胺碘酮治疗老年充血性心力衰竭伴快速房颤的疗效观察
张荣生,顾 翔
胺碘酮治疗老年充血性心力衰竭伴快速房颤的疗效观察
张荣生1,顾 翔2*
(1武警江苏省总队医院心血管内科,扬州 225003;2苏北人民医院心血管内科,扬州 225003)
观察胺碘酮治疗老年充血性心力衰竭(CHF)伴快速心房纤颤(AF)的疗效。选择2012年9月至2013年9月在苏北人民医院住院治疗的老年CHF伴快速AF患者70例,纽约心脏联合会分级(NYHA)Ⅱ~Ⅳ级,心室率≥120次/min;随机分为胺碘酮组和去乙酰毛花苷组,每组各35例。在常规治疗基础上,胺碘酮组首次剂量给予胺碘酮150mg缓慢静注,随后1.5mg/min微量泵维持;去乙酰毛花苷组首次剂量给予去乙酰毛花苷0.4mg或0.2mg缓慢静注,1h后无效者追加0.2mg。观察用药后不同时刻的心室率变化、药物平均起效时间、复律成功比例、B型利钠肽(BNP)变化、不良反应及随访效果。两组患者用药后1,2,24h的心室率与用药前比较差异有统计学意义(<0.01),胺碘酮组用药后30min的心室率与用药前比较差异亦有统计学意义(<0.01);用药后2h胺碘酮组患者心室率较用药前下降47%, 去乙酰毛花苷组下降28%;胺碘酮组与去乙酰毛花苷组用药后30min,1h,2h,24h心室率间差异有统计学意义(<0.01);胺碘酮组和去乙酰毛花苷组的治疗有效率分别为79.8%和72.3%(>0.05);胺碘酮组和去乙酰毛花苷组的复律成功率分别为34.3%和8.6%(<0.01);两组患者间BNP变化差异无统计学意义(>0.05);出院3个月后随访,两组患者治疗后(口服药物包括可达龙、美托洛尔、地高辛)仍为AF的比率分别为60.0%(21/35)和82.9%(29/35);而不良反应发生率分别为8.6%和11.4%(>0.05)。胺碘酮是治疗老年CHF合并AF有效的药物之一,副反应轻,使用安全。
胺碘酮;去乙酰毛花苷;心力衰竭;心房颤动
充血性心力衰竭(congestive heart failure,CHF)是指心脏当时不能搏出同静脉回流及身体组织代谢所需相称的血液供应。往往由各种疾病引起心肌收缩能力减弱,从而使心脏的血液输出量减少,不足以满足机体的需要,并由此产生一系列症状和体征。当其并发快速心房纤颤(atrial fibrillation,AF)时,常易引起严重的血流动力学障碍,使心力衰竭加重,形成恶性循环进而增加病死率,迅速复律或控制心室率对有效改善血流动力学及控制心力衰竭具有重要意义[1−3]。胺碘酮是以Ⅲ类药作用为主的心脏离子多通道阻滞剂,兼具Ⅰ、Ⅱ、Ⅳ类抗心律失常药物的电生理作用,是目前无明显负性肌力作用的广谱抗心律失常药,其静脉制剂是治疗危重症患者快速心律失常的常用药物之一[4−6]。本研究旨在对比观察静脉应用胺碘酮与去乙酰毛花苷治疗CHF伴快速AF患者的临床疗效。
1 对象与方法
1.1 研究对象
选择2012年9月至2013年9月在苏北人民医院住院治疗的CHF且合并快速AF患者70例,纽约心脏联合会(New York Heart Association,NYHA)分级Ⅱ~Ⅳ级,心室率≥120次/min;男37例,女33例,随机分为胺碘酮组和去乙酰毛花苷组,每组各35例。胺碘酮组心功能Ⅱ级7例,Ⅲ级17例,Ⅳ级11例,其中冠心病13例,扩张型心肌病5例,高血压性心脏病12例,风湿性心脏病5例;去乙酰毛花苷组心功能Ⅱ级8例,Ⅲ16级,Ⅳ级11例,其中冠心病11例,扩张型心肌病3例,高血压性心脏病17例,风湿性心脏病4例。排除有严重肝肾功能不全者,>Ⅱ度房室传导阻滞、窦房阻滞、甲状腺功能亢进或减退、电解质紊乱等患者。两组患者基线资料比较差异均无统计学意义(>0.05;表1)。进行常规体检:心脏X线胸片、心电图、心脏彩超,24h动态心电图、肝肾功能、B型利钠肽(brain natriuretic peptide,BNP)和甲状腺功能检查。
1.2 治疗方法
两组患者在CHF常规治疗(低盐低脂饮食、吸氧、利尿剂等)基础上,胺碘酮组应用胺碘酮(赛诺菲−圣德拉堡民生制药有限公司,规格3ml/支,0.15g/3ml)150mg于10min内静脉注射,随后使用微量泵以1.5mg/min维持。去乙酰毛花苷组应用去乙酰毛花苷(无锡市第七制药有限公司,规格2ml/支,0.4mg/2ml),首剂0.4mg(近1个月内未用去乙酰毛花苷类患者)或0.2mg(近1个月已用毛花苷类患者),于10min内静脉注射,1h后无效者追加0.2mg。持续多参数心电、血压监测,治疗观察期为24h。24h后复查BNP。出院后3个月内每个月复诊1次,嘱患者有心慌时立即至最近医院查心电图,复诊详细询问患者出院后一般情况,同时查心电图、肝肾功能、血清电解质。
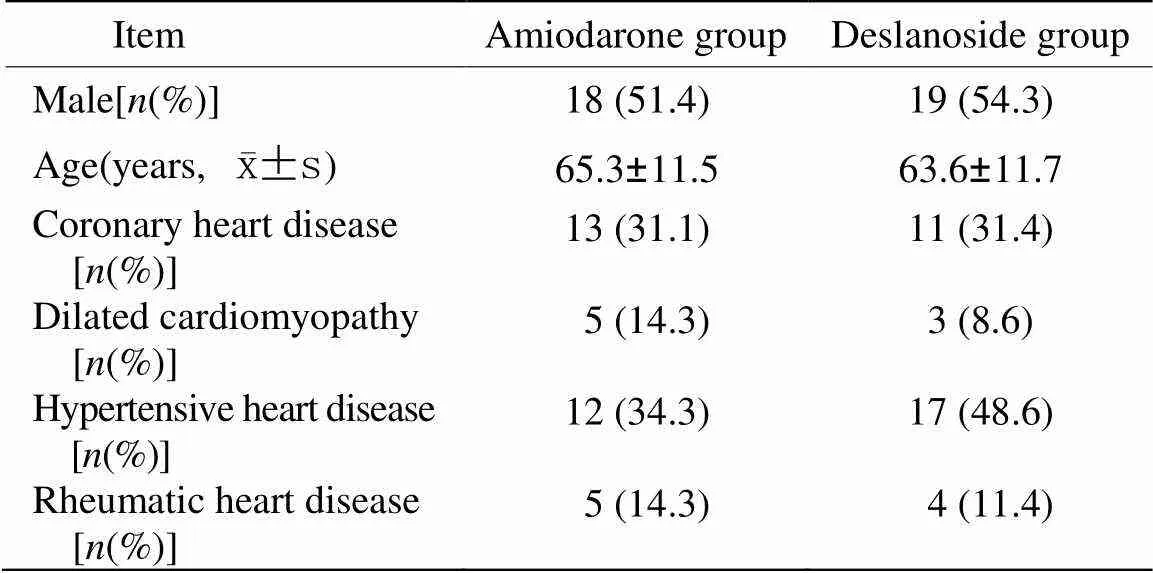
表1 两组患者用药前基线资料比较
1.3 观察指标
记录用药后30min,1h,2h和24h的心室率、血压及临床表现;同时记录两组患者用药后药物起效时间及不良反应;出院后3个月内仍为AF的比率。
1.4 疗效判定
有效:心室率降至<100次/min,或较基线水平下降>20%,或转复为窦性心律;无效:心室率仍>100次/min,且与基线水平比较心室率下降<20%。
1.5 统计学处理
采用SPSS17.0统计软件包进行统计学分析。计量资料以均数±标准差表示,多组间比较采用检验,组间两两比较采用检验,两组间比较采用检验;计数资料比较采用2检验。以<0.05为差异具有统计学意义。
2 结 果
2.1 两组患者用药前后心室率的变化
两组患者用药后1,2,24h的心室率与用药前比较均显著下降(值=3.768~10.359,<0.01),胺碘酮组用药后30min心室率与用药前比较亦有显著降低(=4.237,<0.01);两组患者用药后30min,1h,2h,24h的心室率间差异均有统计学意义(<0.01);此外,用药2h后胺碘酮组患者心室率较用药前下降47%,去乙酰毛花苷组心室率较用药前下降28%(表2)。
2.2 两组患者疗效比较
胺碘酮组与去乙酰毛花苷组患者治疗有效率分别是79.8%(28/35)和72.3%(25/35),差异无统计学意义(2=0.317,>0.05);胺碘酮组与去乙酰毛花苷组的平均起效时间分别为(25.8±11.5)min和(45.3±12.7)min,差异有统计学意义(=5.736,<0.01)。胺碘酮组与去乙酰毛花苷组用药期间转复窦性心律分别为12例(34.3%)和3例(8.6%),差异有统计学意义(=6.873,<0.01)。两组患者用药后24h BNP变化差异无统计学意义(>0.05;表3)。出院后随访3个月内两组患者仍为AF的比率分别为60.0%(21/35)和82.9%(29/35),因两组患者出院后使用的维持窦性心律的药物不同[包括可达龙、美托洛尔(倍他乐克)、地高辛],故未能比较。
2.3 两组患者不良反应比较
胺碘酮组出现不良反应者3例,其中1例用药后30min出现血压下降退出试验,血压由用药前的120/75mmHg(1mmHg=0.133kPa)降至86/55mmHg,立即停药密切观察并应用多巴胺后血压恢复正常;2例转复窦性心律后发生一过性Ⅱ度房室传导阻滞及窦性心动过缓,密切观察后逐渐恢复正常。不良反应发生率为8.6%(3/35)。去乙酰毛花苷组出现不良反应者4例,其中2例用药后约1.5h转复窦性心律并出现窦性心动过缓,心室率均降至约55次/min,立即停药密切观察见心率渐恢复正常,且仍为窦性心律;另外2例用药1h后出现低血压退出试验,血压由用药前的125/75mmHg和118/70mmHg分别降至90/55mmHg和85/50mmHg,停药观察30min后自行恢复正常。不良反应发生率为11.4%(4/35)。两组患者不良反应发生率间差异无统计学意义(>0.05)。两组患者均无心力衰竭加重和严重恶性心律失常发生。
3 讨 论
心室率快速型AF使CHF患者的血流动力学障碍进一步加重,因此需要立即干预,使用迅速有效地复律及控制心室率的药物是延缓心力衰竭发展及降低病死率的主要措施[7−10]。洋地黄类药物一直是治疗CHF伴心室率快速型AF患者的首选药物,主要机制是该类药物具有正性肌力和拟迷走神经性负性频率作用,可直接增强心肌收缩力,提高心脏每搏输出量,从而改善心功能;同时使窦房结自律性降低,房室交界区有效不应期延长,有效地降低心室率,但是起效相对较慢,对迅速控制心室率快速型AF有一定的局限性[11]。胺碘酮属Ⅲ类抗心律失常药,作用机制:主要抑制K+外流,延长动作电位时限,还具有抑制Ca2+内流和非竞争性抑制β受体的作用[4−5,12],广泛应用于各类心律失常的治疗,对于CHF伴心室率快速型AF患者复律及心室率控制具有明显效果[13]。
在本实验观察中,迅速控制CHF伴心室率快速型AF患者方面,去乙酰毛花苷平均起效时间明显长于胺碘酮,说明胺碘酮起效较迅速。同时用药后2h胺碘酮组患者心室率较用药前下降47%,去乙酰毛花苷组下降28%;两组复律比例差异有统计学意义,但总有效率差异无统计学意义。所以在迅速有效控制CHF伴心室率快速型AF时可优先考虑静注胺碘酮治疗,其转复窦性心律的效果优于去乙酰毛花苷,患者转复位窦性心律后心慌胸闷等主观症状明显改善,但评价心功能有多种指标,包括症状、体征以及一些客观检查依据,如6min步行距离、BNP、左室射血分数等,《欧洲心脏病学协会(ESC)慢性心力衰竭诊断和治疗指南》指出BNP与心力衰竭密切相关,可提高NYHA分级的客观性并反映心力衰竭的严重程度[14],可作为治疗监测。两组患者治疗24h后BNP均有下降,但两组之间差异无统计学意义,提示胺碘酮治疗CHF伴快速AF患者虽起效迅速、复律比例高,但对于心功能的改善并不一定优于去乙酰毛花苷,当然其亦有多种不良反应[15−17],需密切监测。

表2 两组患者用药前、后心室率变化情况
Compared with before the medication in the same group,**<0.01; compared with amiodarone group,##<0.01
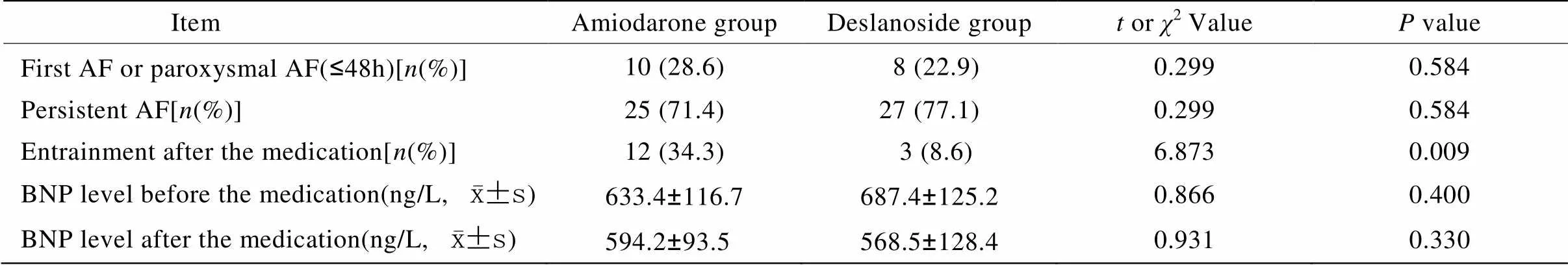
表3 两组患者复律和心功能情况
AF: atrial fibrillation; BNP: brain natriuretic peptide
从本研究总体来看,静脉应用胺碘酮短时治疗CHF伴心室率快速型AF较静脉应用去乙酰毛花苷更为迅速、有效,但需进一步观察心功能改善情况及其不良反应的发生。
[1] Wyse DG, Waldo AL, DiMarco JP,. A comparison of rate control and rhythm control in patients with atrial fibrillation[J]. N Engl J Med, 2002, 347(23): 1825−1833.
[2] Lubitz SA, Benjamin EJ, Ellinor PT. Atrial fibrillation in congestive heart failure[J]. Heart Fail Clin, 2010, 6(2): 187−200.
[3] Workman AJ, Smith GL, Rankin AC.Mechanisms of termination and prevention of atrial fibrillation by drug therapy[J]. Pharmacol Ther, 2011, 131(2): 221−241.
[4] Miles RH, Passman R, Murdock DK. Comparison of effectiveness and safety of ranolazineamiodarone for preventing atrial fibrillation after coronary artery bypass grafting[J]. Am J Cardiol, 2011, 108(5): 673−676.
[5] Chinushi M, Iijima K. Rate control is a better initial treatment for patients with atrial fibrillation and heart failure—rhythm control. rate control: which is better in the management of atrial fibrillation? (Rate-side)[J]. Circ J, 2011, 75(4): 970−978.
[6] Burashnikov A, Belardinelli L, Antzelevitch C. Acute dronedarone is inferior to amiodarone in terminating and preventing atrial fibrillation in canine atria[J]. Heart Rhythm, 2010, 7(9): 1273−1279.
[7] Van Gelder IC, Haegeli LM, Brandes A,. Rationale and current perspective for early rhythm control therapy in atrial fibrillation[J]. Europace, 2011, 13(11): 1517−1525.
[8] Tadros R, Khairy P, Rouleau JL,. Atrial fibrillation in heart failure: drug therapies for rate and rhythm control[J]. Heart Fail Rev, 2014, 19(3): 315−324.
[9] Silvet H, Hawkins LA, Jacobson AK. Heart rate control in patients with chronic atrial fibrillation and heart failure[J]. Congest Heart Fail, 2013, 19(1): 25−28.
[10] Kirchhof P, Lip GY, Van Gelder IC,. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options—a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference[J]. Europace, 2012, 14(1): 8−27.
[11] Davy JM, Razcka F, Beck L,. Pharmacological treatment of atrial fibrillation[J]. Arch Mal Coeur Vaiss, 2004, 4(4): 63−70.
[12] Mun HS, Shen C, Pak HN,. Chronic amiodarone therapy impairs the function of the superior sinoatrial node in patients with atrial fibrillation[J]. Circ J, 2013, 77(9): 2255−2263.
[13] Hohnloser SH, Crijns HJ, van Eickels M,. Dronedarone in patients with congestive heart failure: insights from ATHENA[J]. Eur Heart J, 2010, 31(14): 1717−1721.
[14] Remme WJ, Swedberg K, Task Force for the Diagnosis and Treatment of Chronic Heart Failure, European Society of Cardiology. Guidelines for the Diagnosis and Treatment of Chronic Heart Failure[J]. Eur J Heart, 2001, 22(17): 1527−1560.
[15] Doyle JF, Ho KM. Benefits and risks of long-term amiodarone therapy for persistent atrial fibrillation: a meta-analysis[J]. Mayo Clin Proc, 2009, 84(3): 234−242.
[16] Saklani P, Skanes A. Novel anti-arrhythmic medications in the treatment of atrial fibrillation[J]. Curr Cardiol Rev, 2012, 8(4): 302−309.
[17] Lakshmanadoss U, Lindsley J, Glick D,. Incidence of amiodarone hypersensitivity in patients with previous allergy to iodine or iodinated contrast agents[J]. Pharmacotherapy, 2012, 32(7): 618−622.
(编辑: 周宇红)
Efficiency of intravenous amiodarone in congestive heart failure patients with atrial fibrillation
ZHANG Rong-Sheng1, GU Xiang2*
(1Department of Cardiology, Jiangsu Provincial Corps Hospital, Chinese People’s Armed Police Forces, Yangzhou 225003, China;2Department of Cardiology, Northern Jiangsu People’s Hospital, Yangzhou 225003, China)
To determine the effect of intravenous amiodarone on the rapid ventricular rate in the patients with atrial fibrillation (AF) and congestive heart failure (CHF).Seventy elderly patients with AF and CHF [ventricular rate >120beats/min, New York Heart Association (NYHA) class Ⅱ−Ⅳ] hospitalized in the Northern Jiangsu People’s Hospital from September 2012 to September 2013 were recruited in this study. They were randomized into 2 groups: amiodarone group (=35, loading dosage of 150mg followed by a dose of 1.5mg/min) and deslanoside group (=35, loading dosage of 0.4 or 0.2mg in slow injection, and more 0.2mg should be medicated if no effect occurred in 1h later). The ventricular rate, mean response time, successful cardioversion ratio, brain natriuretic peptide (BNP) level, the adverse effects and follow-up outcomes were recorded after medication.The ventricular rate was significantly decreased in 1, 2 and 24h after treatment than before in both groups (<0.01), even in 30 min in amiodarone group (<0.01). In 2h after treatment, the ventricular rate was reduced by 47% in amiodarone group and by 28% in deslanoside group when compared with the rate before treatment. Significant difference was found in the rate between the 2 groups in 30min, 1h, 2h and 24h after treatment (<0.01). The total effective rate on controlling rapid AF was 79.8% (amiodarone group) and 72.3% (deslanoside group) respectively (>0.05). In 3 months after discharged from hospital, the ratio of persistent AF after the medications (amiodarone, metoprorol and digoxin) was 60.0% (21/35) and 82.9% (29/35), respectively, and the rate of adverse effect was 8.6% and 11.4%, respectively, in the 2 groups (>0.05).Amiodarone is of safety, rapid effect and mild adverse effect in treatment of CHF patients with coexisting AF.
amiodarone; deslanoside; heart failure; atrial fibrillation
R592; R541.6; R541.7
A
10.11915/j.issn.1671-5403.2015.06.104
2015−01−23;
2015−05−04
顾 翔,E-mail: rongrong1981537@sina.com

