Invasion and Morphological Variation of the Non- Indigenous Barnacle Chthamalus challengeri (Hoek, 1883) in Yangshan Port and its Surrounding Areas
LIU Yan, XUE Junzeng, LIN Junda, and WU Huixian, *
Invasion and Morphological Variation of the Non- Indigenous Barnacle(Hoek, 1883) in Yangshan Port and its Surrounding Areas
LIU Yan1), XUE Junzeng1), LIN Junda2), and WU Huixian1), *
1)Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources, Shanghai Ocean University, Ministry of Education, Shanghai 201306, P.R. China 2)Department of Biological Sciences, Florida Institute of Technology, Melbourne, Florida 32901, USA
Invasive species generally possess unique characteristics that allow them to survive the invasion process in order to establish and spread in new habitats. Successful invaders must resist both physical and physiological stresses associated with the changing environment. A common littoral barnacle,Hoek, 1883 (Crustacea, Cirripedia), which is native to Japan, South Korea and northern China, has become established in the high-littoral zone adjacent to Yangshan Port, Shanghai, China. A comparison of the morphology ofspecies from Zhoushan archipelago with previous description indicates the occurrence ofThe new immigrant becomes a dominant species in certain high-intertidal habitats of the adjacent area to of Yangshan Port.was found in part of sampling sites in Zhoushan in 2010; however, it dispersed to all the eleven sampling sites in 2012. Densities ofhad increased over 10 times in the last 2 years, with the highest mean value reaching 39533±6243ind.m−2in the new habitat. The specific ratios of both operculum area () to base area () and average height of parietal plates () to length of base () revealed thatdisplays morphological changes to resist stronger currents in the new habitats for invasion.
; marine alien species; morphological variation; Yangshan Port; invasion
1 Introduction
Biological invasions are one of the four serious global marine ecological problems, with tremendous potential effects on public health, biodiversity, economy and agriculture (Sax and Brown, 2000; Torres., 2012). The introduction of non-indigenous species has increased exponentially and shows no sign of slowing down in the past 200 years (Rilov and Crooks, 2008). Marine species can spread far away from their habitat through ballast water, hull fouling, aquaculture, fisheries and recreational boating (Bax., 2003). Ocean-going ships play an important role for spreading fouling species from estuaries and ports (Apte., 2000). Since the beginning of Great Navigation Epoch, fouled ships have plied the oceans. Marine organisms crossed substantial distance and biogeographical barriers which was effectively facilitated by ships changing trading routes, increased navigation speed and traffic volume and relatively long port residence times (Carlton, 1985). The success of invasion depends on the ability of the invader and the response of species in the receiving community to their new environments (Smith, 2009). Previous studies indicated that some species can modify ecologically crucial phy- siological, morphological and history features and behavior within a lifetime in response to the changing environment (Harvell, 1986; West-Eberhard, 2003; Kistner and Dybdahl, 2013; Zenni., 2014).
The barnacleDarwins (=) which arrived to Europe from Australasian during World War II is the first well documented transoceanic barnacle invasion (Bishop, 1947; Crisp and Chipperfield, 1948; Stubbings, 1950; Crisp, 1958). A typical example of transoceanic spread is the invasion by the western Atlantic speciesof Hawaiian shores (Southward., 1998)., common along the west coast of North America, had been introduced to Japan during the last 20 to 40 years (Kado, 2003; Geller., 2008). A more recent incident of barnacle invasion is the titan barnacle,(Darwin, 1954) was introduced by shipping from west Atlantic to Australia (Yamaguchi., 2009). Previous research have shown that cirripedia can be transported as fouling on the hull of vessels or as larvae in ballast water (Torres., 2012). Many barnacles have taken advantages of strong tolerance to the complicated environment to reach new regions, where they often successfully colonized floating objects and harbor installations (Bishop, 1951; Southward, 1975).
When China enters the World Trade Organisation (WTO) and becomes more involved in the global economy, the major seaports are pivotal places where the international shipping and terminal operators interact (Wang and Slack, 2004). Yangshan Port is the biggest deep water port for container ships in the confluence of Yangtze River Estuary an Hangzhou Bay in China since 2005. It establishes business relations with more than 500 ports in nearly 200 countries. The Yangshan port is built on the islands of Greater and Lesser Yangshan island, part of Zhoushan archipelago, with fill from land reclamation (Notteboom, 2006). A classic paradigm in ecology is that islands are easier to be invaded than mainland locations by both terrestrial and marine organisms (Carlquist and Janish, 1965; MacDonald and Cooper, 1995; Elton, 2000). Oceanographic structures in Yangshan Port and its surrounding areas are very complex, with water from the Yangtze River Estuary, Hangzhou Bay and East China Sea, which provides important and domiant nutritive salts in coastal waters and creats the world-renowned Zhoushan fishery. Yangshan Port and its surrounding areas are the ideal habitat for certain marine organisms. Considering the increase in transatlantic leisure ship traffic and commercial maritime traffic stopping at Yangshan Port, these ocean-going vessels acts as ‘mobile stepping stones’ for fouling organisms and plankton from other harbours and estuaries, Yangshan Port is exposed to a high risk of species introduction. During phylogeographic research on intertidal biological investigation in the adjacent ocean area of Yangshan Port in 2010, a species ofdistinct from the two East China Sea species,.(Liu and Ren, 2007) and.(Liu and Ren, 2007), was also found in the southern coast of the Yangtze River Estuary. This species was identified as.(Hoek, 1883) and constitute the first record of this species in the adjacent sea areas of Yangshan Port..is native to the southern Hokkaido to Ryukyus of Japan (Utinomi, 1949; Utinomi, 1954; Utinomi, 1962; Utinomi, 1970), South Korea (Kim and Kim, 1980) and the Yellow Sea and Bohai Bay of China (Dong., 1980; Southward and Newman, 2003; Cao., 2013).
Following the discovery of.in the adjacent sea area of Yangshan Port, several surveys were conducted around the archipelago of Zhoushan to confirm its introduction. The present work reported on its distribution, density and morphological adaptation to the new habitat.
2 Materials and Methods
2.1 Study Area
is native to the northwest Pacific of Japan, South Korea and the Yellow Sea and Bohai Sea of China. We selected the Nanaehamain Hakodate Bay of Japan (41˚47´N, 140˚42´E) and the north of Yangtze River Estuary (34˚44´N-40˚13´N, 119˚35´E-121˚37´ E) to observe the ecological characteristics of.in its native habitat (Fig.1A).
The archipelago of Zhoushan (29˚32´N-31˚04´, 121˚30´E-132˚25´E) consists of dozens of islands forming three groups including western (Shengsi), central (Dinghai, Daishan, Zhoushan) and eastern (Liuheng) islands (Fig.1B).
2.2 Sample Collection Method and Area
.were collected from the 5 sites across the native range in Nanaehama of Japan (JP: SN1), Bohai Sea (BH: SN2 & SN3) and the Yellow Sea (YS: SN4 & SN5) (Fig.1A). In addition, alien species samples were collected from the 11 sites in the archipelago of Zhoushan (ZS: SZ1-SZ11) (Fig.1B). All the samples were collected in February, 2010 and February, 2012.
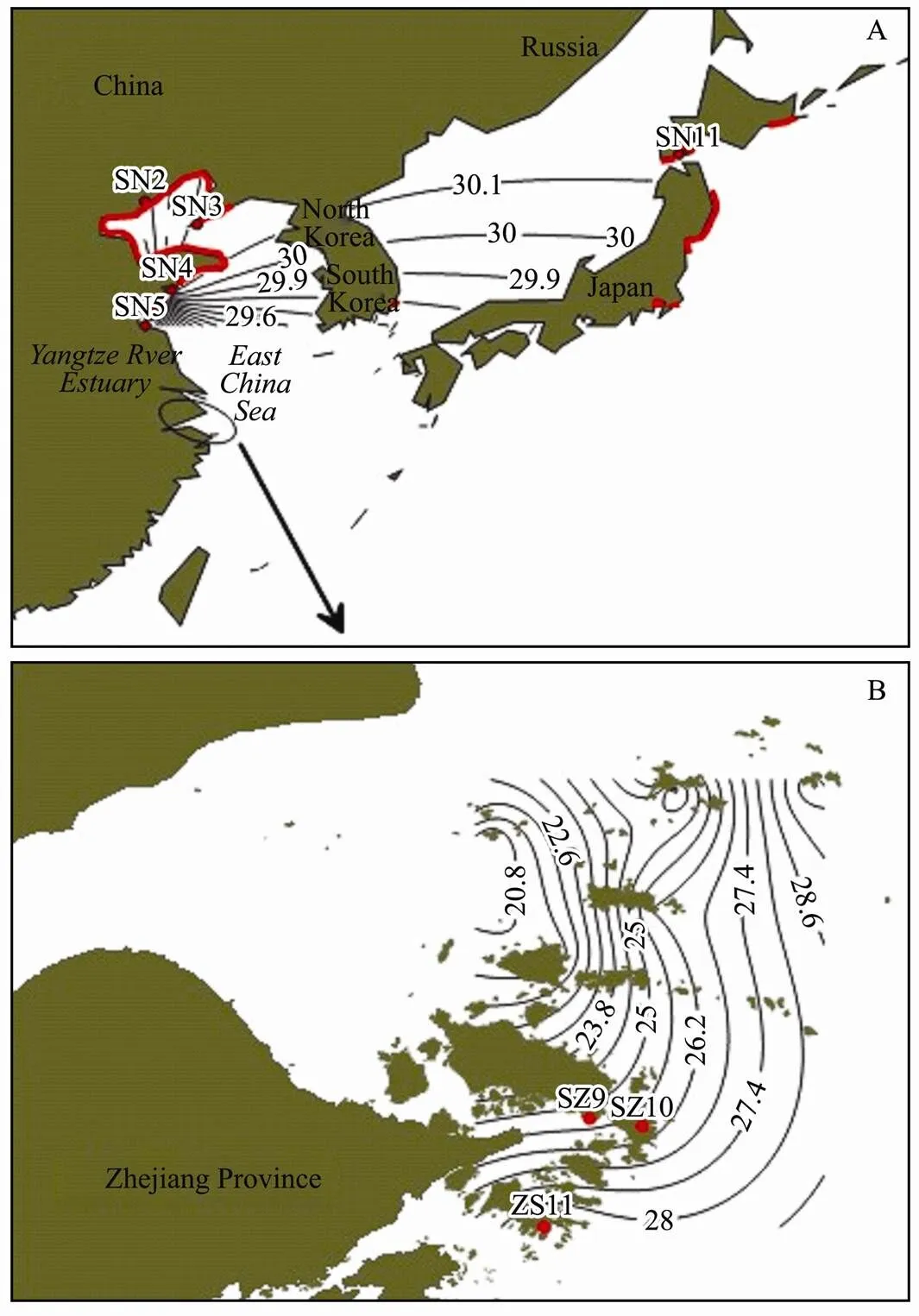
Fig.1 Chthamalus challengeri distribution in its native habitat (A) and the new habitat in adjacent sea area of Yangshan Port (B). Red lines are the native habitats of C. challengeri; the red filled with black edge circles are the sampling sites in its native habitats; Red spots are the sampling sites in the adjacent sea area of Yangshan Port.
A sampling location with high population density of.at at each site was selected, and the shell size and age were used to assess the present extent of the invasion by.using 1 of 3 quadrats (10×10, 25×25, 100×100cm), depending on the visual estimation density. The density of.at each sampling site was measured 3 or 4 times and the average value was recorded. All species in the quadrat were photographed, recorded and collected for later identification.
Identification of barnacles was performed according to Liu and Ren (mainly based on size, shape and number of parietal plates, calcareus) (Liu and Ren, 2007). The barnacles were dissected, all samples were identified based on morphological characteristics (Southward and Crisp, 1963).
Length () and width () of operulum, length () and width () of basis, height of carinolateral (1,2), crina (3) and rostrum (4) and thickness of walls () were measured to the nearest 0.03mm using digital caliper (Fig.2). We measured more than 50 individuals at every sampling site. All sampling sites were divided into four groups to analyze the measured parameters: JP (SN1), BH (SN2 & SN3), YS (SN3, SN4 & SN5) and ZS (SZ1-SZ9).
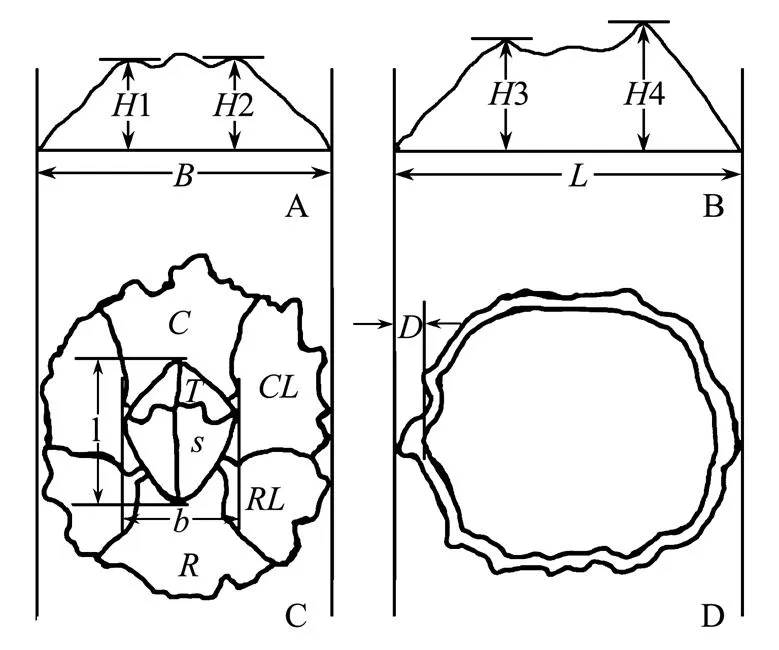
Fig.2 Measured parameters and morphological appearance of C. challengeri mode pattern. H1&H2: height of carinolateral, B: width of base; H3: height of carina, H4: height of rostrum, L: length of base; length of opecrulum, b: width of opecrulum, T: tergum, S: scutum, C: carina, CL: carinolateral, RL: rostrolateral, R: rostrum; D: thickness of walls.
2.3 Analysis Software and Process
Operculum area (), basis area () and volume () ofwere calculated with the following formula (Cai., 1987; Wang., 2011), whereis the average value of,,and:
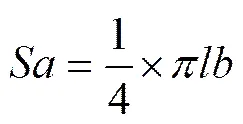
,
Statistical analysis of the data was carried out using SPSS16.0 for windows (Norusis, 2008).
3 Results
3.1 Morphological Features of.in the New Habitat
.collected in the adjacent sea area of Yangshan Port has 6 shell plates (Fig.3C). The shell plates were grey or maroon. The appearance of its individual external form is conical for single and cylindric for cluster growth (Figs.3A, C). The basis was membranous without whorls of accessory plates. Alae of rostrum was well developed and rarely a compound form. Scutum was shaped triangular, with the width longer than the height of it. The above characters above showed evidence of this species belonging to Chthamalidae, Chthamainae,. Sutures of plates were generally with fine denticles. Sculpture of opercular valves was remarkable, which was different to.,.and.with smooth and flat operaulum valves. Inner surface of scutum had adductor muscle ridges, the occludent margin was protuberant (Fig.3B, a1, a2). However, there was no adductor muscle ridge for inner surface of scutum for species of.(Liu and Ren, 2007). Tergum was narrow and long (Fig.3B, b1, b2). Mandible had 4 teeth, with the 2nd-3rd teeth generally with accessory denticles (Fig.3D). Maxillule had anunambiguous indention (Fig.3E). The terminal segments of cirrus had several serrate spines; however, these spines had no large lower teeth (Fig.3F).
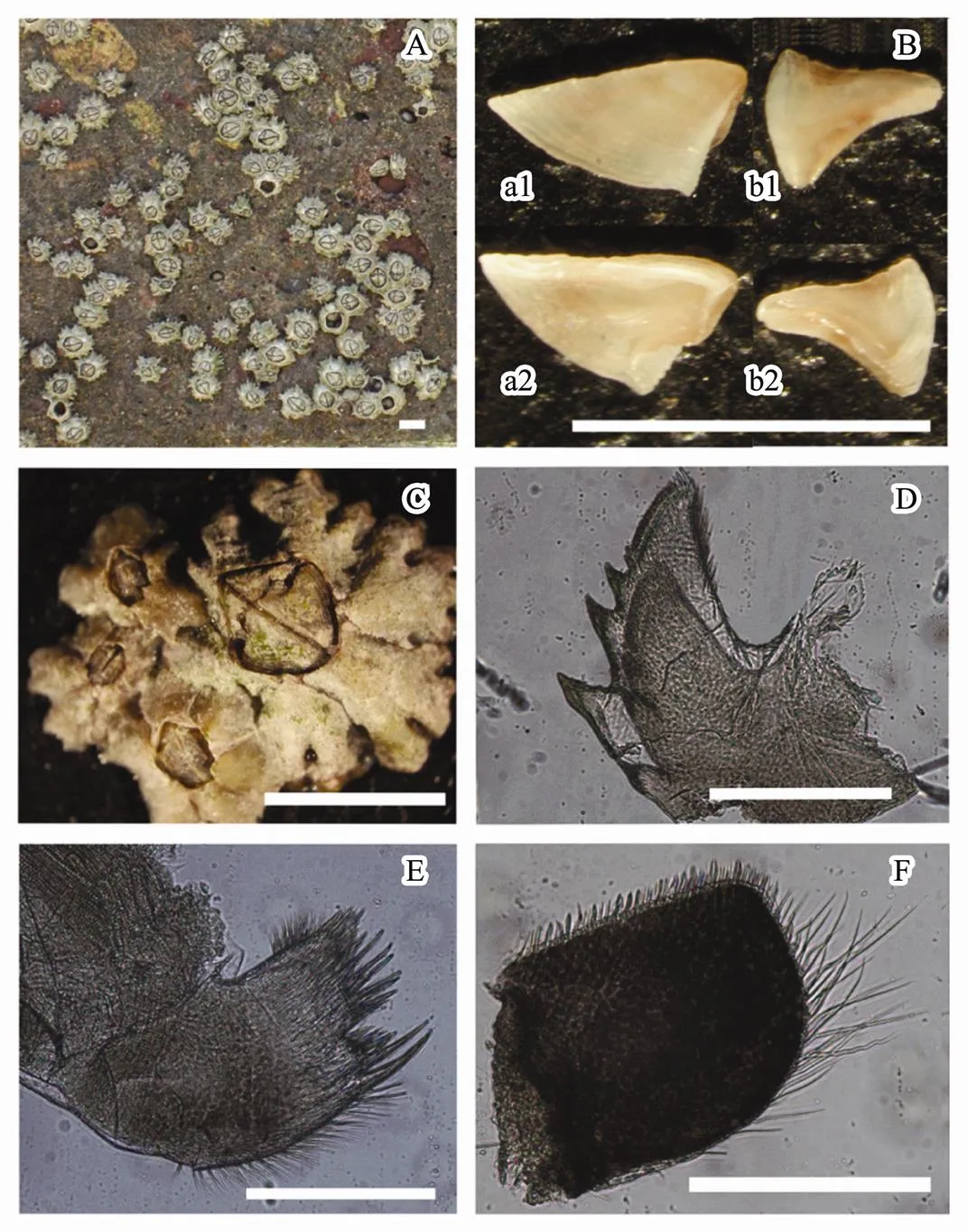
Fig.3 The morphological characteristics for C. challenger in the adjacent water area of Yangshan Port. A) population; B) terga and scuta (a1: scutum external; a2: scutum internal; b1: tergum external; b2: tergum internal); C) individual; D) mandible; E) maxillule; F) cirrus; A, B, C scale bar are 5mm; D, E, F scale bar are 200μm.
3.2 The Distribution ofin Native and New Habitats
Densities of.at the stations in the native habitat (SN1-SN5) varied from (7232±542)ind.m−2(SN1, 2010) to (51205±216)ind.m−2(SN4, 2010) (Table 1). In 2012, densities of.maintained the similar values which ranged from (7864 ± 411)ind.m−2to (5083±2141)ind.m−2(see Table 1)..was then a common high-shore barnacle species in its native habitat.
.was found only in SZ1, SZ4, SZ5, SZ6, SZ7, SZ8 and SZ10 in the archipelago of Zhoushan in 2010. In 2010, densities of.in the sampling sites ranged from (6±2) ind.m−2to (28±11) ind.m−2. The highest density occurred in SZ5 and SZ8. However, when we collected.at each station of this area in 2012, densities had increased for more than 10 times, of which the highest mean value reached to (39533 ± 6243)ind.m−2 in SZ5 (Table 1). The fastest-growing population was in SZ11, with the density of (21432 ± 4000)ind.m−2(and which was not collected in 2010) (Table 1)..was the absolutely dominant species at the stations of SZ5, SZ6 and SZ11 in 2012, and the endemic barnaclesand.almost disappeared in these high tidal regions. The results demonstrate that density of.had grew rapidly in Zhoushan archipelago from 2010 to 2012.
The size-frequency histograms of different basal lengths for.were consistent from 2010 to 2012 in its native habitat (Fig.4A, B, C). However, it showed a significant difference trade in Zhoushan archipelago..basal lengths ranged from 2.5mm-4.5mm in Zhoushan archipelago in 2010, and 30% of them were about 3.5mm (Fig.4D). Nevertheless, the histogram peak occurred in the length of 6.5mm in 2012, and the basal length ranged from 3mm-12mm (Fig.4D)..basal length was increasing continuously in Zhoushan archipelago from 2010 to 2012.
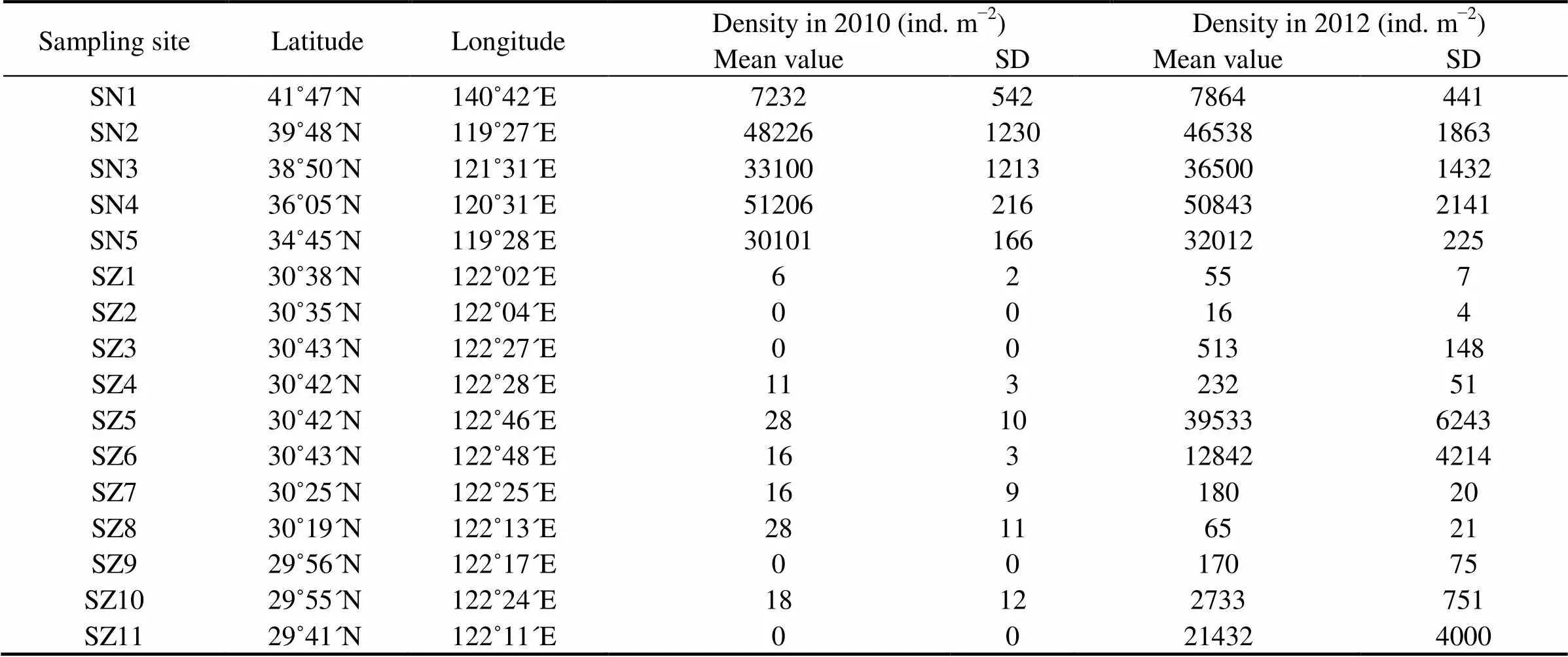
Table 1 Sampling sites and densities of C. challengeri
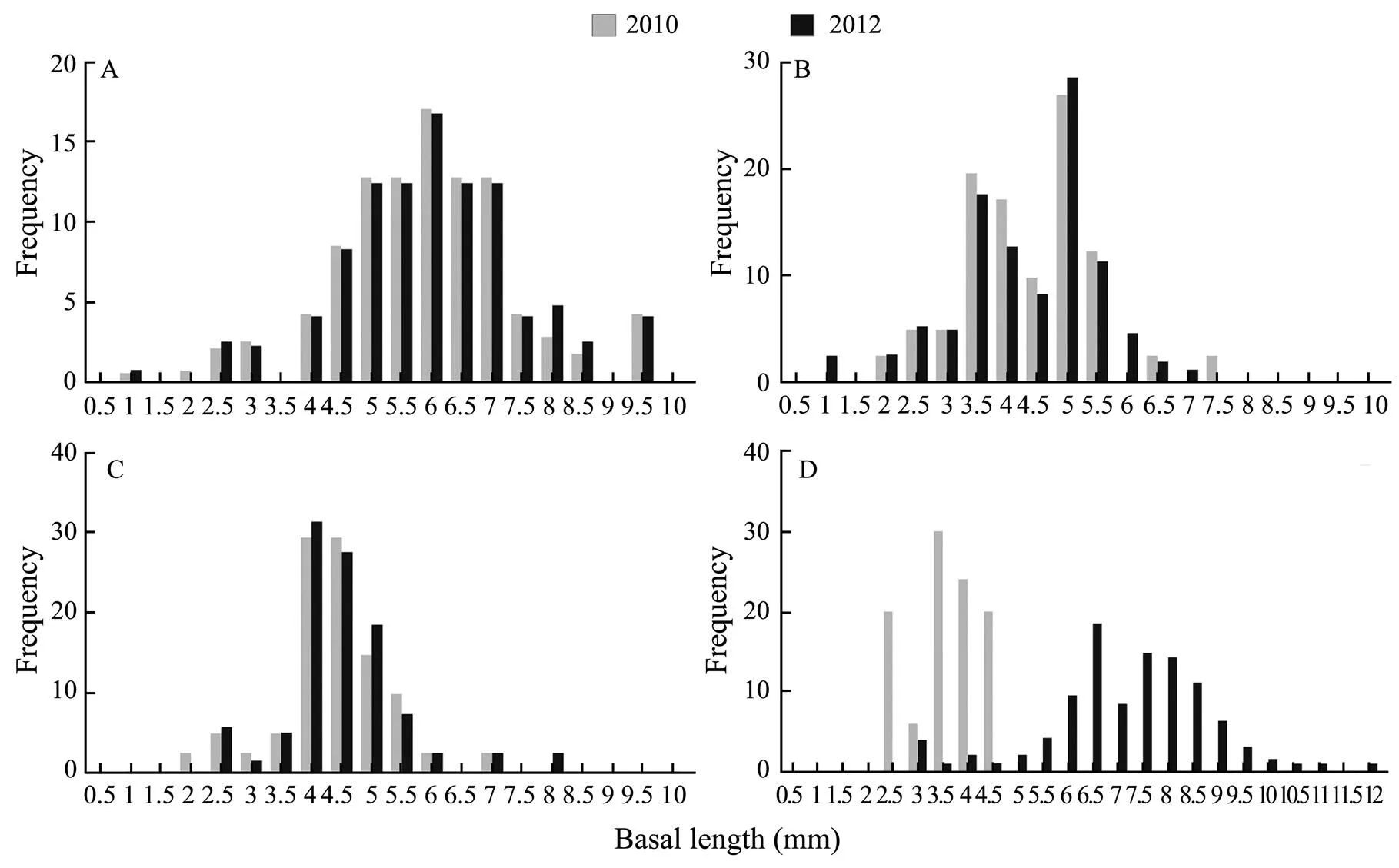
Fig.4 Size-frequency histogram of the basal length for C. challengeri in different locations from 2010 to 2012. A) JP; B) BH; C) YS; D) ZS.
3.3 Morphological Variation of.in New Habitat
The parameters of length and width of operculum and basis, height of carinolateral, carina and rostrum and thickness of walls were measured in this study (Table 2). The results showed that the basis area and volume of mature individuals in ZS were larger than those of the samples collected in other regions. For other parameters, ZS samples ranged between JP and YS.
We calculated the ratios of/and/to assess the morphological variation of.in the new habitat and its ecological adaptability to the changed environment. The specific ratios of/and/showed a significant down trend due to the change of latitude from north to south (<0.05)..in adjacent sea area of Yangshan Port developed the unique morphological characteristics. The ratio of/in the new habitat was significantly lower than that in native habitat (<0.05) (Fig.5A), showing that.in new habitat have low height, so it can increase its ability to withstand the water surge. Furthermore, the ratio of/showed the same trend to/(Fig.5B), which reveals thatin the new habitat had smaller operculum area than that in the native habitat where they have identical area for basis. This morphological character change suggests thathas strong ability of water retention and drought resistance in new habitat.
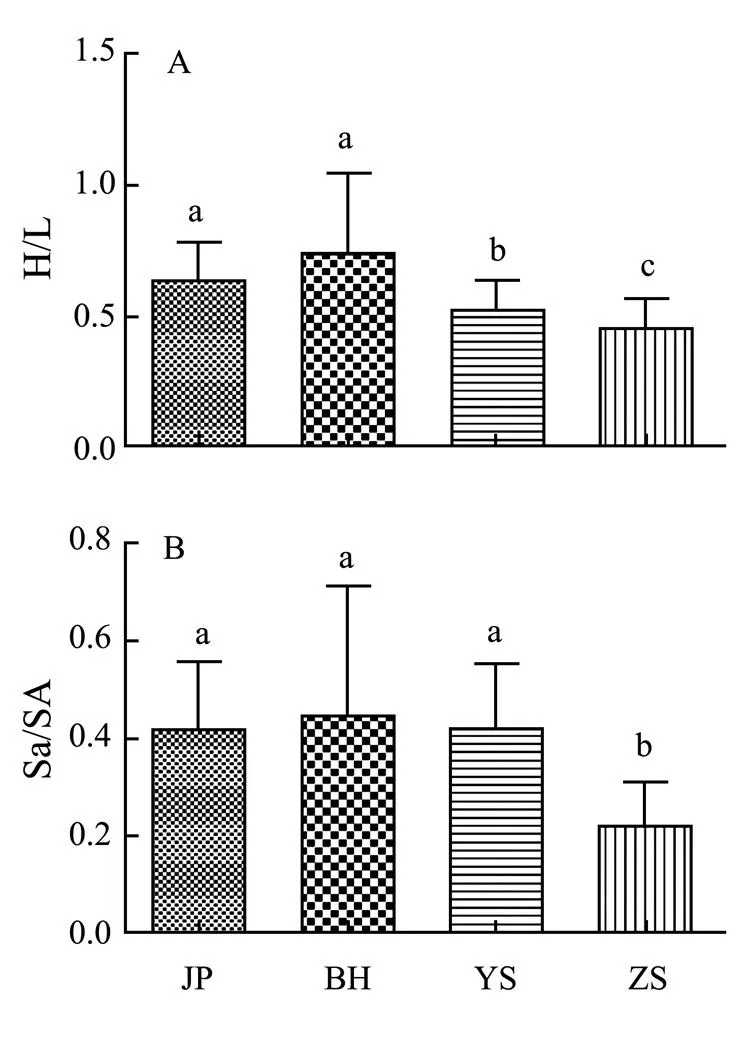
Fig.5 The ratios of some measured parameters for C. challengeri at different sampling sites. Based on one way ANOVA, P<0.05.

Table 2 Measured parameters of C. challengeri
Notes:, Parameter of individuals;, length of operculum;, width of operculum;, mean value of plates height;, length of basis;, width of basis;, thickness of walls;, operculum area;, basis area;, volume.
4 Discussion
In the northwestern Pacific,is a common high tide zone barnacle in temperate waters.is reliably reported only from southern Hokkaido to Ryukyus of Japan (Utinomi, 1949; Utinomi, 1954; Utinomi, 1962; Utinomi, 1970), South Korea (Kim and Kim, 1980) and the Yellow Sea and Bohai Bay of China (Dong, 1980; Southward and Newman, 2003; Cao, 2013) (Fig.1A). Liu and Ren (2007) made a special mention ofin ‘Fauna Sinica’, which recorded that ‘is dominant intertidal species along Bohai Bay to north Yellow Sea, but has not been collected in anywhere of the south coast of the Yangtze River Estuary ever’. Cai (1993) summarized the results of marine biological survey in the Zhejiang sea area from the 1950’s to 1990’s, and noted that cirripedia of lithofacies substrate intertidal zone in Zhejiang including,.,.,,.,,,.,.and.. The species composition in the rocky intertidal zone of Zhoushan is basically consistent with that in the past work till the early 20th century (Yang and Chen, 1996; You, 1997; Yang and Chen, 1998; Shao, 1999; Yang and Chen, 1999; Zeng, 1999a; Zeng, 1999c; Zeng, 1999b; Shao, 2001; Zhu, 2006; Liao, 2007) and there is no report on the appearance ofin this area.
Xue. (2010) collectedin the south of Yangtze River Estuary for the first time during the ecological investigation of Yangshan Port in 2010. Comparing the morphology ofspecies from Zhoushan archipelago with that ofcollected from its native habitat (Liu and Ren, 2007), we could identify the species from new habitat as.. The genetic distances and population genetic structure of.were investigated using mitochondrial cytochrome c oxidase subunit I (I) gene sequences in the preliminary work. The population genetic distance of samples collected from Zhoushan sea area withI sequences of.downloaded from NCBI is 0.994%, which shows a great possibility that the new species from Zhoushan sea area is the species of.(Liu, 2014)..has been found on natural substrata at Yangshan Port (SZ1 & SZ2), Sijiao (SZ3 & SZ4), Gouqi (SZ5), Shengshan (SZ6), Qushan (SZ7), Daishan (SZ8), Zhoushan Island (SZ9), Zhujiajian (SZ10) and Liuheng (SZ11). The evidence is against natural dispersal of Chinese faunistic elements southward across the Yangtze River Estuary (Liu and Ren, 2007). Therefore it is most likely that.has reached the new habitat by remote dispersal, probably by human agency. However, considering the present distribution of.in the adjacent sea area of Yangshan Port and the usual lag time between notable abundance and an introduction, its occurrence was possibly earlier, but at first more localized..was found in Kinmen and Matsu islands as a new record species which was collected in 2007 and first reported in 2009 (Chan., 2009; Chan and Lee, 2012; Cheang., 2012). Ifhave settled in Kinmen and Matsu islands as native species, there was great chance for it to inhabit the costal area of Fujian long ago for researchers to identify. However, there was no record ofbeing collected along the coast of Fujian Province before (Huang and Cai, 1961; Chen, 1989; Chen., 1994; Zhou., 2001a; Zhou., 2001b; Huang., 2006; Lv., 2008; Fang., 2009). We consider the appearance of.in the south of Yangtze River Estuary as the invasion of non-indigenous species..might be introduced to the new habitat earlier than 2007 based on the record time in Kinmen and Matsu islands. A successful invader must be able to survive at all stages leading to and following its introduction in complicated changing environmental conditions (Smith, 2009). With the lower metabolic rate, cold-water species usually have a longer larval developmental time, and are likely to be dispersed over greater geographical distances than warm-water species (O’Connor., 2007). Laboratory experiment data showed that larval of.could complete development to settlement in temperature ranging from 10℃ to 25℃, and the larval duration was from 12d (in 25℃) to 30d (in 10℃) (unpublished data by the authors). The strong tolerance to complicated environment of.larval can increase the survival rate in the transformation in ballast water or long distance dispersal. This may be the key factor for its successful invasion.
Adaptive responses have been viewed primarily to result from evolutionary changes in fixed traits in population (Thompson, 1998; Cox, 2004). The morphogenesis of barnacle is a sign of adaptation response to the changing environment (Barnes and Healy, 1965). The high specific ratio of average height of walls () to length of basis () can reflect the strong capability of surge withstanding, which has been proved in the study of(Barnes and Healy, 1965),(Barnes and Healy, 1969),(Otway and Anderson, 1985),(Bell, 1979),(Pentcheff, 1991),(Wang., 2011),.(Lu., 1998). Previous studies have shown that laigh morphology for barnacles contributes to the resistance to ocean current and wind waves. Wind waves usually result from the wind blowing over a stretch of water surface. There is a positive correlation relationship between wind waves and wind velocity (Wanninkhof and Bliven, 1991). The annual average wind velocity in adjacent ocean area of Yangshan Port is 6ms−1-8ms−1, which is higher than that in native habitat of 4ms−1-6ms−1(Cai, 2010), so it can be predicted that the annual wind waves in new habitat are slightly stronger than those in native habitat. The morphology of.in Zhoushan archipelago is lower than that in the northern ocean area, which can be interpreted as the adaptation in morphology, so it can significantly improve the capability of surge withstanding.
High tide zone barnacles should be exposed in air for half of their life, therefore water retention become quite important for their survival and development. Most water is evaporated from operculum and a small amount evaporated from walls of barnacles, so barnacle operculum area is directly related to evaporation of water (Lu., 1998; Wang., 2011). Wang. (2011) stuied the specific ratio of operculum area () to basis area () for.in different sunshine conditions, the results showed that operculum area became smaller with stronger sunshine. Previous researches showed that barnacle would lose water faster in the higher temperature environment with increasing sunlight intensity, and the smaller operculum area can improve the ability of water retention (Wang, 2011). On the coasts of Bohai Sea, Yellow Sea and Nanaehama, annual mean surface water temperature was 10℃-15℃(Hickox, 2000; Cai, 2010), 13℃-16℃(Chen, 2009) and 5℃-8℃(Iwaki, 1975), respectively, in the last 40 years. The annual mean air temperatures along these coasts were about 10℃(Hickox, 2000; Cai, 2010), 15℃(Chen, 2009) and 6℃(Wang, 2004). In adjacent sea areas of Yangshan Port the annual mean surface water temperature and air temperature are about 20℃-24℃(Cai, 2010) and 20℃(Chen., 2009) respectively, which is higher than the temperature in the native habitat of.. The operculum area of.in the new habitat is smaller than that in native habitat, which perhaps aids it in a stronger drought resistance ability to prevent evaporation. This could be an adaptive response of.to survive in the new high-shore intertidal habitat with higher surface water temperature and air temperature than in the native habitat. In addition, this could also provide favorable conditions for further dispersal and population expansion for..
Further studies should address phylogeography and population dynamics of this alien barnacle to understand its introduction pathway, dispersal mechanism and impact on native species. Although this introduction may be relatively benign, it occurrence shows that there is a need to establish further research programs to prevent and control the potentially harmful introduction to the marine fauna of the adjacent sea areas of Yangshan Port.
Acknowledgements
This work was supported by China’s National Special Research Fund for Non-Profit Marine Sector (No. 2013 418027), Marine Biology Program of Shanghai Leading Academic Disipline (No. J50701) and Marine Special Fund of Shanghai Committee of Science and Technology (No. 11dz1205000) and a Doctoral Research Fund from Shanghai Ocean University (No. A2-0302-14-300084)..
Apte, S., Holland, B. S., Godwin, L. S., and Gardner, J. P. A., 2000. Jumping ship: A stepping stone event mediating transfer of a non-indigenous speciesa potentially unsuitable environment., 2 (1): 75-79.
Barnes, H., and Healy, M. J. R., 1965. Biometrical studies on some common cirripedes I.: Measure- ments of the scuta and terga of animals from a wide geographical range., 45 (3): 779-789.
Barnes, H., and Healy, M., 1969. Biometrical studies on some common cirripedes. II discriminant analysis of measurements on the scuta and terga of,.,.Darwin,.Darwin, and.Darwin (.)., 4 (1): 51-70.
Bax, N., Williamson, A., Aguero, M., Gonzalez, E., and Geeves, W., 2003. Marine invasive alien species: A threat to global biodiversity., 27 (4): 313-323.
Bell, P. D., 1979. Some factors affecting distribution and related morphology variations in(Cirripedia, Thoracica)., 64 (2): 179-182.
Bishop, M. W., 1951. Distribution of barnacles by ships., 167: 531.
Bishop, M. W. H., 1947. Establishment of an immigrant barnacle in British coastal waters., 159 (501): 427- 444.
Cai, R. S., 2010.. China Ocean Press, Beijing, 6-12 (in Chinese with English abstract).
Cai, R. X., Mao, W. D., and Huang, Z. G., 1987. The mor- phological variation ofPilsbry (Cru- stacean, Cirripedia)., 33 (2): 166-173 (in Chinese with English abstract).
Cai, R. X., 1993. Survey Report of Marine Biological Resources in Islands of Zhejiang Province. Zhejiang Aquatic Bureau, Zhejiang Province, 4-72 (in Chinese).
Cao, W. H., Yan, T., Li, Z. F., Li, J., and Cheng, Z. Q., 2013. Fouling acorn barnacles in China-A review., 31: 699-711.
Carlquist, S., and Janish, J. R., 1965.. Natural History Press, Boston, 1-44.
Carlton, J. T., 1985. Transoceanic and interoceanic dispersal of coastal marine organisms: The biology of ballast water., 23: 313-371.
Chan, B. K. K., Prabowo, R. E., and Lee, K. S., 2009.,:. National Taiwan Ocean University, Keelung, 165- 166.
Chan, B. K. K., and Lee, P. F., 2012. Chapter 7 Biogeography of Intertidal Barnacles in Different Marine Ecosystems of Taiwan–Potential Indicators of Climate Change? In:. Lawrence, S., ed., InTech, Croatia, 119-136.
Cheang, C. C., Tsang, L. M., Ng, W. C., Williams, G. A., Chu, K. H., and Chan, B. K., 2012. Phylogeography of the cold-water barnaclein the north-western Pacific: Effect of past population expansion and contem- porary gene flow., 39 (10): 1819- 1835.
Chen, G. T., Yang, X. L., Yang, J. Y., and Gao, A. G., 1994. Ecological and environment qualitative study in the intertidal zone and land area of Nanji archipelago., 12 (2): 1-15 (in Chinese with English abstract).
Chen, P. J., 1989. The preliminary study on the ecology of the intertidal zone in the north to Minjiang River, Fujian I- The biomass and its distribution.,(2): 16-25 (in Chinese with English abstract).
Chen, S. Y., Wang, J. S., Shi, Y. Y., and Guo, Z. X., 2009. The changes of annual mean temperature in monsoon area of East China., 31 (3): 462-471.
Cox, G. W., 2004.:,,,. Island Press, Washington D. C., 12-14.
Crisp, D. J., and Chipperfield, P. N. J., 1948. Occurrence of(Darwin) in British waters., 161: 64.
Crisp, D. J., 1958. The spread ofDarwin in northwest Europe., 37 (2): 483-520.
Dong, Y. M., Chen, Y., and Cai, R. X., 1980. Preliminary study on the Chinese cirripedian fauna (Crustacea)., 2: 124-131 (in Chinese).
Elton, C. S., 2000.. University of Chicago Press, Chicago, 21-24.
Fang, S. H., Lv, X. M., Zhang, Y. P., and Hong, Y. C., 2009. Spatio-temporal distribution and secondary production of macrobenthos in intertidal zone of Dongwu of Meizhou Bay, Fujian Province., 28 (3): 392-398 (in Chinese with English abstract).
Geller, J., Sotka, E. E., Kado, R., Palumbi, S. R., and Schwindt, E., 2008. Sources of invasions of a northeastern Pacific acorn barnacle,, in Japan and Argentina., 358: 211.
Harvell, C. D., 1986. The ecology and evolution of inducible defenses in a marine bryozoan: Cues, costs, and consequences., 128 (6): 810-823.
Hickox, R., Belkin, I., Cornillon, P., and Shan, Z. Q., 2000. Climatology and seasonal variability of ocean fronts in the East China, Yellow and Bohai Seas from satellite SST data., 27 (18): 2945-2948.
Huang, Z. G., and Cai, R. X., 1961. Studies on the ecology of fouling organism of Amoy Harbor., 8 (3): 221-250 (in Chinese with English abstract).
Huang, Z. G., Hong, R. B., and Zhang, L. F., 2006. Study of species in Xiamen Bay., 45 (sup.2): 10-15 (in Chinese with English abstract).
Iwaki, T., 1975. Breeding and settlement ofHoek on the southern coast of Hokkaido., 1 (26): 1-10.
Kado, R., 2003. Invasion of Japanese shores by the NE Pacific barnacleand its ecological and biogeo- graphical impact., 249: 199- 206.
Kim, I. H., and Kim, H. S., 1980. Systematic studies on the cirripeds (Crustacea) from Korea 1:(Cirripedia, Thoracica, Balonomorpha).,23 (3): 161-194.
Kistner, E. J., and Dybdahl, M. F., 2013. Adaptive responses and invasion: The role of plasticity and evolution in snail shell morphology., 3 (2): 424-436.
Liao, Y. B., Zeng, J. N., Chen, Q. Z., Gao, A. G., Shou, L., and Xu, X. Q., 2007. Macrobenthos community patterns in the intertidal zone of the Shengsi archipelago in spring and autumn., 53 (6): 1000-1010 (in Chinese).
Liu, R. Y., and Ren, X. Q., 2007.. Science Press, Beijing, 280-300 (in Chinese with English abstract).
Liu, Y., Wu, H. X., and Xue, J. Z., 2014. The invasion and its impact for the spread ofin Zhoushan Sea area., 38 (7): 1047-1055 (in Chinese with English abstract).
Lu, J. P., Lu, W., Hu, L. C., Wang, W. J., and Cai, R. X., 1998. The morphological variation of Tetraclita (Crustacean, Cirripedia)., 16 (1): 31-38 (in Chinese with English abstract).
Lv, X. M., Fang, S. H., Zhang, Y. P., and Wu, P. R. 2008. Community structure and secondary production of macro- benthos in the intertidal zone of Haitan Strait, Fujian Province., 54 (3): 428-435 (in Chinese with English abstract).
MacDonald, I. A. W., and Cooper, J., 1995. Insular lessons for global biodiversity conservation with particular reference to alien invasions. In:. Vitousek, P. M.,., eds., Springer, Berilin, 189-203.
Norusis, M., 2008. SPSS 16.0 statistical procedures companion. Prentice Hall Press, New Jersey, 11-14.
Notteboom, T., 2006. Strategic challenges to container ports in a changing market environment., 17: 29-52.
O'Connor, M. I., Bruno, J. F., Gaines, S. D., Halpern, B. S., Lester, S. E., Kinlan, B. P.and Weiss, J. M., 2007. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation., 104 (4): 1266-1271.
Otway, N. M., and Anderson, D. T., 1985. Variability of shell growth and morphology of the wall-plate junctions of the intertidal barnacle(Cirripedia: Tetrac- litidae)., 85 (2): 171-183.
Pentcheff, N. D., 1991. Resistance to crushing from wave-borne debris in the barnacle., 110 (3): 399-408.
Raniello, R., Lorenti, M., Brunet, C., and Buia, M. C., 2004. Photosynthetic plasticity of an invasive variety ofin a coastal Mediterranean area: Light harvesting capacity and seasonal acclimation., 271: 113-120.
Rilov, G., and Crooks, J. A., 2008.:. Springer, Berlin, 1-77.
Sax, D. F., and Brown, J. H., 2000. The paradox of invasion., 9 (5): 363-371.
Shao, X. Y., You, Z. J., Cai, R. X., Lu, J. P., and Wang, Y. J., 1999. Studies on ecology of intertidal zone around Zhejiang Province islands I. Species composition and distribution.(), 18 (2): 113-119 (in Chinese with English abstract).
Shao, X. Y., You, Z. J., Cai, R. X., and Lu, J. P., 2001. Studies on ecology of intertidal zone around islands of Zhejiang Province II. Quantities and distribution.(), 20 (4): 279-286 (in Chinese with English abstract).
Smith, L. D., 2009. Chapter 10 The role of phenotypic plasticity in marine biological invasions. In:. Springer, Berlin, 177-202.
Southward, A. J., and Crisp, D. J., 1963. Catalogue of main marine fouling organisms (found on ships coming into European waters)., Paris, 8-10.
Southward, A. J., 1975. On the evolutionary significance of the mode of feeding of Pogonophora.,(13): 77-85.
Southward, A. J., Burton, R. S., Coles, S. L., Dando, P. R., DeFelice, R., Hoover, J., Parnell, P. E., Yamaguchi, T., and Newman, W. A., 1998. Invasion of Hawaiian shores by an Atlantic barnacle., 165: 119- 126.
Southward, A. J., and Newman, W. A., 2003. A review of some common Indo-Malayan and western Pacific species ofbarnacles (Crustacea: Cirripedia)., 83 (4): 797-812.
Stubbings, H. G., 1950. Earlier records ofDarwin in British waters., 166: 277-278.
Thompson, J. N., 1998. Rapid evolution as an ecological process., 13 (8): 329-332.
Torres, P., Costa, A. C., and Dionísio, M. A., 2012. New alien barnacles in the Azores and some remarks on the invasive potential of Balanidae., 66 (4): 513-522.
Utinomi, H., 1949. Studies on the Cirripedian fauna of Japan-VI. Cirripeds from Kyusyu and Ryukyu islands., 1 (2): 19-37.
Utinomi, H., 1954. Invertebrate fauna of the intertidal zone of the Tokara islands-IX. Cirripedia., 4 (1): 17-26.
Utinomi, H., 1962. Studies on the Cirripedian fauna of Japan-VIII. Thoracic Cirripeds from western Kyusyu., 10 (2): 211-239.
Utinomi, H., 1970. Studies on the cirripedian fauna of Japan-IX. Distributional survey of thoracic cirripeds in the southeastern part of the Japan Sea.,(17): 339-372.
Wang, B. Q., Tian, H., Wu, H. X., and Xue, J. Z., 2011. An analysis on morphological plasticity ofto the environment at Yangshan Port., 30 (1): 18-22 (in Chinese with English abstract).
Wang, H. M., Saigusa, N., Yamamoto, S., Kondo, H., Hirano, T., Toriyama, A., and Fujinuma, Y., 2004. Net ecosystem CO2exchange over a larch forest in Hokkaido, Japan., 38 (40): 7021-7032.
Wang, J. J., and Slack, B., 2004. Regional governance of port development in China: A case study of Shanghai International Shipping Center., 31 (4): 357-373.
Wanninkhof, R. H., and Bliven, L., 1991. Relationship between gas exchange, wind speed, and radar backscatter in a large wind-wave tank., 96: 2785- 2796.
West-Eberhard, M. J., 2003.. Oxford University Press, Oxford, 21-22.
Yamaguchi, T., Prabowo, R. E., Ohshiro, Y., Shimono, T., Jones, D., Kawai, H., Otani, M., Oshino, A., Inagawa, S., and Akaya, T., 2009. The introduction to Japan of the Titan barnacle,(Darwin, 1854) (Cirripedia: Balanomorpha) and the role of shipping in its translocation., 25 (4): 325-333.
Yang, W. X., and Chen, Y. S., 1996. Studies on species constitution of benthic biology and fauna and flora characteristics in rocky intertidal zone along Shengsi archipelago.), 20 (4): 82-85 (in Chinese with English abstract).
Yang, W. X., and Chen, Y. S., 1998. Community ecology of intertidal zone of Shengsi archipelago II. Communiry structure of benthic invertebrates in rocky intertidal zone., 9 (1): 75-78 (in Chinese with English abstract).
Yang, W. X., and Chen, Y. S., 1999. Community ecology of intertidal zone of Shengsi islands III. Species distribution of benthos in rocky intertidal zone., 17 (1): 61-65 (in Chinese with English abstract).
You, Z. J., Hong, J. C., Wong, Y. N., Xu, R., Li, J. Z., and Zan, J. S., 1997. Distribution pattern of species on the intertidal rocky shore of the Shenshan island, Zhejiang., 10 (1): 64-71 (in Chinese with English abstract).
Zeng, D. G., Cai, R. X., Huang, Z. G., Shao, X. Y., Wang, H. M., and Fan, Z. Y., 1999a. Studies on marine fouling communities in the East China Sea III. Community structure., 17 (4): 47-50 (in Chinese with English abs- tract).
Zeng, D. G., Cai, R. X., Huang, Z. G., Shao, X. Y., Wang, H. M., and Wang, Y. J., 1999b. Studies on marine fouling communities in the East China Sea II. biomass distribution., 17 (1): 56-59 (in Chinese with English abstract).
Zeng, D. G., Cai, R. X., Huang, Z. G., Shao, X. Y., Wang, H. M., and Wang, Y. J., 1999c. Studies on marine fouling communities in the East China Sea I. Composition and distribution of species., 17 (1): 48- 55 (in Chinese with English abstract).
Zenni, R. D., Lamy, J.-B., Lamarque, L. J., and Porté, A. J., 2014. Adaptive evolution and phenotypic plasticity during naturalization and spread of invasive species: Implications for tree invasion biology., 16 (3): 635-644.
Zhou, S. Q., Guo, F., Tian, Y., Wu, L. S., and Li, R. G., 2001a. Studies on island intertidal benthic ecology in Fujian., 20 (3): 417-425 (in Chinese with Eng- lish abstract).
Zhou, S. Q., Guo, F., Wu, L. S., and Li, R. G., 2001b. The study on the ecology of the benthic community in intertidal zone, Fujian islands., 23 (5): 104-109 (in Chinese with English abstract).
Zhu, S. X., Yang, H. L., Wang, P., and Ying, Q. Z., 2006. Studies on intertidal zone ecology of Zhoushan archipelago during the summer, 2005., 25 (4): 359-372 (in Chinese with English abstract).
(Edited by Ji Dechun)
10.1007/s11802-015-2632-y
(March 19, 2014; revised May 26, 2014; accepted June 13, 2014)
. E-mail:hxwu@shou.edu.cn
ISSN 1672-5182, 2015 14 (3): 575-583
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2015
 Journal of Ocean University of China2015年3期
Journal of Ocean University of China2015年3期
- Journal of Ocean University of China的其它文章
- Monte Carlo Simulation of in situ Gamma-Spectra Recorded by NaI (Tl) Detector in the Marine Environment
- Effects of Waterborne Cu and Cd on Anti-oxidative Response,Lipid Peroxidation and Heavy Metals Accumulation in Abalone Haliotis discus hannai Ino
- A General Overview of the Typical18Frontal-Ventral-Transverse Cirri Oxytrichidae s. l. Genera (Ciliophora, Hypotrichia)
- Application of a Delay-Difference Model for the Stock Assessment of Southern Atlantic Albacore (Thunnus alalunga)
- The Burial of Biogenic Silica, Organic Carbon and Organic Nitrogen in the Sediments of the East China Sea
- Hydrochemistry of the Natural Low pH Groundwater in the Coastal Aquifers near Beihai, China
