Synthesis and Characterization of Pentanol-3-nitraza-5-azidonitrate
GAO Fu-lei,JI Yue-ping,LIU Wei-xiao,WANG Ying-lei,CHEN Bin,LIU Ya-jing,DING Feng
(Xi′an Modern Chemistry Research Institute,Xi′an 71065,China)
1 Introduction
The energetic azido compounds[1-2]including energetic azido plasticizers have received a great deal of attention as they impart additional energy to propellants and explosives[3-6].It has been shown that the energetic azido plasticizers commonly applied,such as azide nitrate ester and 1,3-propanediol-2,2-bis(adzidomethyl) dinitrate (PDADN),can increase burning rate and energy of nitramine modified double base propellant[7-8],thought they have a critical shortcoming of high sensitivity to impact and shock,which implies that it is necessary to look for a new plasticizers with high performance and lower sensitivity.Fortunately,pentanol-3-nitraza-5-azidonitrate (PNAN) with nitramino (—N—NO2),azido (—N3) and nitrate ester group (—ONO2) reported in this work is just such a plasticizer,which is synthesized for the first time via reaction in dimethyl sulfoxide,using 1,5-dinitrato-3-nitraza pentane (DINA) and sodium azide as raw materials (Scheme 1),confirmed its structure and measured its physicochemical properties.
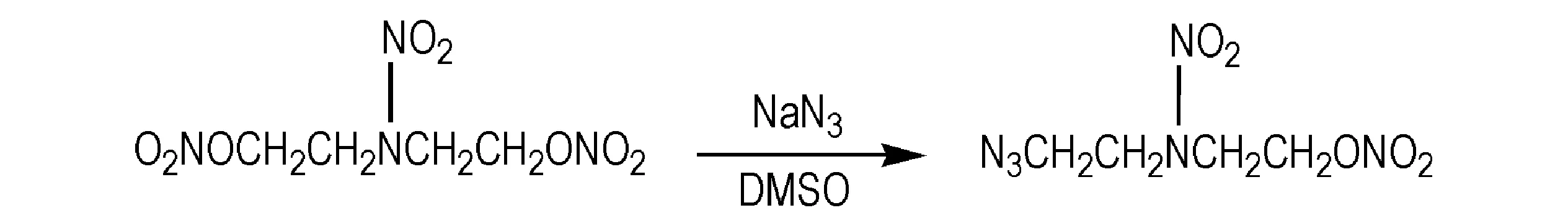
Scheme 1 The synthesis of PNAN
2 Experimental
2.1 Synthesis of PNAN
DINA (20 g,0.083 mol) was slowly added to 40 mL of dimethyl sulfoxide at 50 ℃,and the solution was heated to 65 ℃ with good stirring.After DINA was completely dissolved in dimethyl sulfoxide solution,NaN3(6.5 g,0.1 mol) was added in 2 h,then the mixture was held at 85 ℃ for 3 h.To generate the primary product,NaN3must be treated with an excess of DINA and also added in 2 h for several times.The optimum reaction conditions were as follows:the molar ratio of DINA to NaN3was 1∶0.8,reaction time 3 h,reaction temperature 85 ℃.After cooling an equal quantity of methylene chloride solvent was added and the entire organic portion was washed four times with water to remove dimethyl sulfoxide and inorganic salts.The methylene chloride solution was concentrated to give 18 g of light yellow oil.The products were detected by TLC (Thin Layer Chromatography).After dilution with acetone,the mixture was separated by column chromatography (SiO2,petroleum ether∶EtOAc=4∶1),then 7.14 g of colorless oil with a purity of 98.60% (HPLC) was obtained.The product was PNAN.Fig.1 was the1H NMR spectra of PNAN.1H NMR(CDCl3/TMS)δ:3.659 (t,2H,—CH2N3),3.986 (t,2H,—CH2NNO2),4.195(t,2H,—CH2NNO2) ,4.791(t,2H,—CH2ONO2); IR (KBr,ν/cm-1):2960,1452,1419,882(—CH2),2108(—N3),1521(N—NO2),1226(—ONO2),847(N—O); Anal.Calc (%) for C4N6O5H8:C 21.82,N 38.14,H 3.64; Found (%) C 21.85,N 37.70,H 3.64.
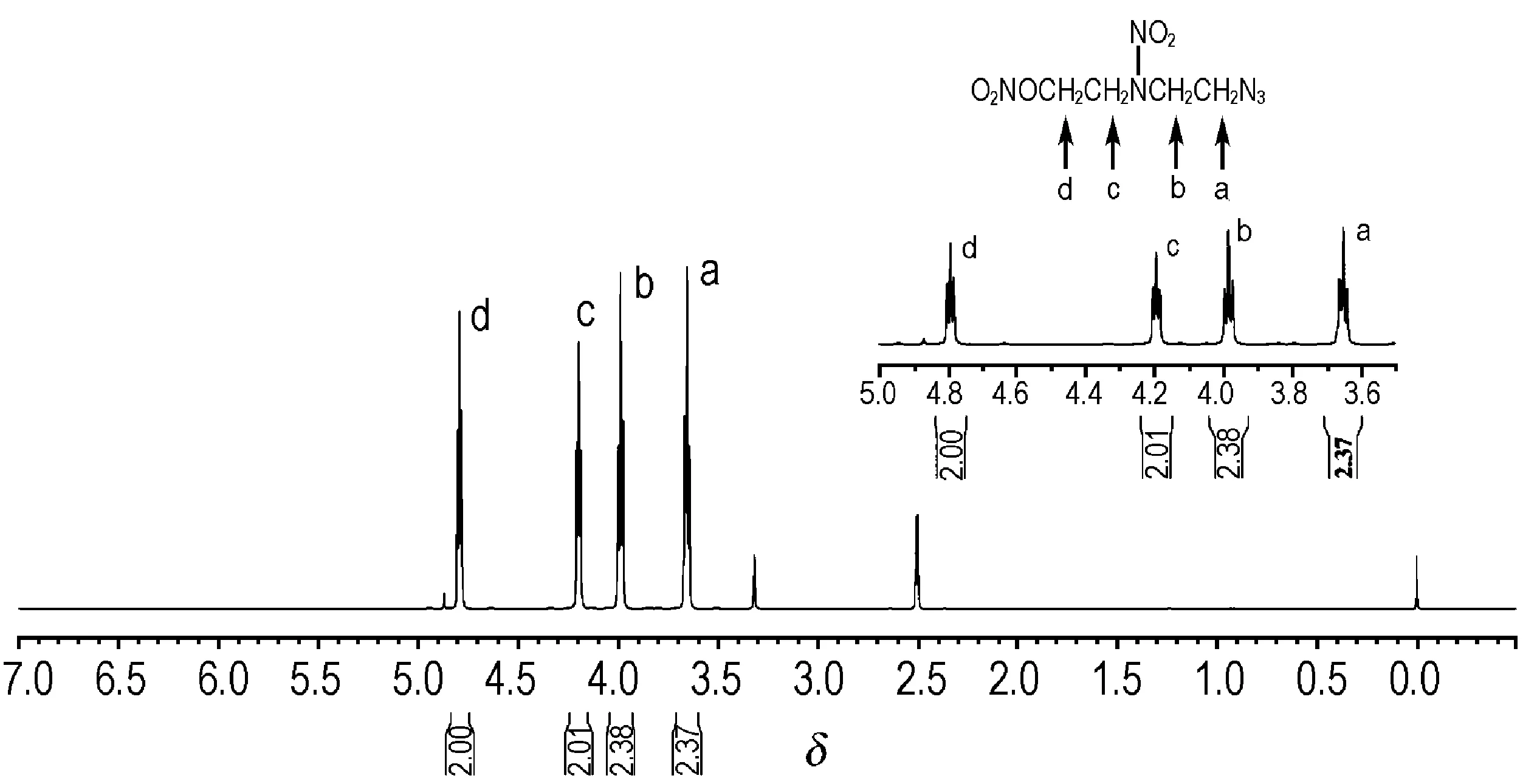
Fig.11H NMR spectra of PNAN
2.2 Properties of PNAN
Some physicochemical properties of PNAN obtained by the tests are all listed in Table 1,including the appearance,density,dissolubility,decomposition temperature,glass transition temperature,viscosity,friction sensitivity and impact sensitivity.In which,the decomposition temperature of 172 ℃ shows that PNAN has good heat-resistance ability.The friction sensitivity of 12% of PNAN is less than that of PDADN (80%[8]).The impact sensitivity of 56% of PNAN is less than that of PDADN (100%[8]),revealing that in comparison with PDADN,PNAN has better mechanical sensitivity.
Table 1 Properties of PNAN

propertiesresultstestconditionappearancecolorlessoileyeballingempiricalformulaC4N6O5H8—density/g·cm-31.46densitybottlemethoddissolubilitysolubleinmethylenechloride,acetone,benzene,dimethylsulfoxide,experimentoxygenbalance/%-43.6—decompositiontemperature/℃172GJB772A-1997,method502.1glasstransitiontemperature/℃-41dynamicthermomechanometryviscosity/mPa·s19.5experimentfrictionsensitivity/%12GJB772A-1997,method602.1impactsensitivity/%56GJB772A-1997,method601.1
3 Results
Pentanol-3-nitraza-5-azidonitrate (PNAN) was synthesized for the first time with a density of 1.46 g·cm-3,decomposition temperature of 172 ℃,glass transition temperature of -41 ℃,viscosity of 19.5 mPa·s,friction sensitivity of 12% and impact sensitivity of 56% ,which is a colorless oil,soluble in methylene chloride,acetone,benzene and dimethyl sulfoxide,but insoluble in water and alcohol.It can be potentially used as an energetic plasticizer of solid propellants.
[1] Simmons R L.Azido nitramine:US 4450110[P].1984.
[2] Flanagan J E.1,5-Diazido-3-nitrazapentane and method of preparation thereof:US 5013856 [P].1991.
[3] JI Yue-ping,GAO Fu-lei,HAN Rui,et al.Estimation and determination of the solubility parameter of 1,5-Diazido-3-nitrazapentane[J].ChineseJournalofEnergeticMaterials(HannengCailiao),2013,21(5):612-615.
[4] JI Yue-ping,LAN Ying,LI Pu-rui,et al.Synthesis and characterization of 1,5-Diazido-3-nitrazapentane (DIANP)[J].ChineseJournalofExplosives&Propellants,2008,31(3):44-46.
[5] WANG Ying-lei,JI Yue-ping,LI Pu-rui,et al.Synthesis of 2-methyl-2-nitro-1,3- diazidopropane[J].ChineseJournalofEnergeticMaterials(HannengCailiao),2010,18(1):11-14.
[6] Raman K V.Ballistic modification of RDX-CMDB propellant[J].PropellantExplosivesPyrotechnics,1988,13:149.
[7] WANG Jin,LI Shu-fen.Effect of azide nitrate ester on combustion behaviour of nitramine modified double base propellant[J].ChineseJournalofExplosives&Propellants,2001,(3):22-24.
[8] ZHANG Li-jie,CUI Jun-min ,ZHANG Chuan,et al.One-pot synthesis of neopentyl gilcol dinitrate[J].ChineseJournalofEnergeticMaterials(HannengCailiao),2010,1(5):541-543.

