hMSH2和nm23在肠癌中的表达及临床意义
沈 冬,张 瑜
(1.江苏省苏州市中医医院检验科 215000;2.江苏省苏州市立医院北区检验科 215008)
·论 著·
hMSH2和nm23在肠癌中的表达及临床意义
沈 冬1,张 瑜2△
(1.江苏省苏州市中医医院检验科 215000;2.江苏省苏州市立医院北区检验科 215008)
目的 探讨人MutS同源蛋白2(hMSH2)和转移抑制基因nm23在肠癌中的表达、相互关系及其临床意义。方法 选取未经过术前放、化疗的肠癌标本48例及癌旁正常黏膜组织15例,XP免疫组织化学法检测hMSH2和nm23蛋白的表达,分析hMSH2和nm23蛋白的表达与肠癌临床病理特征的关系及其相互关系。结果 肠癌组织hMSH2和nm23蛋白阳性表达率分别为35.4%、62.5%,均显著低于正常肠黏膜组织(93.3%),差异有统计学意义(P<0.05);nm23表达与患者性别、年龄及肿瘤分化程度无关,但与肿瘤浆膜浸润、肠癌淋巴结转移密切相关(P<0.05);hMSH2与临床病理参数无相关性;nm23与hMSH2蛋白的表达呈正相关(P<0.05)。结论 nm23蛋白质水平与恶性程度相关,可作为预测入侵和转移的评判指标。hMSH2蛋白的表达与nm23蛋白合并后的表达对肠癌预后有一定指导意义。
nm23; hMSH2; 肠癌; 肿瘤转移
肠癌是消化道最常见的恶性肿瘤,最近几年肠癌的发病率和病死率逐年增加,占所有癌症相关死亡原因的第3位[1]。目前,肠癌发生、发展的确切机制尚不清楚,被视为是多因素、多步骤的复杂过程。例如,癌基因的活化、抑癌基因的失活和错配修复基因的突变等。研究表明,肠癌的发生、发展、转移和预后与不同基因和蛋白的表达密切相关,相关的敏感指标可以更好地指导临床筛查高危患者,从而加强治疗,密切随访,并提高患者的生存期。最近,许多实验研究表明,DNA错配修复(MMR)和转移抑制基因nm23的失活在肿瘤的发生和发展中扮演一个重要的角色[2-6]。本研究利用XP免疫组织化学法检测错配修复基因:人MutS同源蛋白2(hMSH2)和肿瘤转移抑制基因nm23在肠癌组织中的表达,并探讨其与肠癌临床参数的关系。
1 资料与方法
1.1 一般资料 选用肠癌组织48例,为苏州市立医院病理科2010~2012年的存档蜡块,为手术切除肠癌标本、正常肠黏膜组织(据肿瘤5 cm以上)15例,均有详细临床资料,年龄26~86岁,中位年龄62.2岁。全部患者术前均未接受放疗或化疗,所有标本均经2位病理专家进行组织病理证实。
1.2 仪器与试剂 nm23一抗为鼠抗人多克隆(基因公司,工作液);hMSH2一抗为兔抗人多克隆(基因公司,工作液);二抗选用福州迈新公司产品。
1.3 方法 按照传统的方法石蜡包埋组织4 μm切片,采用XP免疫组织化学法,试验中以磷酸盐缓冲液代替一抗作为阴性对照;以试剂公司提供的阳性对照片作为阳性对照。nm23和hMSH2的阳性判定标准按照参考文献[1]标准读片:hMSH2蛋白表达位于细胞核,无着色或小于1%的细胞核着色视为(-),>1%为(+);nm23蛋白表达位于胞浆,无着色为(-),>1%着色为(+)。
1.4 统计学处理 统计学分析采用SPSS18.0分析软件,计数资料比较采用χ2检验,以P<0.05为差异有统计学意义。
2 结 果
2.1 nm23和hMSH2在正常肠黏组织及肠癌组织中的表达 15例正常肠黏膜组织中表达nm23和hMSH2均为14例(93.3%);nm23在48例肠癌中的阳性表达率为62.5%(30/48)(图1A);hMSH2在肠癌中的阳性表达为35.4%(17/48)(图1B)。肠癌组织nm23、hMSH2蛋白的阳性表达均显著低于癌旁正常肠黏组织,差异均有统计学意义(P<0.05)。
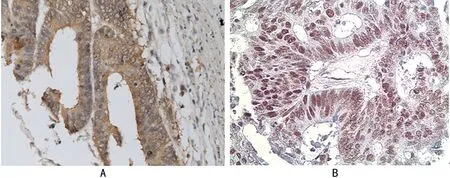
注:A表示nm23肠癌胞浆阳性;B表示hMSH2肠癌细胞核阳性(XP免疫组织化学法高倍放大)。
图1 nm23和hMSH2在肠癌中的阳性表达
2.2 nm23和hMSH2的表达与临床病理特征的关系 见表1。统计学相关性分析表明,nm23蛋白表达与患者的性别、肿瘤分化程度、年龄无明显相关性(P>0.05),但与肿瘤浆膜是否浸润、肠癌淋巴结有无转移密切相关(P<0.05)。hMSH2蛋白的表达与肠癌临床病理参数之间无相关性(P>0.05)。
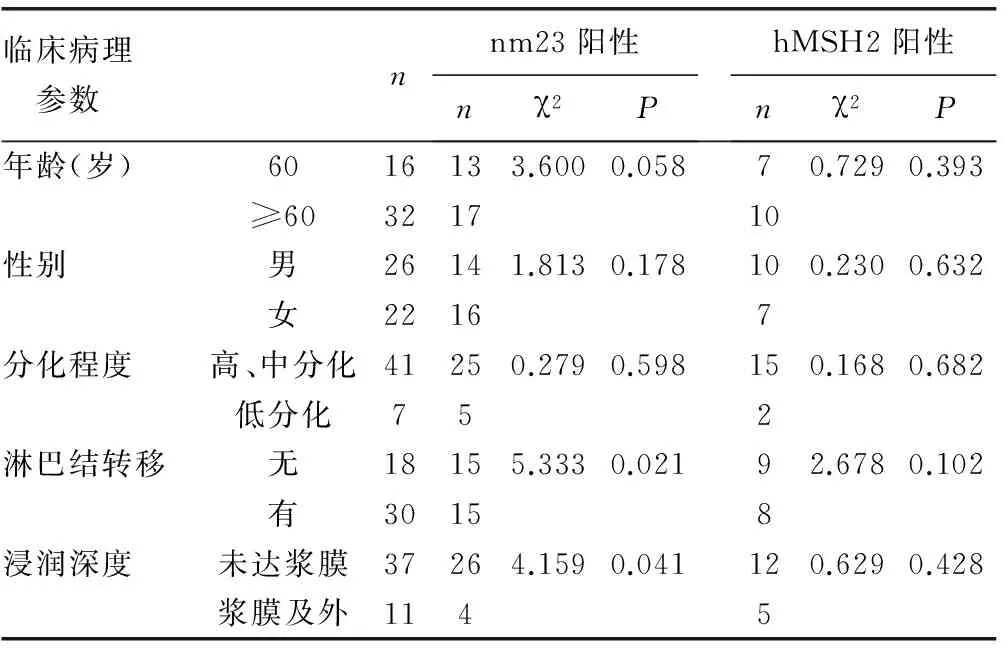
表1 nm23和hMSH2蛋白表达与肠癌临床病理特征的关系
2.3 肠癌组织中nm23和hMSH2蛋白表达相关性分析 见表2。Spearman等级相关分析结果显示,48例肠癌nm23阳性者hMSH2亦阳性为14例,nm23和hHMSH2蛋白在肠癌中的表达呈正相关(r=0.304,P=0.037)。
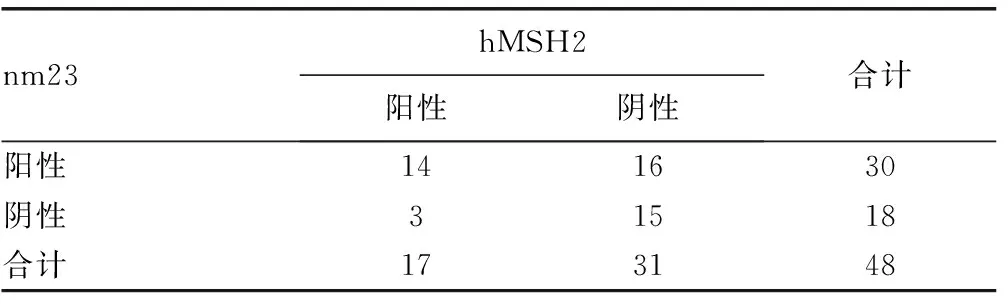
表2 肠癌组织中nm23和hMSH2蛋白表达的相关性
3 讨 论
人类DNA错配修复基因包括hMSH2、人MutS同源蛋白3(hMSH3)、人MutS同源蛋白6(hMSH6)、人MutL同源蛋白1(hMLH1)、人MutL同源蛋白3(hMLH3)和人减数分裂后分离增加蛋白1(hPMS1)6种[7]。其作用机制可能是由错配修复蛋白编码MMR基因构成异二聚体参与错配修复。修复单个碱基错配及单个碱基缺失/插入错配,维持基因组的稳定性[8-10]。MMR基因缺陷会导致错配修复功能损失,增加细胞突变频率,不断扩大和积累突变事件,这样的错误信息通过整个基因组,并最终导致肿瘤的发生和发展[11]。本研究发现,48例肠癌中hMSH2蛋白阴性表达者31例(64%),与Wu等[1]的研究相比较略高,可能与本组标本例数较少有关,但肠癌与周围正常肠黏膜hMSH2蛋白表达差异有统计学意义(P<0.05),肠癌中hMSH2缺失,表明DNA修复能力减弱;同时与Wu等[1]的研究相一致:hMSH2蛋白表达与临床病理参数之间无相关性,但通过Spearman等级相关统计分析显示,hHMSH2和nm23蛋白在肠癌中的表达呈正相关(r=0.304,P=0.037)。表明这二者可能有积极反馈调控机制。
nm23基因是肿瘤转移抑癌基因,其家族包括8个成员中,只有nm23 H1和nm23 H2被证明有能力抑制肿瘤转移[12],是美国国立癌症研究所的Steeg等[13]1988年从7个转移潜力不同的K-1735鼠黑色素瘤细胞株中用消减杂交分离克隆成功的,nm23等位基因的突变、缺失和失表达与许多恶性肿瘤的转移密切相关[14-15]。Steeg等[13]的研究表明,nm23在低转移细胞株中的表达强度是高转移细胞株内的10倍,表明nm23基因在高转移肿瘤中表达降低,这一结果与本研究结果相一致,本研究中nm23蛋白的表达在淋巴结转移组表达率为50.0%(15/30),而在淋巴结未转移组其表达率为83.3%(15/18),说明nm23基因在转移组中表达降低,同时,nm23蛋白在肿瘤浸润达浆膜及浆膜外者的表达率为36.4%(4/11)而在肿瘤浸润深度未达浆膜者中nm23蛋白的表达率为70.2%(26/37),由此表明肿瘤浸润越深其表达强度降低,与文献[1]报道一致。进一步说明nm23基因失表达时,机体对癌细胞约束力下降,从而极大地促进周围组织的侵犯和远处转移。
综上所述,hMSH2错配修复基因的突变会导致广泛的体细胞突变和DNA复制错误,导致因癌基因突变率增加,从而推动相关因素,如下调nm23基因表达。总之,nm23蛋白质水平与恶性程度有关,可以作为一项指标来预测入侵和转移潜能[16]。hMSH2蛋白的表达与nm23蛋白呈正相关,二者合并后的表达对肠癌预后有一定指导意义。
[1]Wu HW,Gao LD,Wei GH.hMSH2 and nm23 expression in sporadic colorectal cancer and its clinical significance[J].Asian Pac J Cancer Prev,2013,14(3):1995-1998.
[2]Luey N,Toon CW,Sioson L,et al.A further investigation of combined mismatch repair and BRAFV600E mutation specific immunohistochemistry as a predictor of overall survival in colorectal carcinoma[J].PLoS One,2014,9(8):e106105.
[3]Guillotin D,Martin SA.Exploiting DNA mismatch repair deficiency as a therapeutic strategy[J].Exp Cell Res,2014,329(1):110-115.
[4]Radovic S,Doric M,Hukic A.Immunohistochemical expression and significance of NM23 suppressor protein in primary gastric adenocarcinoma[J].Bosn J Basic Med Sci,2013,13(2):72-77.
[5]Tripkovic A,Tripkovic I,Tomic S,et al.The role of nm23 gene in colorectal carcinogenesis[J].Acta Clin Croat,2012,51(1):43-49.
[6]Overbeek LI,Ligtenberg MJ,Willems RW,et al.Interpretation of immunohisto chemistry for mismatch repair proteins is only reliable in a specialized setting[J].Am J Surg Pathol,2008,32(8):1246-1251.
[7]Iyer RR,Pluciennik A,Burdett V,et al.DNA mismatch repair:functions and mechanisms[J].Chem Rev,2006,106(6):302-320.
[8]Ting S,Mairinger FD,Hager T.ERCC1,MLH1,MSH2,MSH6,and βⅢ-tubulin:resistance proteins associated with response and outcome to platinum-based chemotherapy in malignant pleural mesothelioma[J].Clin Lung Cancer,2013,14(5):558-567.
[9]Belcheva A,Irrazabal T,Robertson SJ.Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells[J].Cell,2014,158(2):288-299.
[10]Hsu T,Huang KM,Tsai HT,et al.Cadmium(Cd)-induced oxidative stress down-regulates the gene expression of DNA mismatch recognition proteins MutS homolog 2 (MSH2) and MSH6 in zebrafish(Danio rerio) embryos[J].Aquat Toxicol,2013,126(1):9-16.
[11]Hsieh P,Yamane K.DNA mismatch repair:molecular mechanism,cancer,and ageing[J].Mech Ageing Dev,2008,129(7/8):391-407.
[12]Leone A,Flatow U,Richter KC,et al.Reduced tumor incidence,metastatic potential,and cytokine responsiveness of nm23-transfected melanoma cells[J].Cell,1991,65(1):25-35.
[13]Steeg PS,Bevilacqua G,Kopper L,et al.Evidence for a novel gene associated with low tumor metastatic potential[J].Nat Cancer Inst,1998,80(2):200-204.
[14]Dursun A,Akyürek N,Günel N,et al.Prognostic implication of nm23-H1 expression in colorectal carcinomas[J].Pathology,2002,34(6):427-432.
[15]Suzuki E,Ota T,Tsukuda K,et al.nm23-H1 reduces in vitro cell migration and the liver metastatic potential of colon cancer cells by regulation myosin light chain phosphorylation[J].Cancer,2004,108(3):207-211.
[16]Hsu T,Steeg PS,Zollo M,et al.Progress on Nme (NDP kinase/Nm23/Awd) gene family-related functions derived from animal model systems:studies on development,cardiovascular disease,and cancer metastasis exemplified.Naunyn Schmiedebergs Arch Pharmacol[J].2015,388(2):109-117.
The expression of hMSH2 and nm23 in intestinal cancer and its clinical significance
SHENDong1,ZHANGYu2△
(1.DepartmentofClinicalLaboratory,SuzhouHospitalofTraditionalChineseMedicine,Suzhou,Jiangsu215000,China;2.DepartmentofClinicalLaboratory,NorthBranchofSuzhouMunicipalHospital,Suzhou,Jiangsu215008,China)
Objective To study the expression of hMSH2 and nm23 in intestinal cancer,and to explore their inter-relationship and clinical significance.Methods XP immunohistochemistry was used to detect the expression levels of hMSH2 and nm23 in samples including 48 cases of intestinal cancer tissues without radiotherapy or chemotherapy and 15 cases of adjacent normal colon tissues.The relevance of the expression levels of nm23 and hMSH2 and their correlations to the clinicophathologic features of intestinal cancer were analyzed.Results The positive rates of hMSH2 and nm23 were respectively 35.4% and 62.5% in intestinal cancer tissues,which were significantly lower than those in adjacent normal colon tissues(93.3%),with statistical difference (P<0.05).The expression level of nm23 was not significantly associated with the genders,ages or differentiation of the patients,however,it was closely associated with serous coat infiltration and lymphatic metastasis of intestinal cancer (P<0.05).The expression level of hMSH2 was not significantly correlated with these clinicopathologic features.There was a positive correlation between the expression of nm23 and hMSH2 in intestinal cancer (P<0.05).Conclusion The expression level of nm23 was related with the degree of malignancy,which could be used as a potential index to predict the invasion and metastasis.The expression of hMSH2 and nm23 had certain guiding significance for the prognosis of intestinal cancer.
nm23; hMSH2; intestinal cancer; tumor metastasis
沈冬,男,本科,主管技师,主要从事临床检验工作。△
,E-mail:13584884983@163.com。
10.3969/j.issn.1672-9455.2015.24.017
A
1672-9455(2015)24-3655-02
2015-04-21
2015-06-17)
——紫 苏

