Effects of Dietary Stachyose on Growth Performance, Digestive Enzyme Activities and Intestinal Morphology of Juvenile Turbot (Scophthalmus maximus L)
HU Haibin, ZHANG Yanjiao, MAI Kangsen, AI Qinghui, XU Wei, ZHANG Wenbing, LI Yanxian, and LIU Jintao
Key Laboratory of Aquaculture Nutrition and Feed of Ministry of Agriculture; Key Laboratory of Mariculture of Ministry of Education, Ocean University of China, Qingdao 266003, P. R. China
Effects of Dietary Stachyose on Growth Performance, Digestive Enzyme Activities and Intestinal Morphology of Juvenile Turbot (Scophthalmus maximus L)
HU Haibin, ZHANG Yanjiao*, MAI Kangsen, AI Qinghui, XU Wei, ZHANG Wenbing, LI Yanxian, and LIU Jintao
Key Laboratory of Aquaculture Nutrition and Feed of Ministry of Agriculture; Key Laboratory of Mariculture of Ministry of Education, Ocean University of China, Qingdao 266003, P. R. China
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
A 12-week feeding trial was conducted to evaluate the effects of dietary stachyose on the growth performance, digestive enzymes activities and intestinal structures of juvenile turbot (Scophthalmus maximus L). Five isonitrogenous (49.58% crude protein) and isolipidic (10.50% crude lipid) diets were formulated to contain 0 (Control), 0.625% (S-0.625), 1.25% (S-1.25), 2.5% (S-2.5) and 5% (S-5) stachyose, respectively. With the increase of stachyose level, the growth performance and feed utilization of turbot, such as the specific growth rate, final mean body weight, weight gain rate and feed efficiency, increased significantly (P < 0.05) and then stabilized. The feed intake of fish fed S-5 was significantly higher (P < 0.05) than that of fish in other groups. The activities of trypsin, intestinal caseinolytic, stomach and intestinal amylase were significantly influenced by stachyose (P < 0.05). The highest values of trypsin and intestinal caseinolytic activities were observed in group S-1.25, while the highest activity of stomach amylase and the lowest activity of intestine amylase were observed in group S-5. No lesion or damage was found on the distal intestine structures of fish from all treatments, while the height of simple folds in the distal intestine was significantly increased (P< 0.05) when 1.25% or 2.5% stachyose was added in the diets. These results indicated that moderate level of stachyose (1.25%) improves the growth performance, feed utilization, digestive enzyme activities and the distal intestine structures of juvenile turbot.
stachyose; growth; digestive enzyme; intestinal morphology; turbot.
1 Introduction
Plant ingredients are the most important and cost-effective alternatives of fish meal in the aqua-feed industry (Naylor et al., 2009; USDA, 2009; Olsen and Hasan, 2012). However, high levels of plant protein in diets depressed the growth performance and feed utilization of fish, especially the carnivorous fish (Day and Plascencia-Gonzalez, 2002; Chou et al., 2004; Deng et al., 2006; Hernández et al., 2007). These negative effects of plant protein on fish were mainly due to the anti-nutritional factors (ANFs) in plant materials (Mussatto et al., 2007; Choct et al., 2010).
Oligosaccharide is generally considered as an ANF because of its negative effects on the growth performance and feed utilization of animals (Choct et al., 2010). Of all species of oligosaccharides, stachyose is one of the most predominant ANF in plant ingredients, for example, the stachyose content in soybean meal is up to 5%–6% (Cern-ing-Beroard and Filiatre, 1976; Francis et al., 2001; Choct et al., 2010; Sørensen et al., 2011). Structurally, stachyose is an α-galacto-oligosaccharide consisting of two molecules of α-(1, 6) linked galactose that bound to a terminal sucrose unit. Usually it can’t be digested by animals due to the absence of α-galactosidase, whereas it can be fermented by intestinal bacteria (Mul and Perry, 1994; Zhang et al., 2003; Choct et al., 2010). Several studies have reported that stachyose depresses the growth performance and feed utilization of fish (Kaushik et al., 1995; Refstie et al., 1998; Cai, 2006), piglets (Pan et al., 2002; Zhang et al., 2003) and broiler (Irish and Balnave, 1993; Jiang et al., 2006).
Though stachyose is an ANF, it also shows some beneficial properties. It can serve as the substrate for the growth of anaerobic bacteria to improve the gut health, and produce short chain fatty acids (SCFA) or other nutrients that are beneficial to host (Mussatto et al., 2007). Stachyose has been used as a probiotic that improves the health of humans (Hayakawa et al., 1990; Gibson et al., 2000), piglets (Risley and Lohrmann 1998), chicken (Spring et al., 2000) and fish (Deng et al., 2009a). All of these positive effects of stachyose are attracting more andmore attentions from aqua feed manufacturers, and thus more information about stachyose is needed.
Turbot (Scophthalmus maximus L) is a marine flatfish with high commercial values, and is originally farmed in Europe. Since 1990s, it is gradually appreciated by Chinese consumers and now is extensively cultured in China. The aim of the present study was to investigate the effects of dietary stachyose on the growth performance, digestive enzyme activities and distal intestinal morphology of juvenile turbot.
2 Materials and Methods
2.1 Experimental Diets
The compositions of the 5 experimental diets were presented in Table 1. The basal diet was formulated to contain 49.58% (dry matter, DM) crude protein and 10.5% (DM) crude lipid. Stachyose was added to the basal diet to obtain five levels of dietary stachyose (0, 0.625%, 1.25%, 2.5%, 5% DM) (91.45% stachyose, Xi’an Rongsheng Bio Technology Co., Ltd.), named control, S-0.625, S-1.25, S-2.5, S-5, respectively.
Ingredients were ground into fine powder through 200 µm mesh. All ingredients were thoroughly mixed with the oil, and then water was added to produce stiff dough. The dough was pelleted using F-26 (II) screw extruder (South China University of Technology, China) through a 4.5-mm die. The moist pellets were dried for about 8 h in a ventilated oven at 50℃. The dry pellets were broken up, sieved into proper pellet size and stored at -20℃ until used.
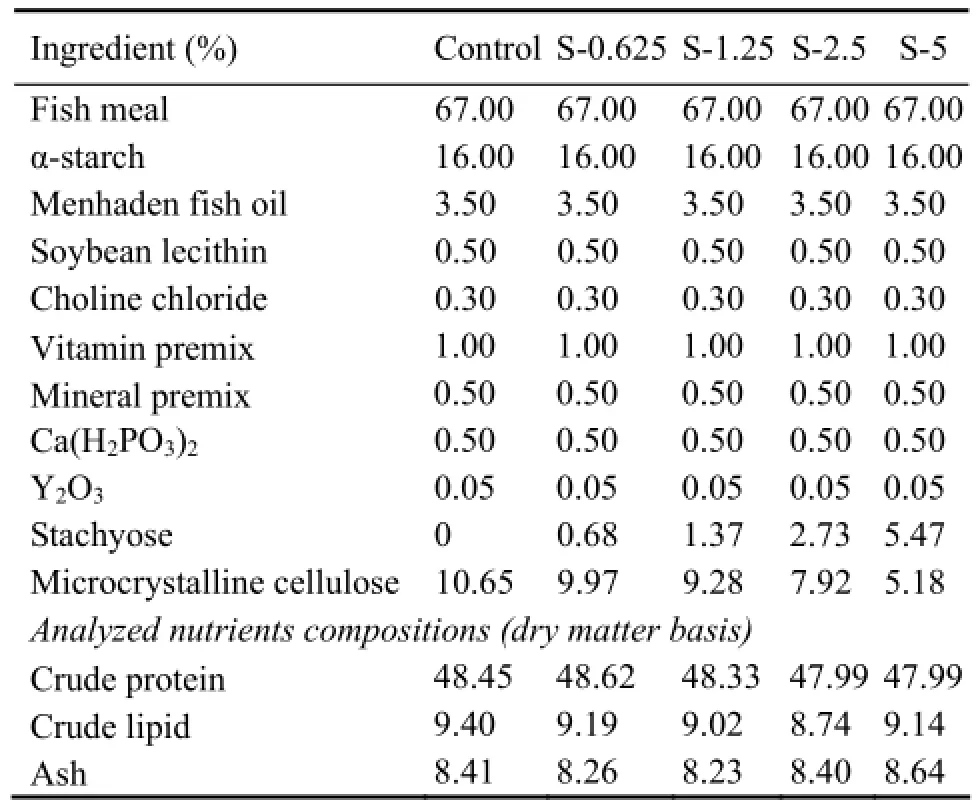
Table 1 Formulation and proximate composition of the experimental diets (% dry matter)
2.2 Feeding Trial
Juvenile turbot (S. maximus) were obtained from a commercial farm in Laizhou, China. Prior to the experiment, fish were acclimated to a commercial diet with 50% crude protein and 11% crude lipid (Great Seven Bio-Tech Co. Ltd, Qingdao, China) for two weeks. Then the fish were fasted for 24 h and weighed. A total of 450 fish (initial weight 4.63 g ± 0.01 g) were randomly distributed to 15 cylindrical fiberglass tanks (200 L) in an indoor rearing system with flow-through water, 30 each. Diets were randomly assigned to tanks, 3 each.
The feeding trial lasted for 12 weeks. Fish were slowly hand-fed to apparent satiation twice daily (at 7:30 and 19:30). The uneaten pellets were removed by siphoning daily and the feed consumption was recorded weekly. The number and weight of dead fish were recorded.
During the feeding period, the water temperature was controlled at 15–19℃, pH at 7.5–8.0, the salinity at 30–33, the ammonia nitrogen lower than 0.4 mg L-1, the nitrite lower than 0.1 mg L-1, and dissolved oxygen higher than 7.0 mg L-1. Aeration was continuous.
2.3 Sample Collection
A sample of 20 fish at the onset of feeding experiment and 4 fish per tank at the end were collected and stored at -20℃ for the determination of whole body composition. At the end of the feeding trial, fish were fasted for 24 h, anesthetized with eugenol (1:10000) (purity 99%, Shanghai Reagent Corp, Shanghai, China), and then counted and weighed. Intestine and stomach samples for digestive enzymes analysis were taken from other ten fish per tank and frozen immediately in liquid nitrogen before being stored at -80℃.
From each tank three fish were dissected to obtain liver and intestine samples. The body weight, body length, liver weight and visceral weight of these fish were mea- sured to calculate condition factor, hepatosomatic index and viscerosomatic index. Then the middle segments of the distal intestines from these fish were sampled (0.5 cm) for histological analysis. The rings were cut-open and rinsed in saline (9 g L-1NaCl) to remove eventual remaining gut contents. Bonn's stationary liquid (mixture of saturated water solution of picric acid, 40% formalin, and acetic acid at a ratio of 15:5:1) was used to fix the samples for the future use.
2.4 Chemical Analysis
2.4.1 Composition analysis
Feed ingredients, experimental diets and whole fish samples were analyzed in duplicates for contents of moisture, crude protein, crude lipid and ash using standard methods of AOAC (1995). Samples were dried to a stabilized weight at 105℃ to determine moisture levels. Crude protein was determined by measuring nitrogen (N×6.25) using the Kjeldahl method (2300-Auto-analyzer, FOSS, Denmark), crude lipid by ether extractionusing Soxhlet method (36680-analyer, BUCHI, Switzerland), and ash by combustion at 550℃.
2.4.2 Digestive enzyme activity
Pepsin activity was determined colorimetrically according to Anson (1938) with slight modifications. Bovine hemoglobin (Sigma Chemical Co., St. Louis, MO, USA) was used as the substrate. The soluble fraction was determined by Folin-phenol reagent (AppliChem, Darmstadt Germany). One unit of protease activity was defined as 1 μg tyrosine liberated by hydrolyzing bovine hemoglobin in 1 min at 37℃. Enzyme activity was expressed as units per mg tissue protein.
Intestinal caseinolytic activity was determined colorimetrically according to Lowry et al. (1951) and Song et al. (2011) with slight modifications. Casein (Sigma Chemical Co., St. Louis, MO, USA) was used as the substrate. The soluble fraction was determined by Folin-phenol reagent. One unit of protease activity was defined as 1 μg tyrosine liberated by hydrolyzing casein in 1 min at 37℃. Enzyme activity was expressed as units per mg tissue protein.
Trypsin activity was determined colorimetrically as described by Holm et al. (1988) and Tseng et al. (1982) with slight modifications. This method used the Na-benzoyl-arginine-p-nitroanilide (BAPNA) (Sigma Chemical Co., St. Louis, MO, USA) as the substrate. One unit was defined as 1 μmol p-nitroanilide (PNA) by catalyzing BAPNA in 1 min at 37℃. Enzyme activity was expressed as units per g tissue protein.
Intestinal and stomach amylase activities were determined according to the Somogy-Nelson colorimetric method described by Hidalgo et al. (1999) with slight modifications. Starch (Sigma Chemical Co., St. Louis, MO, USA) was used as the substrate. One unit was defined as the amount of enzyme catalyzing 10 mg starch hydrolyzed in 30 min at 37℃. Enzyme activity was expressed as units per mg tissue protein.
2.4.3 Intestine morphology
After fixation, the distal intestines were successively dehydrated in ethanol, equilibrated in xylene and embedded in paraffin wax according to standard histological procedures (Baeverfjord and Krogdahl, 1996). Then the samples were sliced into 7 μm longitudinal sections following the axis of gut lumen using a Lecia Jung RM 2016 rotary microtome (German) and stained with Hematoxylin-Eosin (H&E).
The distal intestines slides were examined under a Nikon eclipse Ti-S microscope (Japan) for lesions following the description of Baeverfjord and Krogdahl (1996), Escaffre et al. (2007) and Bonaldo et al. (2011). The intestine structure was shown in Fig.1. The height of the simple fold (hSF), the height of the complex fold (hCF), the total length of the intestinal epithelium over 500
μm distance (lIE), the height of the microvillus (hMV), and the thickness of the muscularis (tM) were chosen as the easily assessable markers for the morphological changes of the distal intestine. The macro-morphological parameters were measured by a semi-automatic computer-assisting system as follows: all simple folds and complex folds were measured for hSF and hCF separately, and 8, 20, and 20 measurements were measured for lIE, hMV, and tM respectively per fish. Because of the variable numbers of observations per individual fish, mean values of simple folds and complex folds per fish were used in the subsequent analysis.

Fig.1 The intestinal structure. hSF, the height of the simple fold; hCF, the height of the complex fold; lIE, the total length of the intestinal epithelium over 500 μm distance; hMV, the height of the microvillus estimated (height of the PAS positive stained zone); tM, the thickness of the muscularis.
2.5 Calculations
2.5.1 Growth responses
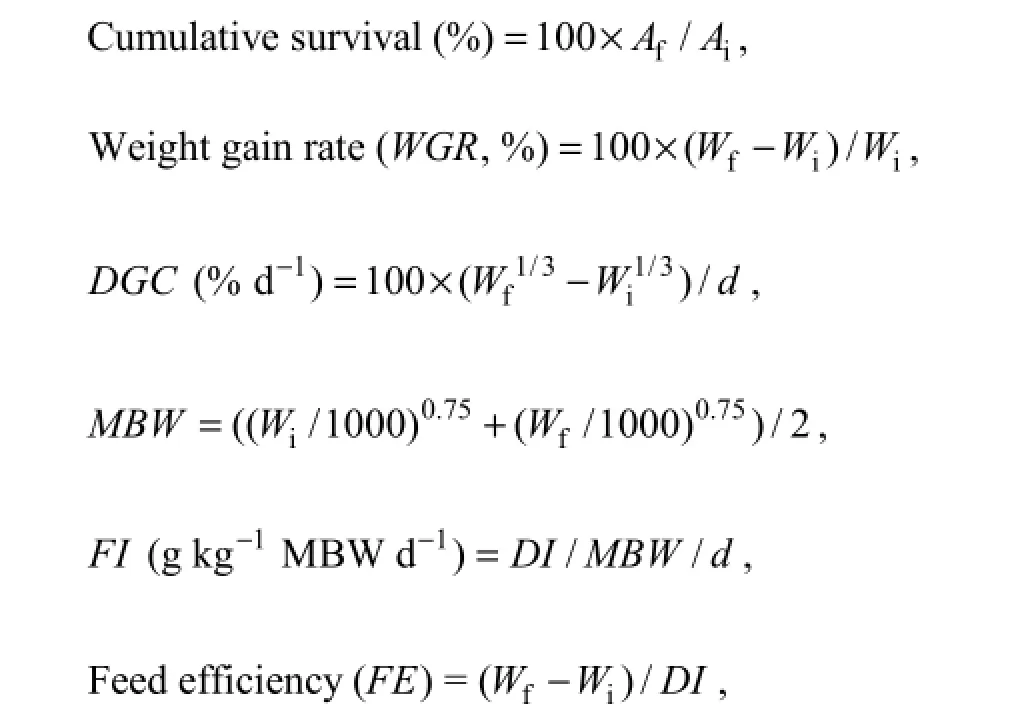
where Aiand Afis the initial and final amount of fish, respectively; Wiand Wfis the initial and final mean body weight (g), respectively; d is the feeding days, DGC is daily growth coefficient, MBW is mean metabolic body weight, FI is feed intake, DI is the dry feed intake per fish (g, DM fish-1).
2.5.2 Body index
Condition factor (C F , %) = 100 × Wb/Lb3,
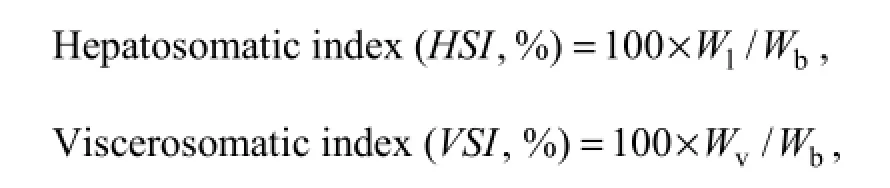
where Wbis the fish body weight (g), Wland Wvis the fish liver and viscera weight (g) respectively, Lbis the fish body length (cm).
2.6 Statistical Analysis
Data from each treatment were subjected to one-way analysis of variance (ANOVA) using SPSS 16.0 for windows. When overall differences are significant (P < 0.05), Tukey’s test is used to compare the means among treatments.
3 Results
3.1 Survival, Growth Performance and Somatic Indexes
No significant difference (P > 0.05) was observed on the survival of fish from all treatments (Table 2). The growth performance, including the final mean body weight (Wf), weight gain rate (WGR) and specific growth rate (SGR), significantly increased (P < 0.05) and then stabilized (P > 0.05) with the increase of dietary stachyose level. The highest SGR, Wfand WGR were observed ingroup S-1.25 (P < 0.05). Fish in group S-1.25 showed significantly higher (P < 0.05) feed efficiency values (FE) than that of fish fed with S-0.625 and S-5 diets. The feed intake (FI) of group S-5 was significantly (P < 0.05) higher than that of fish from other treatments. There was no significant difference (P > 0.05) on the condition factor (CF), hepatosomatic index (HSI) or viscerosomatic index (VSI) of fish among treatments.

Table 2 Effects of dietary stachyose on survival, growth performance, feed utilization and somatic indexes of juvenile turbot
3.2 Body Composition
The whole-body moisture, protein, lipid or ash content of fish from all treatments showed no significant difference (P > 0.05) (Table 3).
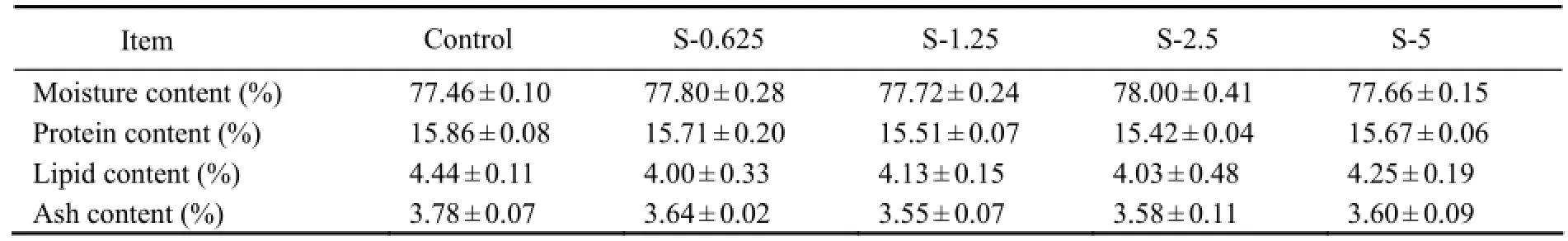
Table 3 Effects of dietary stachyose on whole-body compositions of juvenile turbot
3.3 Digestive Enzyme Activity
The activities of trypsin and intestinal caseinolytic first increased (P < 0.05), and then stabilized (P < 0.05) with the increasing of the dietary stachyose level, with the highest value observed in group S-1.25 (Table 4). The activity of stomach amylase significantly increased (P < 0.05) by the addition of stachyose, and fish from group S-5 showed the highest value (P < 0.05). Fish from the control showed significantly higher (P < 0.05) intestinal amylase activity than that of fish fed other diets. With the increased dietary stachyose level, the activity of pepsin firstly increased slightly and then stabilized, and no difference was observed (P > 0.05).
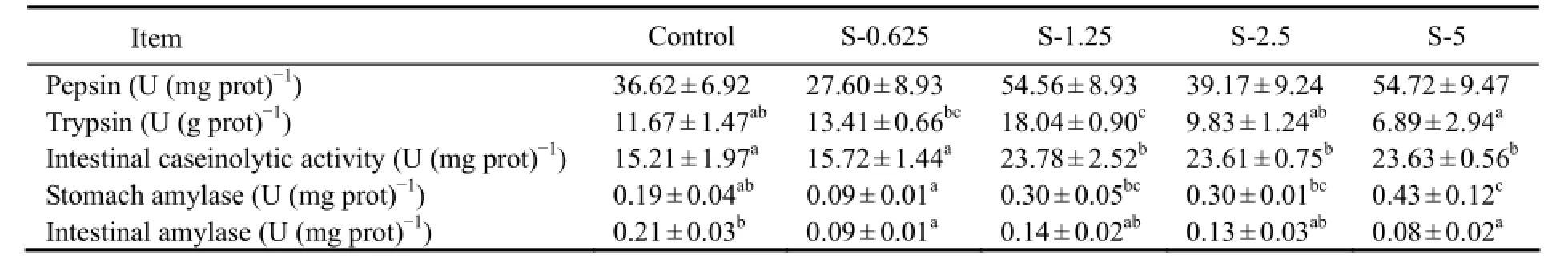
Table 4 Effects of dietary stachyose on digestive enzymes of juvenile turbot
Notes: Values are means ± S.E (n =3); Values within the same row with different letters are significantly different (P < 0.05).
3.4 Morphology and Morphometry of Distal Intestine
No lesion or damage was found in the distal intestine of fish from all treatments (Fig.2). The mucosa were highly developed and showed two kinds of folds: simple folds and complex folds characterized by multiple branches. The epithelium of the mucosal folds consisted of a single layer of epithelium cells, and there were numerous goblet cells and intraepithelial lymphocytes among them. The microvillous border and mucus covered on the apical surface of epithelium cell. The epithelium cell showed various degrees of cytoplasmic supranuclear vacuolation, and its nuclei were evenly polarized and basally located. The lamina propria and submucosa existed below the mucosa and presented similar width.
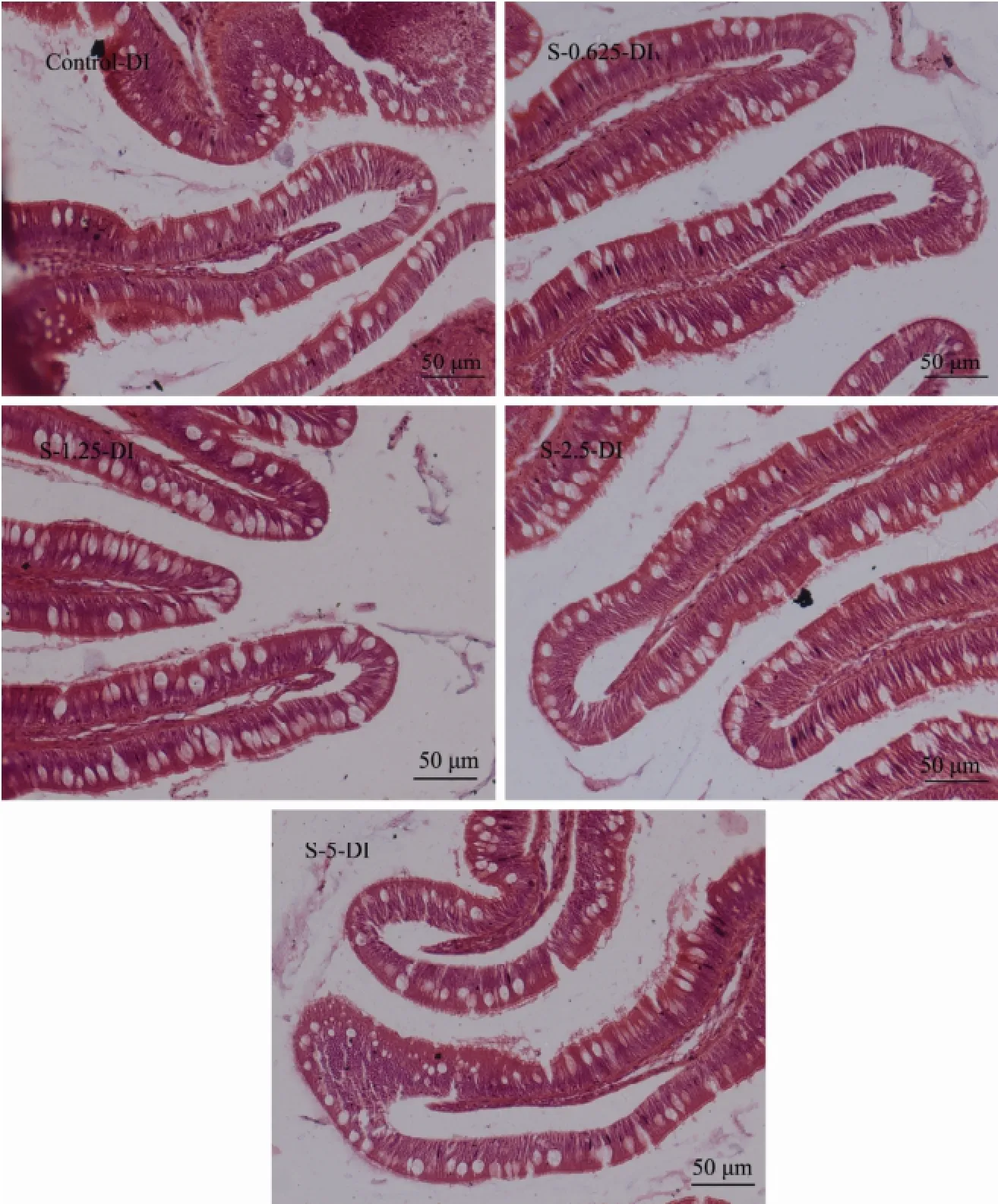
Fig.2 Effects of dietary stachyose on distal intestine morphology of juvenile turbot. Control-DI, distal intestine of fish from control treatment; S-0.625-DI, distal intestine of fish from S-0.625 treatment; S-1.25-DI, distal intestine of fish from S-1.25 treatment; S-2.5-DI, distal intestine of fish from S-2.5 treatment; S-5-DI, distal intestine of fish from S-5 treatment.

Table 5 Effects of dietary stachyose on distal intestine morphometry of juvenile turbot
The height of simple fold from distal intestine (Table 5) was significantly increased (P < 0.05) when 1.25% and 2.5% stachyose were added in diets. The height of the complex fold (hCF), total length of intestinal epithelium over 500 μm distance (lIE), thickness of muscularis estimated (hMV) or thickness of muscularis (tM) in the dis-tal intestine of fish from all treatments showed no significant differences (P > 0.05). However, with the increase of dietary stachyose content, the hCF and lIE were first slightly increased and then stabilized (P > 0.05).
4 Discussion
Stachyose is generally considered as an anti-nutritional factor (ANF) because it depresses the growth performance and feed utilization of animals (Irish and Balnave, 1993; Pan et al., 2002; Zhang et al., 2003; Jiang et al., 2006; Deng et al., 2009a) via its detrimental effects including flatulence and intestine disturbance (Mussatto et al., 2007; Choct et al., 2010; Hart et al., 2010), as well as diarrhea (Wiggins, 1984; Pan et al., 2002; Zhang et al., 2001). However, in the present study, limited negative effects of dietary stachyose (0.625%–5%) were observed on the growth performance, feed utilization, digestive enzyme activities and distal intestine structures of turbot. Recent studies also reported that the addition of 3% stachyose in diet showed no detrimental effect on the growth performance, feed utilization, digestive enzymes activities and distal intestine structure of Atlantic salmon (Sørensen et al., 2011). Feeding allogynogenetic silver crucian carp and piglets with 3.4% dietary stachyose (Cai et al., 2012) and 1%–2% dietary stachyose (Zhang et al., 2001), respectively, also showed similar results. Sørensen et al. (2011) and Cai et al. (2012) suggested that the addition of stachyose in fish diet alone may not result in the soybean meal-induced negative effects. The negative effects of soybean meal might be due to the interaction of oligosaccharides with other ANFs. As stachyose could only be utilized by the gut bacteria, the higher stachyose fermentation capability of the intestinal microflora may contribute to these results (Jiang et al., 2006). However, it has been reported that higher level stachyose could depress growth performance and feed utilization of fish (Kaushik et al., 1995; Refstie et al., 1998; Cai, 2006), piglets (Pan et al., 2002; Zhang et al., 2003) and broiler (Irish and Balnave, 1993; Jiang et al., 2006). Different factors might contribute to the various results observed in different studies, e.g., the experiment duration, the source, type and concentration of stachyose, the basal diet, the species and age of experimental animal, rearing environment and tolerance of ANF of animals (Choct et al., 2010; Sørensen et al., 2011).
Stachyose has been accepted as a functional food or a probiotic because of its positive effects on human and animals, and has been applied as a food ingredient (Refstie et al., 2005; Mussatto et al., 2007; Grisdale-Helland et al., 2008; Sørensen et al., 2011). In this study, improved growth performance and feed utilization, increased activities of different digestive enzymes, and increased areas and cells for the digestion and absorption of nutrients in the distal intestine were observed. The similar results were observed in Atlantic salmon (3% starchyose) (Sørensen et al., 2011), Japanese flounder (2.61% starch- yose) (Deng et al., 2009b), and allogynogenetic silver crucian carp (3.4% stachyose) (Cai et al., 2012). This might be due to the beneficial effects of the degradation products of stachyose by intestinal microorganisms, such as short chain fatty acids (SCFA), which could up-regulate gene expression of digestive enzymes (Lilleeng et al., 2007; Mi et al., 2011; Sørensen et al., 2011). These nutrients could also improve the growth of certain microorganisms in the intestine, which may produce exogenous digestive enzymes to improve the digestive ability of host (Moriarty, 1996, 1998; Mussatto et al., 2007). The improved intestinal microflora could improve gut health and protect intestinal structure of the host, and the nutrients produced by microorganisms could also improve intestinal cell proliferation and differentiation and decrease apoptosis (Macfarlane et al., 2008; Sørensen et al., 2011). All these reasons might lead to the improved digestive ability and intestinal structure by stachyose in this study. Consequently, the beneficial effects of stachyose on intestinal properties could improve the growth performance and feed utilization.
5 Conclusions
In this study, a moderate level of stachyose (1.25%) added to the diet improved growth performance, digestive enzymes activities and distal intestinal morphology of turbot. High levels of dietary stachyose (2.5%–5%) showed limited negative effects. The present results provide further insight into the use of stachyose in aquafeed.
Acknowledgements
This study was supported by the Research Fund for the Doctoral Program of Higher Education of China (No. 20120132120025), National Program on Key Basic Research Project (973 Program, 2014CB138600), the Promotive Research Fund for Excellent Young and Middle-aged Scientists of Shandong Province (No. BS2013 SW007) and Modern Agro-Industry Technology Research System (No. nycytx-50-G07). The authors would like to thank Houguo Xu, Rantao Zuo, Xiaodong Wang, Dandan Xu and Songlin Li for their help in this study.
Anson, M. L., 1938. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. The Journal of General Physiology, 22 (1): 79-89.
Association of Official Analytical Chemists (AOAC), 1995. Official Methods of Analysis of Official Analytical Chemists International. 16th edition. Association of Official Analytical Chemists, Arlington, VA, 16-26.
Baeverfjord, G., and Krogdahl, Å., 1996. Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: A comparison with the intestines of fasted fish. Journal of Fish Diseases, 19 (5): 375-387.
Bonaldo, A., Parma, L., Mandrioli, L., Sirri, R., Fontanillas, R., Badiani, A., and Gatta, P. P., 2011. Increasing dietary plant proteins affects growth performance and ammonia excretion but not digestibility and gut histology in turbot (Psettamaxima) juveniles. Aquaculture, 318 (1): 101-108.
Cai, C. F., Wang, W. J., Ye, Y. T., Krogdahl, Å., Wang, Y. L., Xia, Y. M., and Yang, C. G., 2012. Effect of soybean meal, raffinose and stachyose on the growth, body composition, intestinal morphology and intestinal microflora of juvenile allogynogenetic silver crucian carp (Carassius auratus gibelio♀ × Cyprinus carpio ♂). Aquaculture Research, 43: 128-138.
Cai, Y. H., 2006. Effects of antinutritional factors in soybean on the growth performance and digestive physiology of Japanese flounder, Paralichthys olivaceus. Master thesis. Ocean University of China (in Chinese with English abstract).
Cerning-Beroard, J., and Filiatre, A., 1976. A comparison of the carbohydrate composition of legume seeds: Horse beans, peas, and lupines. Cereal Chemistry, 53: 968-978.
Choct, M., Dersjant-Li, Y., McLeish, J., and Peisker, M., 2010. Soy oligosaccharides and soluble non-starch polysaccharides: A review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Asian-Australasian Journal of Animal Sciences, 23 (10): 1386-1398.
Chou, R. L., Her, B. Y., Su, M. S., Hwang, G., Wu, Y. H., and Chen, H. Y., 2004. Substituting fish meal with soybean meal in diets of juvenile cobia Rachycentron canadum. Aquaculture, 229 (1): 325-333.
Day, O. J., and Plascencia GonzÁlez, H. G., 2000. Soybean protein concentrate as a protein source for turbot Scophthalmus maximus L. Aquaculture Nutrition, 6 (4): 221-228.
Deng, J. M., Mai, K. S., Ai, Q. H., Zhang, W. B., Wang, X., Xu, W. J., and Liufu, Z. G., 2006. Effects of replacing fish meal with soy protein concentrate on feed intake and growth of juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture, 258 (1): 503-513.
Deng, J. M., Mai, K. S., Ai, Q. H., and Zhang, W. B., 2009a. Effects of soybean oligosaccharides on nutritional characters of Japanese flounder (Paralichthys olivaceus): I. Feeding rate, growth and metabolize enzyme activities. Acta Hydrobiologica Sinica, 33: 7-14 (in Chinese with English abstract).
Deng, J. M., Mai, K. S., Ai, Q. H., and Zhang, W. B., 2009b. Effects of soybean oligosaccharides on nutritional characters of Japanese flounder (Paralichthys olivaceus): II. Digestibility and digestive physiology. Acta Hydrobiologica Sinica, 33: 369-375 (in Chinese with English abstract).
Escaffre, A. M., Kaushik, S., and Mambrini, M., 2007. Morphometric evaluation of changes in the digestive tract of rainbow trout (Oncorhynchus mykiss) due to fish meal replacement with soy protein concentrate. Aquaculture, 273 (1): 127-138.
Francis, G., Makkar, H. P., and Becker, K., 2001. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture, 199 (3): 197-227.
Gibson, G. R., Ottaway, P. B., and Robert, A. R., 2000. Prebiotics: New Developments in Functional Foods. Chandos Publishing Limited, Oxford, 1-108.
Grisdale-Helland, B., Helland, S. J., and Gatlin III, D. M., 2008. The effects of dietary supplementation with mannanoligosaccharide, fructooligosaccharide or galactooligosaccharide on the growth and feed utilization of Atlantic salmon (Salmo salar). Aquaculture, 283 (1): 163-167.
Hart, S. D., Bharadwaj, A. S., and Brown, P. B., 2010. Soybean lectins and trypsin inhibitors, but not oligosaccharides or the interactions of factors, impact weight gain of rainbow trout (Oncorhynchus mykiss). Aquaculture, 306: 310-314.
Hayakawa, K., Mizutani, J., Wada, K., Masai, T., Yoshihara, I., and Mitsuoka, T., 1990. Effects of soybean oligosaccharides on human faecal flora. Microbial Ecology in Health and Disease, 3 (6): 293-303.
Hernández, M. D., Martínez, F. J., Jover, M., and García García, B., 2007. Effects of partial replacement of fish meal by soybean meal in sharpsnout seabream (Diplodus puntazzo) diet. Aquaculture, 263 (1): 159-167.
Hidalgo, M. C., Urea, E., and Sanz, A., 1999. Comparative study of digestive enzymes in fish with different nutritional habits. Proteolytic and amylase activities. Aquaculture, 170 (3): 267-283.
Holm, H., Hanssen, L. E., Krogdahl, Å., and Florholmen, J., 1988. High and low inhibitor soybean meals affect human duodenal proteinase activity differently: In vivo comparison with bovine serum albumin. The Journal of Nutrition, 118 (4): 515.
Irish, G. G., and Balnave, D., 1993. Non-starch polysaccharides and broiler performance on diets containing soyabean meal as the sole protein concentrate. Crop and Pasture Science, 44 (7): 1483-1499.
Jiang, H. Q., Gong, L. M., Ma, Y. X., He, Y. H., Li, D. F., and Zhai, H. X., 2006. Effect of stachyose supplementation on growth performance, nutrient digestibility and caecal fermentation characteristics in broilers. British Poultry Science, 47 (4): 516-522.
Kaushik, S. J., Cravedi, J. P., Lalles, J. P., Sumpter, J., Fauconneau, B., and Laroche, M., 1995. Partial or total replacement of fish meal by soybean protein on growth, protein utilization, potential estrogenic or antigenic effects, cholesterolemia and flesh quality in rainbow trout Oncorhynchus mykiss. Aquaculture, 133 (3): 257-274.
Lilleeng, E., Froystad, M. K., Ostby, G. C., Valen, E. C., and Krogdahl, Å., 2007. Effects of diets containing soybean meal on trypsin mRNA expression and activity in Atlantic salmon (Salmo salar L). Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 147 (1): 25-36.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J., 1951. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry, 193 (1): 265-275.
Macfarlane, G. T., Steed, H., and Macfarlane, S., 2008. Bacterial metabolism and health-related effects of galacto-oligosac- charides and other prebiotics. Journal of Applied Microbiology, 104 (2): 305-344.
Mi, H. F., Mai, K. S., Zhang, W. B., Wu, C. L., and Cai, Y. H., 2011. Effects of dietary soybean stachyose and phytic acid on gene expressions of serine proteases in Japanese flounder (Paralichthys olivaceus). Journal of Ocean University of China, 10 (3): 234-240.
Moriarty, D., 1996. Microbial biotechnology: A key ingredient for sustainable aquaculture. Infofish International, (4): 29-33.
Moriarty, D. J. W., 1998. Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture, 164 (1): 351-358.
Mussatto, S. I., and Mancilha, I. M., 2007. Non-digestible oligosaccharides: A review. Carbohydrate Polymers, 68 (3): 587- 597.
Mul, A. J., and Perry, F. G., 1994. The role of fructo-oligosaccharides in animal nutrition. In: Recent Advances in Animal Nutrition. Garnsworthy, P. C., and Cole, D. J. A., eds., Nottingham University Press, Nottingham, 57-79.
Naylor, R. L., Hardy, R. W., Bureau, D. P., Chiu A., Elliott, M., Farrell, A. P., Forster, I., Gatlin, D. M., Goldburg, R. J., Hua, K., and Nichols, P. D., 2009. Feeding aquaculture in an era of finite resources. Proceedings of the National Academy of Sciences, 106 (36): 15103-15110.
Olsen, R. L., and Hasan, M. R., 2012. A limited supply of fish-meal: Impact on future increases in global aquaculture production. Trends in Food Science and Technology, 27 (2): 120- 128.
Pan, B. H., Li, D. F., Piao, X. S., Zhang, L. Y., and Guo, L., 2002. Effect of dietary supplementation with α-galactosidase preparation and stachyose on growth performance, nutrient digestibility and intestinal bacterial populations of piglets. Archives of Animal Nutrition, 56 (5): 327-337.
Refstie, S., Storebakken, T., and Roem, A. J., 1998. Feed consumption and conversion in Atlantic salmon (Salmo salar) fed diets with fish meal, extracted soybean meal or soybean meal with reduced content of oligosaccharides, trypsin inhibitors, lectins and soya antigens. Aquaculture, 162 (3): 301-312.
Refstie, S., Sahlström, S., Bråthen, E., Baeverfjord, G., and Krogedal, P., 2005. Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for Atlantic salmon (Salmo salar). Aquaculture, 246 (1): 331-345.
Risley, C. R., and Lohrmann, T., 1998. Growth performance and apparent digestibility of weanling pigs fed diets containing low stachyose soybean meal. Journal of Animal Science, 76 (1): 179.
Song, J., Tao, W. Y., and Chen, W. Y., 2011. Kinetics of enzymatic unhairing by protease in leather industry. Journal of Cleaner Production, 19 (4): 325-331.
Spring, P., Wenk, C., Dawson, K. A., and Newman, K. E., 2000. The effects of dietary mannaoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of salmonella-challenged broiler chicks. Poultry Science, 79 (2): 205-211.
Sørensen, M., Penn, M., El-Mowafi, A., Storebakken, T., Cai, C. F., Øverland, M., and Krogdahl, Å., 2011. Effect of stachyose, raffinose and soya-saponins supplementation on nutrient digestibility, digestive enzymes, gut morphology and growth performance in Atlantic salmon (Salmo salar, L). Aquaculture, 314 (1): 145-152.
Tseng, H. C., Grendell, J. H., and Rothman, S. S., 1982. Food, duodenal extracts, and enzyme secretion by the pancreas. American Journal of Physiology-Gastrointestinal and Liver Physiology, 243 (4): G304-G312.
United States Department of Agriculture, 2009. USDA National Nutrient Database for Standard Reference Release. 18.
Wiggins, H. S., 1984. Nutritional value of sugars and related compounds undigested in the small gut. Proceedings of the Nutrition Society, 43 (1): 69-75.
Zhang, L. Y., Li, D. F., Qiao, S. Y., Wang, J. T., Bai, L., Wang, Z. Y., and Han, I. K., 2001. The effect of soybean galactooligosaccharides on nutrient and energy digestibility and digesta transit time in weaning piglets. Asian-Australasian Journal Animal Sciences, 14 (11): 1598-1604.
Zhang, L. Y., Li, D. F., Qiao, S. Y., Johnson, E. W., Li, B. Y., Thacker, P. A., and Han, I. K., 2003. Effects of stachyose on performance, diarrhoea incidence and intestinal bacteria in weanling pigs. Archives of Animal Nutrition, 57 (1): 1-10.
(Edited by Qiu Yantao)
(Received January 20, 2014; revised March 28, 2014; accepted May 27, 2015)
J. Ocean Univ. China (Oceanic and Coastal Sea Research)
DOI 10.1007/s11802-015-2587-z
ISSN 1672-5182, 2015 14 (5): 905-912
http://www.ouc.edu.cn/xbywb/
E-mail:xbywb@ouc.edu.cn
* Corresponding author. Tel: 0086-532-82031627
E-mail: yanjiaozhang@ouc.edu.cn
 Journal of Ocean University of China2015年5期
Journal of Ocean University of China2015年5期
- Journal of Ocean University of China的其它文章
- Seasonal Dynamics of Turbidity Maximum in the Muthupet Estuary, India
- Decadal Variability of Global Ocean Significant Wave Height
- An Observational and Modeling Study of Extratropical Transition of Hurricane Sandy in 2012
- The Nonlinear Bifurcation and Chaos of Coupled Heave and Pitch Motions of a Truss Spar Platform
- Beach Morphology and Coastline Evolution in the Southern Bohai Strait
- Comparison of Meiofaunal Abundance in Two Mangrove Wetlands in Tong’an Bay, Xiamen, China
