Significance of Different Microalgal Species for Growth of Moon Jellyfish Ephyrae, Aurelia sp.1
ZHENG Shan, SUN Xiaoxia, WANG Yantao, and SUN Song
1) Jiaozhou Bay Marine Ecosystem Research Station, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, P. R. China
2) Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, P. R. China
3) University of Chinese Academy of Sciences, Beijing 100049, P. R. China
Significance of Different Microalgal Species for Growth of Moon Jellyfish Ephyrae, Aurelia sp.1
ZHENG Shan1),2),3), SUN Xiaoxia1),*, WANG Yantao1),2),3), and SUN Song1),2)
1) Jiaozhou Bay Marine Ecosystem Research Station, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, P. R. China
2) Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, P. R. China
3) University of Chinese Academy of Sciences, Beijing 100049, P. R. China
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2015
The scyphozoan Aurelia aurita (Linnaeus) sp. l., is a cosmopolitan species-complex which blooms seasonally in a variety of coastal and shelf sea environments around the world. The effects of different microalgal species on the growth of newly-released Aurelia sp.1 ephyrae were studied under laboratory conditions. We fed ephyrae with four different microalgal species (diatom, autotrophic dinoflagellate, heterotrophic dinoflagellate, and chlorophyta) plus Artemia nauplii for 12 - 24 d at 18℃. Results showed that the growth rate diverged significantly for Artemia nauplii compared to other food types. In addition, there was no significant variation between the growth rates for Skeletonema costatum and Prorocentrum donghaiense, and no significant variation was found in the growth rates for N. scintillans and P. subcordiformis. Artemia nauplii could support the energy requirement for the newly-released ephyrae to develop to meduase, and the ephyrae with Artemia nauplii showed a significant average growth rate of 25.85% d-1. Newly-released ephyrae could grow slightly with some species of microalgae in the earliest development stage. Chain diatom Skeletonema costatum and autotrophic dinoflagellate Prorocentrum donghaiense, could not support the growth of the ephyrae, while heterotrophic dinoflagellate Noctiluca scintillans and chlorophyta Platymonas subcordiformis could support the growth of the ephyrae. However, none of the ephyrae fed with the tested phytoplankton could mature to medusae.
Aurelia sp.1; ephyrae; phytoplankton; growth
1 Introduction
Jellyfish blooms are increasing in many coastal waters worldwide, and cause detrimental effects on human enterprises (Purcell et al., 2007; Condon et al., 2012; McNamara et al., 2013). Carnivorous jellyfish are both competitors and predators of fish (reviewed by Purcell, 1985; Arai, 1988; Purcell and Arai, 2001), thus the increase of jellyfish populations may be a potential threat for sustainable fisheries (Uye, 2008; Pauly et al., 2009). Aurelia aurita, which is one of the most common species in East Asian coastal waters, has resulted in problems in operation of power plants and in commercial fisheries (Han and Uye, 2010).
A. aurita has a complex life cycle from benthic asexual stage (polyp) to planktonic sexual stage (medusa). Polyps reproduce asexually by budding and producing podocysts to form colonies of millions of individuals. Moreover, individual polyps can metamorphose into strobilae, which release great numbers of ephyrae during strobilation.Hence, numerous ephyrae mature into adult medusae, even cause jellyfish outbreak.
As a predator, A. aurita has been extensively studied both in the field and in the laboratory (see Båmstedt et al., 1994 and references therein).It is shown in the literature that ambient conditions and food supply are correlated with spatial and temporal variations for both the polyp and the medusa stages (Hernroth and Gröndahl, 1985; Schneider and Behrends, 1994; Olesen, 1995; Nielsen et al., 1997; Purcell et al., 1999; Mills, 2001; Malej et al., 2007). Water temperature affects both feeding and growth by regulating the metabolism in basic physiological activities (Olensen, 1995). Food availability controls the growth; consequently, the average size of A. aurita varies along with the variation in food supply (Schneider and Behrends, 1994). It is likely that the suitable food conditions during the period of scyphistoma growth and strobilation give away to poor food conditions during the period of medusa growth and development (Båmstedt et al., 2001).
A. aurita, usually starting its planktonic life in spring, grows from a 2-mm-diameter ephyra to an adult medusa of more than 25 cm diameter (Lucas, 1996). Technically, ephyrae make contributions to jellyfish blooming fromtwo aspects: considerable survival and rapid growth. Food availability is of significant importance for newly released ephyrae, as suitable food can ensure the rapid growth of ephyrae and avoid the risk of being preyed. As far as we know, most published reports on feeding and utilization for growth have focused on the developed medusa, and simply a few papers referred to the ephyra stage. Yet it is supposed that there should be differences between ephyrae and mature medusae in prey selection and utilization. Olesen et al. (1994) reported that A. aurita ephyrae preyed upon abundant rotifers. Sullivan et al. (1997) observed the gut contents of A. aurita ephyrae sampled from field as well as laboratory and reported the high diversity and selection of prey. Båmstedt et al. (1997) showed that sufficient Artemia nauplii play a significant role in the growth of A. aurita ephyrae. Møller and Riisgård (2007) assessed feeding and growth of A. aurita ephyrae with Artemia sp., Balanus sp., Brachionus sp. and Rathkea octopunctata. However, all the food types in the research mentioned are restricted to the animal food. Few literatures have been published on the feeding and utilization of microalgae by A. aurita ephyrae.
Ephyrae are released by polyps in spring, which is the same time when phytoplankton blooms. Phytoplankton blooms, especially dinoflagelates blooms, have increased over the past several decades in spring in the East China Sea (Zhou et al., 2008). Southward (1955) suggested A. aurita fed on phytoplankton. Kerstan (1977) found many species of diatoms in the gut of A. aurita ephyrae. Båmstedt et al. (2001) showed that A. aurita ephyrae feed on one specific microalgal species. Zheng et al. (2012) reported that Aurelia sp.1 ephyrae can prey on diatom Skeletonema costatum (Greville) Cleve and dinoflagellate Prorocentrum donghaiense Lu, and presented quantitative data on predation rate. Huang et al. (2014) have described that the concentration of Alexandrium catenella can influence the behavior and growth of Aurelia sp. ephyrea. If the availabity of phytoplankton is high, ephyrae gowth might be enhanced, which is crucial to jellyfish blooms. Therefore, our purpose was to evaluate whether different microalgal species could be used by newly released Aurelia sp.1 ephyrae.
2 Materials and Methods
2.1 Source and Culturing Conditions
We used non-fed ephyrae that were recently strobilated from the laboratory culture of Aurelia sp.1 polyps in the Institute of Oceanology (Chinese Academy of Sciences). The polyps were incubated at 20℃ in filtered seawater with a salinity of 32. They were fed with Artemia sp. nauplii twice a week and the seawater was weekly changed. To stimulate strobilation, the polyps were incubated while temperature was lowered from 20℃ to 13℃, and then increased to 15℃. During the strobilation, Artemia sp. nauplii were not provided for the polyps and ephyrae. Once the ephyrae were released, the healthy ones of the same size were used in the experiments.
The ephyrae were cultured with six treatments: (1) none, (2) Skeletonema costatum (Greville) Cleve (diatom), (3) Prorocentrum donghaiense Lu (autotrophic dinoflagellate), (4) Noctiluca scintillans (Macartney) Kofoid & Swezy (heterotrophic dinoflagellate), (5) Platymonas subcordiformis (Chlorophyta), and (6) Artemia sp. nauplii (as positive control). The phytoplankton was obtained from the Key Laboratory of Marine Ecology & Environmental Sciences (Chinese Academy of Sciences). The phytoplankton used in experiments was of exponential growth phase. S. costatum and P. donghaiense were cultured in f/2 medium (Guillard and Ryther, 1962), and a 14 h light: 10 h dark cycle with 2000 lux light intensity at 20℃. P. subcordiformis was cultured in SE medium in the same temperature and light condition. N. scintillans was taken from samples collected by vertical plankton hauls in the Jiaozhou Bay. Artemia sp. nauplii used were all acquired from a daily batch of new hatched eggs.
2.2 Experimental Protocol
Each treatment consisted of three replicated glass beakers with 1.0 L filtered (0.45 μm pore size) seawater and five ephyrae. The filtered seawater was at a salinity of 32.We renewed the seawater in all beakers every day and added all types of food in a given amount (Table 1). The concentrations of food were close to the maxima occurring naturally in the field (Zhou et al., 2008), and were similar in terms of carbon content, corresponding to the about 1mg C m-3(according to previous results in the laboratory) that was added to the experimental beakers. Food items were gently distributed by equal and slow bubbling in all beakers. All the treatments were kept in the dark in an incubator with the temperature of 18℃; moreover, a storage tank was kept to make sure that the experimental water adjusted to the set temperature. The choice of temperature was based on the results of Båmstedt et al. (1999, 2001) and the need to facilitate the most possible food source.
These experiment treatments lasted for 12 - 24 d. The ephyrae were gently pipetted from the glass beakers into Petri dishes and measured, then put back after the beakers were renewed with water and food.

Table 1 Concentration of different food used in the experiment
Food abundance in all treatments was high enough to eliminate any effect caused by the insufficient food supply.
2.3 Statistical Analysis
The diameters (between opposite lappet tips) of ephyrae were measured under a dissection microscope with an ocular graticule every 3 d. To evaluate the variations of individual body size, we calculated the growth rate (% d-1) by using the formula of Båmstedt et al. (1997):
% growth d-1=ln[(D2/D1)3]/(t2– t1)×100 %,
where D1and D2are the mean diameters (mm) from each group at t1and t2(d), respectively.
One-way ANOVA tests were used to test for significant differences among the effects of the different species on the average growth rates over the entire experiments. A post hoc test (Tukey HSD test) was used to identify the differences among the phytoplankton species. The program SPSS 16.0 was used in the statistical calculations.
3 Results
The experiment treatments lasted for 24 d except for two cases. The reason why the treatments were eliminated by day 12 was the maturity of ephyrae fed on Artemia nauplii and the mortality of ephyrae cultured with P. donghaiense.
3.1 Survival
Fig.1 shows that there were clear differences among the food treatments in the survival of ephyrae. The variations in survival of ephyrae of microalgal species were obvious. During the experiments with ephyrae fed on nothing, N. scintillans, Artemia sp. nauplii and P. subcordiformis maintained high survival (86.7% - 100%). As for ephyrae cultured with S. costatum, the survival slowly declined to 93.3% by day 21, and then sharply dropped to less than 50%. The survival of ephyrae fed with P. donghaiense showed a marked decline which started from day 9 and ended up with 46.7% by day 12.

Fig.1 Variations of different microalgal species in survival of Aurelia sp.1 ephyrae.
3.2 Variations of Diameters and Growth Rates
In all treatments of the experiment, the initial average size of ephyrae was (3.67 ±0.03) mm in diameter. The variations of ephyrae diameter in all treatments were shown in Fig.2. Through the experiment, the average growth rates for different days, calculated by the formula mentioned above, are shown in Table 2. The growth of ephyrae varied in the three different ways mentioned below. Results of One-way ANOVA testing the effect of food type on the average growth rate (% d-1) over the first 12 d are shown in Table 2. A post hoc test (Tukey’S HSD-test) showed that the growth rate diverged significantly for Artemia nauplii compared to other food types (Table 3). In addition, there was no significant difference between the growth rates for S. costatum and P. donghaiense, and no significant difference was found in the growth rates between N. scintillans and P. subcordiformis.

Fig.2 Effects of different microalgal species on the growth of Aurelia sp.1 ephyrae, n=3 in each treatment.
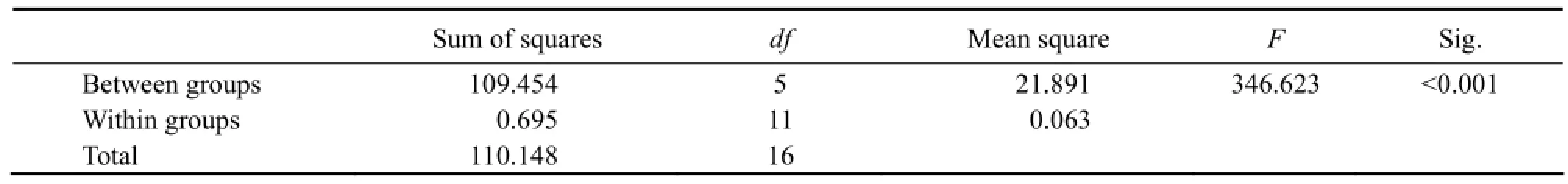
Table 2 Results of One-way ANOVA testing the effect of food type on the average growth rate (% d-1) over the first 12 d
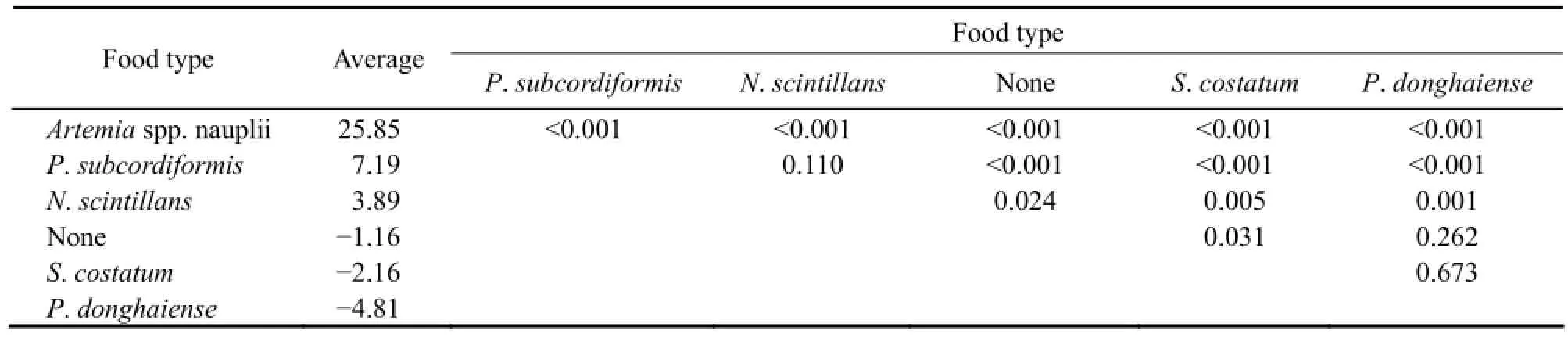
Table 3 Results of post hoc tests (Tukey HSD test) on the average growth rate (% d-1) over the first 12 d in all treatments
1) Increasing. Ephyrae fed with Artemia nauplii showed the strongest growth and grew up to medusa by day 12 with a rate of about 25.85% overall, growing from (3.68 ± 0.02) mm to (10.35 ±1.42) mm.
2) Decreasing. Ephyrae fed with nothing, S. costatum and P. donghaiense showed a slow decline in growth rate in the entire period. Ephyrae, suffering from starvation, shrank from (3.69 ± 0.41) mm to (2.74 ± 0.71) mm at a rate of about -3.74% over 24 d. Similarly, the ephyrae fed with S. costatum decreased at a rate of -6.89% in growth rate, with a decline from (3.61 ± 0.29) mm to (2.08 ± 0.54) mm in diameter. Ephyrae fed with P.donghaiense decreased at a rate of -4.81% in growth rate, with a decline from (3.65 ± 0.37) mm to (3.01 ± 0.90) mm in diameter.
3) Increasing and then decreasing. Ephyrae fed with N. scintillans grew from (3.69 ± 0.21) mm to (4.31 ± 0.59) mm at a rate of about 3.89% in the first 12 d, and then shrank to (3.09 ± 0.39) mm at a growth rate of -8.34% in the next 12 d. Similarly, ephyrae fed with P. subcordiformis grew from (3.68 ± 0.02) mm to (4.90 ± 0.46) mm at a rate of about 7.19% in the first 12 d, and then shrank to (3.74 ± 0.52) mm at a growth rate of -6.78% in the next 12 d.
4 Discussion
Generally, results in our experiment showed that newly-released ephyrae can take some species of microalgae for growth in the earliest development stage. In our results, chain diatom S. costatum and autotrophic dinoflagellate P. donghaiense did not support the growth of ephyrae, while dinoflagellate N. scintillans and chlorophyta P. subcordiformis could support the growth for the ephyrae in the earliest phase. However, none of these microalgae species can support ephyrae to mature. There might be two reasons: (1) Swimming ability of phytoplankton could affect the feeding behavior of ephyrae. (2) Food quality of different species of phytoplankton might be important for the growth of ephyrae.
Firstly, diatom S. costatum cannot support the growth of ephyrae. Sullivan et al. (1997) suggested that the swimming speed and size of prey were of great importance and determined the actual ingestion rate. S. costatum is incapable of swimming and the chains of cells usually fall downwards. On the contrary, three other species of phytoplankton in our experiment are capable of swimming and thus have the higher probability of being captured. Although species of diatom were found in the gut of A. aurita medusa and ephyrae both in the fields (Kerstan, 1977) and in the laboratory (Båmstedt, 1990; Zheng et al., 2012), it might be the result of indiscriminate feeding by A. aurita medusa and ephyrae in the seawater. As coelenterates are unable to disrupting cell walls of phytoplankton mechanically (Pitt et al., 2009b), S. costatum is likely to be hard to be used by ephyrae. Furthermore, it has been showed that diatoms lack in few specific fatty acids and sterols (Jonasdottir et al., 1995; Klein Breteler et al., 1999), which are essential for development, growth and reproduction of zooplankton. As all above, S. costatum is not a sufficient food source and cannot support ephyrae to grow.
Secondly, P. donghaiense do harm to the A. aurita ephyrae. Compared with other treatment, the survival of ephyrae fed with P. donghaiense rapidly declined in 12 d. Although P. donghaiense is confirmed as non-toxic species, the abundant exudation of cells is sticky when the cells density is high. Therefore, the normal activities of ephyrae might be inhibited to death. Wang et al. (2003) found that P. donghaiense at a density of 10×104cell mL-1resulted in a strong inhibition of the swimming of rotifers, even death.
Finally, although the ephyrae served with dinoflagellate N. scintillans and chlorophyta P. subcordiformis could grow in the earliest phrase, they were not able to be as mature as those fed on Artemia sp. nauplii. It is obvious that ephyrae benefit more from animal food than from phytoplankton food. It was suggested that ephyrae of Chrysaora quinquecirrha grew much better on ctenophores as food than on rotifers (Olesen et al., 1996). Likewise, Bamstedt (1997) illustrated the priority of ctenophores for the growth of Cyanea capillata ephyrae to Artemia nauplii and copepods. In addition, Bamstedt et al. (2001) compared the effect of one species of phytoplankton to that of four animal foods on the growth of A. aurita ephyrae, and showed that animal supported development better than phytoplankton did. Our observation islikely to be the first report on the effects of different microalgae on growth of ephyrae.
In conclusion, our results show that newly-released ephyrae can take some species of microalgae for growth in the earliest development stage. Chain diatom S. costatum and autotrophic dinoflagellate could not support the growth of ephyrae, while heterotrophic dinoflagellate N. scintillans and chlorophyta P. subcordiformis could in the earliest phase. However, none of ephyrae only fed with phytoplankton as food could mature to medusae.
Acknowledgements
We thank Professor Yan Tian for providing culture strains of Skeletonema costatum, Prorocentrum donghaiense and Platymonas subcordiformis. We also thank Professor Ian R Jenkinson for the assistance with English writing. This research was supported by the National Basic Research Program of China (973 Program) (No. 2011 CB403603), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA0503 0401) and the National Natural Science Founda- tion of Shandong Province, China (No. ZR2012DQ005).
Arai, M. N., 1988. Interactions of fish and pelagic coelenterates. Canadian Journal of Zoology, 66 (9): 1913-1927.
Båmstedt, U., 1990. Trophodynamics of the scyphomedusae Aurelia aurita. Predationrate in relation to abundance, size and type of prey organism. Journal of Plankton Research, 12 (1): 215-229.
Båmstedt, U., Ishii, H., and Martinussen, M. B., 1997. Is the scyphomedusa Cyanea capillata (L.) dependent on gelatinous prey for its early development? Sarsia, 82: 269-273.
Båmstedt, U., Wild, B., and Martinussen, M., 2001. Significance of food type for growth of ephyrae Aurelia aurita (Scyphozoa) Marine Biology, 139 (4): 641-650.
Condon, R. H., Graham, W. M., Duarte, C. M., Pitt, K. A., Lucas, C. H., Haddock, S. H. D., Sutherland, K. R., Robinson, K. L., Dawson, M. N., Decker, M. B., Mills, C. E., Purcell, J. E., Malej, A., Mianzan, H., Uye, S. I., Gelcich, S., and Madin, L. P., 2012. Questioning the rise of gelatinous zooplankton in the World’s oceans. Bioscience, 62 (2): 160-169.
Guillard, R. R. L., and Ryther, J. H., 1962. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Canadian Journal of Microbiology, 8 (2): 229-239.
Han, C. H., and Uye, S., 2010. Combined effects of food supply and temperature on asexual reproduction and somatic growth of polyps of the common jellyfish Aurelia aurita s.l. Plankton Benthos Research, 5 (3): 98-105.
Hernroth, L., and Gröndahl, F., 1985. On the biology of Aurelia aurita (L.): 2. Major factors regulating the occurrence of ephyrae and young medusae in the Gullmar fjord, western Sweden. Bulletin of Marine Science, 37 (2): 567-576.
Huang, X. G., Zeng, Y., Huang, B. Q., and Li, S. X., 2014. Effect of Alexandrium catenella (Dinophyta) concentration on the behavior and growth of Aurelia sp. ephyrae. Journal of Plankton Research, 36 (2): 591-595.
Ianora, A., Poulet, S. A., and Miralto, A., 1995. A comparative study of the inhibitory effect of diatoms on the reproductive biology of the copepod Temora stylifera. Marine Biology, 121 (3): 533-539.
Ishii, H., 2001. The influence of environmental changes upon the coastal plankton ecosystems, with special reference to mass occurrence of jellyfish. Bulletin of Plankton Society of Japan, 48: 55-61 (in Japanese with English abstract).
Jonasdottir, S. H., Fields, D., and Pantoja, S., 1995. Copepod egg production in Long Island Sound, USA, as a function of the chemical composition of seston. Marine Ecology Progress Series, 119: 87-98.
Kerstan, M., 1977. Untersuchungen zur Nahrungsökologie von Aurelia aurita Lam. Diplomarbeit, Universität Kiel, Kiel, 1-95.
Klein Breteler, W. C. M., Schogt, N., Baas, M., Schouten, S., and Kraay, G. W., 1999. Trophic upgrading of food quality by protozoans enhancing copepod growth: Role of essential lipids. Marine Biology, 135 (1): 191-198.
López-Sandoval, D. C., Rodríguez-Ramos, T., Cermeño, P., and Marañón, E., 2013. Exudation of organic carbon by marine phytoplankton: Dependence on taxon and cell size. Marine Ecology Progress Series, 477: 53-60.
Lucas, C. H., 1996. Population dynamics of Aurelia aurita (Scyphozoa) from an isolated brackish lake, with particular reference to sexual reproduction. Journal of Plankton Research, 18 (6): 987-1007.
McNamara, M. E., Lonsdale, D. J., and Cerrato, R. M., 2013. Top-down control of mesozooplankton by adult Mnemiopsis leidyi influences microplankton abundance and composition enhancing prey conditions for larval ctenophores. Estuarine, Coastal and Shelf Science, 133: 2-10.
Malej, A., Turk, V., and Lučić, D., 2007. Direct and indirect trophic interactions of Aurelia sp. (Scyphozoa) in a stratified marine environment (Mljet Lakes, Adriatic Sea). Marine Biology, 151: 827-841.
Miller, R. J., 1970. Distribution and energetics of an estuarine population of the ctenophore, Mnemiopsis leidyi. Ph.D thesis, North Carolina State University, Raleigh, 1-44.
Mills, C. E., 2001. Jellyfish blooms: Are populations increasing globally in response to changing ocean conditions? Hydrobiologia, 451: 55-68.
Møller, L. F., and Riisgård, H. U., 2007. Feeding, bioenergetics and growth in the common jellyfish Aurelia aurita and two hydromedusae, Sarsia tubulosa and Aequorea vitrina. Marine Ecology Progress Series, 346: 167-177.
Nielsen, A. S., Pedersen, A. W., and Riisgård, H. U., 1997. Implications of density driven currents for interaction between jellyfish (Aurelia aurita) and zooplankton in a Danish fjord. Sarsia, 82 (4): 297-305.
Olesen, N. J., 1995. Clearance potential of jellyfish Aurelia aurita, and predation impact on zooplankton in a shallow cove. Marine Ecology Progress Series, 124: 63-72.
Olesen, N. J., Frandsen, K., and Riisgård, H. U., 1994. Population dynamics, growth and energetics of jellyfish Aurelia aurita in a shallow fjord. Marine Ecology Progress Series, 105: 9-18.
Olesen, N. J., Purcell, J. E., and Stoecker, D. K., 1996. Feeding and growth by ephyrae of scyphomedusae Chrysaora quinquecirrha. Marine Ecology Progress Series, 137: 149-159.
Pauly, D., Graham, W., Libralato, S., Morissette, L., and Palomares, M. L., 2009. Jellyfish in ecosystems, online databases, and ecosystem models. Hydrobiologia, 616: 67-85.
Pitt, K. A., Welsh, D. T., and Condon, R. H., 2009a. Influence of jellyfish blooms on carbon, nitrogen and phosphorus cyclingand plankton production. Hydrobiologia, 616: 133-149.
Pitt, K. A., Connolly, R. M., and Meziane, T. 2009b. Stable isotope and fatty acid tracers in energy and nutrient studies of jellyfish: A review.Hydrobiologia, 616: 119-132.
Purcell, J. E., 2007. Environmental effects on asexual reproduction rates of the scyphozoan Aurelia labiata. Marine Ecology Progress Series, 348: 183-196.
Purcell, J. E., and Arai, M. N., 2001. Interactions of pelagic cnidarians and ctenophores with fishes: A review. Hydrobiologia, 451: 27-44.
Purcell, J. E., White, J. R., Nemazie, D. A., and Wright, D. A., 1999. Temperature, salinity and food effects on asexual reproduction and abundance of the scyphozoan Chrysaora quinquecirrha. Marine Ecology Progress Series, 180: 187-196.
Schneider, G., and Behrends, G., 1994. Population dynamics and the trophic role of Aurelia aurita medusae in the Kiel Bight and western Baltic. ICES Journal of Marine Science, 51 (4): 359-367.
Southward, A. J., 1955. Observations on the ciliary currents of the jellyfish Aurelia aurita L. Journal of the Marine Biological Association of the United Kingdom, 34: 201-216.
Stoecker, D. K., Michaels, A. E., and Davis, L. H., 1987. Grazing by the jellyfish Aurelia aurita on microzooplankton. Journal of Plankton Research, 9: 901-915
Sullivan, B. K., Suchman, C. L., and Costello, J. H., 1997. Mechanics of prey selection by ephyrae of the scyphomedusa Aurelia aurita. Marine Biology, 130: 213-222.
Toyokawa, M., Furota, T., and Terazaki, M., 2000. Life history and seasonal abundance of Aurelia aurita medusae in Tokyo Bay, Japan. Plankton Biology and Ecology, 47: 48-58.
Uye, S., 2008. Blooms of the giant jellyfish Nemopilema nomurai: A threat to the fisheries sustainability of the East Asian Marginal Seas. Plankton Benthos Research, 3 (Suppl): 125-131.
Wang, L., Yan, T., Tan, Z. and Zhou, M., 2003. Effects of Alexandrium tamarense and Prorocentrum donghaiense on rotifer Brachionus plicatilis population. Chinese Journal of Applied Ecology, 14 (7): 1151-1155 (in Chinese with English abstract).
Zheng, S., Sun, X., and Sun, S., 2012. The grazing of Aurelia sp.1 on Skeletonema costatum and Prorocentrum donghaiense. Oceanologia et Limnologia Sinica, 43 (3): 445-450 (in Chinese with English abstract).
(Edited by Ji Dechun)
(Received October 9, 2014; revised April 14, 2015; accepted April 22, 2015)
J. Ocean Univ. China (Oceanic and Coastal Sea Research)
DOI 10.1007/s11802-015-2775-x
ISSN 1672-5182, 2015 14 (5): 823-828
http://www.ouc.edu.cn/xbywb/
E-mail:xbywb@ouc.edu.cn
* Corresponding author. Tel: 0086-532-82898599
E-mail: xsun@qdio.ac.cn
 Journal of Ocean University of China2015年5期
Journal of Ocean University of China2015年5期
- Journal of Ocean University of China的其它文章
- The Mechanism of the Acclimation of Nannochloropsis oceanica to Freshwater Deduced from Its Transcriptome Profiles
- A Carboxymethyl Cellulase from a Marine Yeast (Aureobasidium pullulans 98): Its Purification, Characterization, Gene Cloning and Carboxymethyl Cellulose Digestion
- Effects of Dietary Stachyose on Growth Performance, Digestive Enzyme Activities and Intestinal Morphology of Juvenile Turbot (Scophthalmus maximus L)
- Pharmacokinetics and Biodegradation of Chitosan in Rats
- Pharmacokinetics and Biodegradation Performance of a Hydroxypropyl Chitosan Derivative
- Changes in Plasma Osmolality, Cortisol and Amino Acid Levels of Tongue Sole (Cynoglossus semilaevis) at Different Salinities
