<0.05 were considered to be statistically signif i cant.
3.Results and discussion
3.1.Flow property(angle of repose)
Prior to compression,blends(F1-F5)were evaluated for their fl ow property.From the values of angle of repose,it is evident that blends having graft copolymers showed better fl ow properties than that of blends having acetylated starch and native starch(Table 2).For formulation containing maize starch(F1)and acetylated starch(F2), fl ow property was falling in the passable range,in which values of angle of repose were found to be 32.34°±0.11 and 30.41°±0.23 respectively(31-34°is said to be a passable range).While formulation with St-g-PMMA(F3)and Ast-g-PMMA(F4)had good fl ow property, wherein values of angle of repose were 25.73±0.17 and 23.45°±0.19 respectively.Hypromellose(F5)containing blend depicted excellent fl ow property(wherein angle of repose was 19.11°±0.20).
3.2.Standard physical test of tablets
Formulations of salbutamol sulphate(F1-F5)with mentioned array of excipients had thickness ranging from 3.19 to 3.41 mm(Table 3).Drug content was found to be uniform among differentbatches ofthe tablets and was foundbetween 100.98 and 104.21%.Hardness and percentage friability of the tablets of all the batches were found amid 3.27-4.01 kg/cm2and 0.53-0.84%,respectively.Tablets with all the aforesaid compositions passed USP criteria for friability(<1.00%w/w). Outcome from friability assessment revealed good mechanical strength of the tablets.The percentage of weight variation of individual tablet to that of average weight was found within ±5%w/w,which f i ts in USP criteria for weight variation.
3.3.In-vitro release study
The in-vitro release prof i le of salbutamol sulphate matrix tablets in SGF followed by SIF is shown in Fig.1.Study was conducted for the period of 14 h and a higher percentage of drug release was observed for matrices containing native starch(F1)compared with the ones having acetylated starch (F2)and graft copolymers(F3&F4).The release of drug was prolonged up to 14 h for matrices containing graft copolymers (F3&F4)and up to 6 h for matrices containing acetylated starch(F2)whereas in case of starch>85%drug was released within 2 h.Signif i cant retardation in drug release behavior of graft copolymer matrices could be attributed by a better swelling property of the polymers,while a fast drug release behavior of starch was attributed by the burst release tendency of starch causing tablets to break after immersing in dissolution media.
In SGF media,a remarkable decrement in drug release was observed in graft copolymer matrices,when St-g-PMMA was used as a carrier then 10.7%of the drug was released in initial 2 h,13.8%of the drug was release in case of Ast-g-PMMA whereas tablets containing HPMC K 100M and commercial sustained release tablets(Asthalin SA-8 mg)showed%drug release 23.6%&30.2%respectively.Comparison of drug release pattern of graft copolymers matrices(F3 and F4)with HPMC K 100M containing tablets&commercial sustained release tablets(Asthalin SA-8 mg)in SIF media revealed that formulation F3 and F4 released upto 74.4%and 82.5%of the drug in successive12 h while at same time point HPMC K 100M tablets released 76.8%and Asthalin SA-8 mg released 87.8%of the drug.Retardation in drug release rate for graft copolymer formulations(F3&F4)was almost equivalent to that of commercially used controlled release polymer i.e.HPMC K 100M and commercially available sustained release tablets (Asthalin SA-8 mg).Thus it can be inferred based on release prof i leofgraftcopolymerandcommerciallyavailable formulation that graft copolymer has a potential application as a control release excipient in modif i ed drug delivery.
3.4.Analytical method validation
The developed methodwas validated followingICH guidelines [20].The in-vitro release study was validated to salbutamol tablets through the determination of specif i city,linearity, precision and accuracy.
3.4.1.Speci fi city
The speci fi city of the in-vitro release test was evaluated through the analysis of placebo tablets(Fig.2A).The specifi city test by HPLC demonstrated that the excipients from tablets do not interfere in the drug peak because retention times of placebo(3.02 min)and salbutamolsulphate(5.22 min) are quite different(Fig.2B).
3.4.2.Linearity
Linearity of the method was evaluated at fi ve concentration levels ranging from 2 to 10 μg/ml(y=30874x-6522)with correlation coef fi cient of 0.9968.
3.4.3.Precision
The precision of the in-vitro release study were evaluated by analyzing intra-day precision and inter-day precision.The% RSD for intra-day precision is 1.68%and inter-day precision is 0.98%.According to ICH norms,in all condition%RSD was<2 which shows method is precise.
3.4.4.Accuracy
Recovery studies were performed to validate the accuracy of developed method.To the preanalysed sample solution,a de fi nite concentration of standard drug was added and then its recovery was analyzed.The method was found to be accurate with%recovery of 98.42-101.53%.
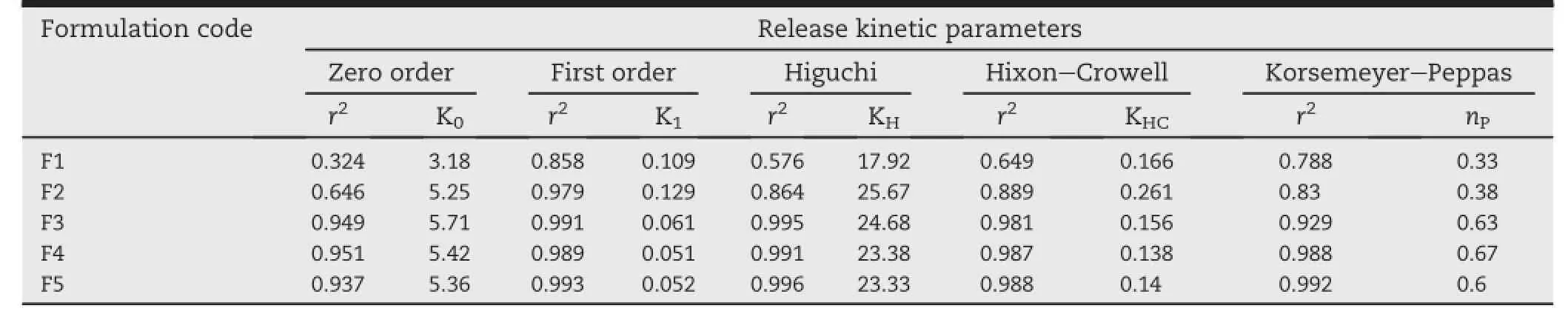
Table 4-Comparative release kinetics parameter of all the batches of controlled release tablets.
3.5.Kinetics and mechanism of drug release
The drug release mechanism was determined by f i tting the invitro release prof i le in various release kinetic models and the values of release exponent(nP),kinetic constant(K)and regression coeff i cient are shown in Table 4.Zero order,f i rst order,Higuchi,Hixon-Crowell and Korsmeyer-Peppas are the major models to identify the drug release from sustained release formulations and criteria of selecting the most appropriate model was based on the its goodness of f i tting. Starch and acetylated starch tablets(F1&F2)showed a f i rst order release,with regression value of 0.858 and 0.979 respectively.In-vitro release prof i le of graft copolymers matrix formulations(F3&F4)and HPMC K 100M containing tablets (F5)are best expressed by the Higuchi model,as the plots showed high linearity with regression value of 0.995,0.991, and 0.996 followed by f i rst order kinetics with regression value of 0.991,0.989,and 0.993 respectively.Two factors,however, diminish the applicability of Higuchi's equation to matrix systems.This model fails to explain the inf l uence of swelling of the matrix upon hydration and gradual erosion of the matrix.Therefore,the in-vitro release data were also f i tted to the well-known exponential Korsmeyer-Peppas equation and valueofreleaseexponent(nP)explains thereleasemechanism of the drug from the tablets.The observed‘nP’values for release prof i les of formulation F3,F4 and F5 were fall in between 0.50 and 0.89 indicated anomalous release behavior coupled with diffusion and erosion.The release exponents for formulation F1 and F2 are less than 0.5 indicating quasi f i ckian diffusion mechanism of drug release.
3.6.In-vivo study
The results of the plasma drug concentration at different time intervals,after administration of formulation F1,F3,F4,F5 andAsthalin SA-8 mg tablet containing 8 mg of salbutamol sulphate to rabbits,are presented in Table 5.SS was detected and quantif i ed in plasma by using HPLC method and mean plasma concentration curve of formulated tablets and commercial tablets were plotted as depicted in Fig.3.
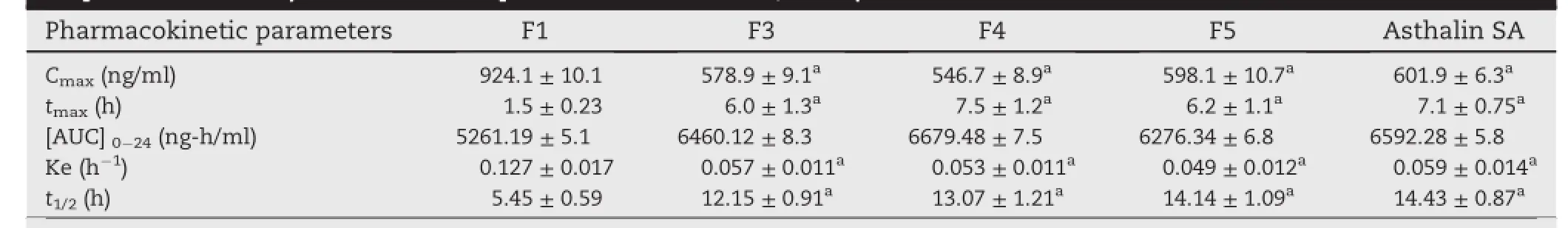
Table 5-Pharmacokinetic parameters of formulated(F1,F3,F4&F5)and marketed tablets(Asthalin SA)of salbutamol sulphate in rabbits(All values are expressed as mean±SD,n=3).
Graft copolymer matrices(F3&F4)and starch matrix(F1) showed signif i cantly different(P<0.05)Cmaxvalues of about 578.9,546.7 and 924.1 ng/ml,respectively.Decrement of Cmaxvalue in case of controlled release tablets indicated and justif i ed sustained and prolonged release potential of graft copolymer.Observed mean plasma[AUC]0-24values for F3 (6460.12 ng h/ml)and F4(6679.48 ng h/ml)was signif i cantly (P<0.05)higher than F1(5261.19 ng h/ml)which indicated improvement in relative bioavailability of graft copolymer matrices.The tmaxvalue of graft copolymer matrices F3(6 h) and F4(7.5 h)was signif i cantly(P<0.05)higher than starch matrix F1(1.5 h),which indicates the slow absorption rate in graft copolymer tablets due to extended release effect of hydrophobic polymer matrix.When elimination rate constants(Ke)forabovementionedformulationswere compared,it was found that formulation F1 has Ke value was 0.127,this Ke value signif i cantly went down in F3(Ke=0.057) and F4(Ke=0.053),indicating slow elimination rate of the drug from body in graft copolymer formulations.The elimination half life(t½)of the F3(12.15 h)and F4(13.07 h)was more than F1(5.45 h),which conf i rmed prolonged availability of SS in body.However there was no signif i cant difference in the pharmacokinetic parameters(tmax,Cmax,AUC,Ke,and t1/2)for graft copolymers matrices,HPMC K100M matrix tablets and commercial tablets.Results revealed that the graft copolymer matrices provided comparable sustained and prolonged effect to that of HPMC K 100M matrix tablets and Asthalin SA-8 mg(commercial tablets),so graft copolymers can be used as potential excipients in controlled drug delivery system.
3.7.Bioanalytical method validation
3.7.1.Selectivity
The chromatogram of salbutamol sulphate extracted from plasma is shown in Fig.4.With the chromatogram it is clear that retention times of plasma(2.03 min)and salbutamol sulphate(5.21 min)are quite different means plasma components are not showing interference with the drug elution.
3.7.2.Limit of detection(LOD)and Limit of quantif i cation (LOQ)
LOD and LOQ for salbutamol sulphate were 33.97 ng/ml and 101.91 ng/ml,respectively.
3.7.3.Linearity
Standard calibration curve in rabbit plasma was found to be linear at concentrations ranging from 100 to 1200 ng/ml (Y=0.0318x+1.4027)with correlation coeff i cient of 0.9968.
3.7.4.Accuracy
Recovery studies were performed to validate the accuracy of developed method.To preanalysed sample solution,a de fi nite concentration of standard drug was added and then its recovery was analyzed.The method was found to be accurate if %recovery is 100±15%and%CV is<15%.The%recovery of drug in plasma found in the range of 89.59-92.41%and coeffi cient of variation was 2.21-3.81.Since the%recovery and% CV is within the range,it shows accuracy of method.
3.7.5.Precision
Precision of the method was assessed by intra-day and interday analysis of six replicates for each concentration at 3 different concentration levels(100,600,and 1200 ng/ml, respectively).The coeff i cient of variation(CV)at each concentration level was expressed as precision.The method proved to be precise because the%CV for is not more than 5.61%at three different concentration levels.
3.8.In-vitro-in-vivo correlation
In-vitro-in-vivo correlation was established by plotting the graph of fraction absorbed in-vivo against fraction dissolved in-vitro.In this study,Faand Fddata of graft copolymerformulations(F3&F4)wereanalyzedanda reliablecorrelation (R2>0.97)was observed between fraction dissolved in-vitro and fraction absorbed in-vivo shown in Fig.5.The prediction error of the Cmaxand AUC of the correlation were found to be -9.62%and 12.02%.The low prediction error indicates the reliability of model towards carrying out predictions;hence it can be selected as a bio relevant tool to screen the best formulation and used for waiver approval in future.
4.Conclusion
Release characteristics of salbutamol sulphate was evaluated and assessed using graft copolymer as a carrier matrix and was compared with HPMC K100M containing controlled release matrix system and marketed formulation(Asthalin SA-8 mg).It was revealed that the graft copolymerization improves the fl ow property of native starch and modi fi ed starch.Matrix tablets prepared employing graft copolymers imparted slow release pro fi le of up to 14 h,similar was in case of HPMC K100M matrix formulation.
Statistical model and kinetic data revealed that in graft copolymer based matrices;drug release was governed by diffusion and erosion mechanism.The in-vitro release pro fi les of salbutamol sulphate starch matrix tablets showed>70% drug release within 1 h whereas in graft copolymer matrix tablets same amount of drug was released upto 9 h.This refl ects the potential of graft copolymers to sustain the drug release and the results are well supported by in-vivo pharmacokinetic studies.Pharmacokinetic parameter i.e.tmax(1.5±0.23 h)values of the starch matrix tablets clearly indicates the ability of the formulation to release the drug immediately upon reaching the GIT which is due to the hydrophilic nature and high solubility of starch.The dramatic shift in tmaxof the graft copolymer matrix tablets[F3(6.0±1.3) &F4(7.5±1.2)h]w.r.t starch matrix tablets(1.5±0.23 h)are indicative of the graft copolymer carrier to control the release of the drug even under the gastrointestinal environment. Whereas pharmacokinetic parameters(tmax,Cmax,AUC,Ke, and t1/2)of graft copolymers matrices(F3&F4),HPMC K 100M matrix tablets(F5)and marketed tablets are comparable, indicating that graft copolymers might be a promising vehicle for sustained release preparations in oral therapy.A well invitro-in-vivo correlation was observed for graft copolymer formulations,which indicates their suitability for the waiver approval in future.
Acknowledgements
Authors are grateful to their parental institutes for providing the necessary facilities to accomplish the present research work.
REFERENCES
[1]Uhrich KE,Cannizzaro SM,Langer RS,et al.Polymeric system for controlled drug release.Chem Rev 1999;99:3181-3198.
[2]Volkert B,Lehmann A,Greco T,et al.A comparison of different synthesis routes for starch acetates and the resulting mechanical properties.Carbohydr Polym 2010;79:571-577.
[3]Bhattarai N,Gunn J,Zhang M.Chitosan-based hydrogels for controlled,localized drug delivery.Adv Drug Deliv 2010;62:83-99.
[4]Liu CS,Desai KGH,Meng XH,et al.Sweet potato starch microparticles as controlled drug release carriers: preparation and in-vitro drug release.Dry Technol:An Int J 2007;25:689-693.
[5]Nandhakumar L,Dharmamoorthy G,Rameshkumar S,et al. Ethyl cellulose based timolol maleate microspheres for sustained drug delivery.Int J Pharm Ind Res 2011;1:242-244.
[6]Mahammed N,Deshpande RD,Gowda DV.Modif i ed polysaccharide as drug delivery:review.Int J Pharm Sci Rev 2011;11:42-47.
[7]Santacruz S,Koch K,Svensson E,et al.Three underutilized sources of starch from the Andean region in Ecuador:part I. Physico-chemical characterization.Carbohydr Polym 2002;49:63-70.
[8]Shaikh MM,Lonikar SV.Starch-acrylics graft copolymers and blends:synthesis,characterization,and applications as matrix for drug delivery.J Appl Polym Sci 2009;114:2893-2900.
[9]Greim H,Ahlers J,Bias R,et al.Assessment of structurally related chemicals:toxicity and ecotoxicity of acrylic acid alkyl esters(acrylates),methacrylic acid and methacrylic acid alkyl esters(methacrylates).Chemosphere 1995;31:2637-2659.
[10]Marinich JA,Ferrero C,Jimˊenez-Castellanos MR.Graft copolymers of ethyl methacrylate on waxy maize starch derivatives as novel excipients for matrix tablets: physicochemical and technological characterization.Eur J Pharm Biopharm 2009;72:138-147.
[11]Varshosaz J,Tavakoli N,Kheirolahi F.Use of hydrophilic natural gums in formulation of sustained-release matrix tablets of tramadol hydrochloride.AAPS Pharm Sci Tech 2006;7:E168-74.
[12]Kumar P,Ganure AL,Subudhi BB,et al.Synthesis and characterization of pH sensitive ampiphillic new copolymer of methyl methacrylate grafted on modif i ed starch: inf l uences of reaction variables on grafting parameters.Int J Pharm Pharm Sci 2014;1:868-880.
[13]Bose A,Wong TW,Singh N.Formulation development and optimization of sustained release matrix tablet of Itopride HCl by response surface methodology and its evaluation of release kinetics.Saudi Pharm J 2013;21:201-213.
[14]Revathi R,Ethiraj T,Marreddy JL,et al.Development and validation of a dissolution test for candesartan cilexetil in tablet forms using reverse phase-high performance liquid chromatography.J Pharm Educ Res 2011;2:71-77.
[15]Guidance for Industry.Bioanalytical method validation,U.S. Food and drug administration.Fed Regist 2001;66:49028-49029.
[16]Wagner JG,Nelson E.Percent absorbed time plots derived from blood level and/or urinary excretion data.J Pharm Sci 1963;52:610-611.
[17]Wagner JG,Nelson E.Kinetic analysis of blood levels and urinary excretion in the absorptive phase after single doses of drug.J Pharm Sci 1964;53:1392-1403.
[18]Kim JU,Park CW,Lee BJ,et al.Design and evaluation of nicorandil extended-release tablet.Asian J Pharm Sci 2014:1-6.
[19]Wang X,Yu J,Tang X.In vitro release and pharmacokinetics of f l urbiprofen sustained-release capsules containing coated pellets.Asian J Pharm Sci 2007;2:77-84.
[20]ICH-Q4B Annex.7.Guideline on dissolution test.2010.p.1-4.
*Corresponding author.School of Pharmaceutical Sciences,Siksha O Anusandhan University,Khandagiri Square,Bhubaneswar,Orissa 751030,India.Tel.:+91 9221715720.
E-mail address:pankajnil@yahoo.com(P.Kumar).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.12.003
1818-0876/©2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2015年3期
Asian Journal of Pharmacentical Sciences2015年3期
- Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- UPLC-MS/MS for the determination of azilsartan in beagle dog plasma and its applicationin a pharmacokinetics study
- Characterization of recrystallized itraconazole prepared by cooling and anti-solvent crystallization
- Enhanced bioavailability of rebamipide nanocrystal tablets:Formulation and in vitro/in vivo evaluation
- Liposomes for systematic delivery of vancomycin hydrochloride to decrease nephrotoxicity: Characterization and evaluation
- Chlorogenic acid loaded chitosan nanoparticles with sustained release property,retained antioxidant activity and enhanced bioavailability
