Changes in Plasma Osmolality, Cortisol and Amino Acid Levels of Tongue Sole (Cynoglossus semilaevis) at Different Salinities
WANG Guodong, XU Kefeng, TIAN Xiangli,, DONG Shuanglin, and FANG Ziheng
1) Key Laboratory of Mariculture of Ministry of Education, Ocean University of China, Qingdao 266003, P. R. China
2) National Oceanographic Center of Qingdao, Qingdao 266071, P. R. China
Changes in Plasma Osmolality, Cortisol and Amino Acid Levels of Tongue Sole (Cynoglossus semilaevis) at Different Salinities
WANG Guodong1), XU Kefeng2), TIAN Xiangli1),*, DONG Shuanglin1), and FANG Ziheng1)
1) Key Laboratory of Mariculture of Ministry of Education, Ocean University of China, Qingdao 266003, P. R. China
2) National Oceanographic Center of Qingdao, Qingdao 266071, P. R. China
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
A serial of salinity transferring treatments were performed to investigate the osmoregulation of tongue sole (Cynoglossus semilaevis). Juvenile tongue sole were directly transferred from a salinity of 30 to 0, 10, 20, 30, 40 and 50. Blood sampling was performed for each treatment after 0, 1, 6 and 12 h, as well as after 1, 2, 4, 8, 16 and 32 d. The plasma osmolality, cortisol and free amino acids were assessed. Under the experimental conditions, no fish died after acute salinity transfer. The plasma cortisol level increased 1 h after the abrupt transfer from a salinity of 30 to that of 0, 40 and 50, and decreased from 6 h to 8 d after transfer. Similar trends were observed in the changes of plasma osmolality. The plasma free amino acids concentration showed a ‘U-shaped’ relationship with salinity after being transferred to different salinities for 4 days. More obvious changes of plasma free amino acid concentration occurred under hyper-osmotic conditions than under hypo-osmotic conditions. The concentrations of valine, isoleucine, lysine, glutamic acid, glycine, proline and taurine increased with rising salinity. The plasma levels of threonine, leucine, arginine, serine, and alanine showed a ‘U-shaped’ relationship with salinity. The results of this study suggested that free amino acids might have important effects on osmotic acclimation in tongue sole.
Cynoglossus semilaevis; amino acids; cortisol; osmoregulation; salinity acclimation; time course
1 Introduction
Salinity is one of the most important abiotic factors affecting the growth of fishes and has complex and wideranging biological effects (Boeuf and Payan, 2001; Jamil et al., 2004; Gonzalez, 2012; Webb et al., 2012). Cortisol is an important glucocorticoid that functions in the osmotic acclimation and stress response of teleosts (Mommsen et al., 1999; McCormick, 2001; O’Connor et al., 2011; Pankhurst, 2011). The effects of cortisol on osmoregulatory parameters have been studied in teleosts (Yamashita et al., 2003). In addition, it is showed that high plasma cortisol levels, induced by stressful condition, generally regulate the fish amino acid metabolism in several teleost species (Vijayan et al., 1997). Organic osmolytes, such as amino acids, can be accumulated at higher concentrations in fish without perturbing cell structures and metabolic activities (Gilles, 1997). Changes in the plasma levels of free amino acid may be attributed to the amino acid requirements of fish (Wilson, 2002; Robinson et al., 2011). To date, only a few reports have discussedand integrated the changes of amino acid in seawater acclimation (Bystriansky et al., 2007).
Tongue sole, Cynoglossus semilaevis Güther, 1873, belongs to the Cynoglossidae family in Class Actinopterygii. It is an important native commercial fish that widely distributes throughout the coastal areas of Bohai Sea, Yellow Sea, and East China Sea (Ma et al., 2005). In recent years, tongue sole has been intensively cultured indoor in China because of its high nutritional and economic values (Ma et al., 2007). The knowledge of its ability to acclimate to the different salinity levels is important for the indoor culture. Tian et al. (2011) observed that tongue sole is a euryhaline species, which has the ability of adapting to a wide range of salinity levels (0 to 40). However, until now only a few such studies have been carried out.
The primary objectives of this research were to study the capacity of tongue sole to adapt to a wide range of salinity levels, and to assess the time course of the osmoregulatory and metabolic changes during the acclimation to hyper-osmotic and hypo-osmotic environments. The role of plasma free amino acids with respect to osmoregulation was investigated particularly during the first month after the transfer.
2 Materials and Methods
2.1 Fish Acclimation
The experiment was conducted in Key Laboratory of Mariculture of Ministry of Education, Qingdao, China. Juvenile tongue soles, with the weight of 52.9 g ± 7.0 g, and the size of 20.3 cm ± 1.8 cm, were obtained from Mingbo Aquatic Product Co., Ltd., China.
The juvenile tongue soles were cultured in square fiberglass tanks (240 × 120 × 30 cm) filled with sand-filtered seawater (salinity of 30) at 21℃ ± 1℃ for four weeks to fully ensure thermal and environmental adaptation. During the period of acclimation, 50% of the water in each tank was replaced once a day. The fish were fed to satiation twice a day at 07:30 and 19:30 with commercial dry pellets (INVE, Thailand). The pellet size was 0.8–1.2 mm with protein 48.52%, lipid 17.63%, ash 10.28% and moisture 8.05%.
2.2 Experimental Design and Management
After acclimation, 360 fish were randomly assigned to 6 salinity groups. Each group included 5 tanks with 12 individuals per tank (80 cm × 60 cm × 40 cm). Each salinity group was labeled S0, S10, S20, S30, S40 and S50, which contained the following levels of salinity: 0 (28 mOsm kg-1H2O), 10 (309 mOsm kg-1H2O), 20 (603 mOsm kg-1H2O), 30 (897 mOsm kg-1H2O), 40 (1184 mOsm kg-1H2O), and 50 (1511 mOsm kg-1H2O), respectively. Sampling was conducted for each treatment at 0, 1, 6, and 12 h as well as at 1, 2, 4, 8, 16 and 32 d after transfer. During each sampling, 5 fish per salinity group were sacrificed (there were 2 individuals surplus at each tank after the experiment) after anesthetized with MS-222 (100 ppm; Sigma, Germany). Blood was individually withdrawn from the fish caudal vein using 2 mL syringes, and centrifuged at 3000 r min-1for 3 min at 4℃ in heparinized tubes. The collected supernatant was frozen in liquid nitrogen and stored at -80℃ until further analysis. To minimize the influence of feeding on plasma cortisol levels or other parameters, the fish were fasted for 24 h before sampling.
The experimental salinity levels were achieved either by mixing seawater (salinity of 30) with dechlorinated tap water, or by adding commercial artificial sea salt (Haida Tongyong Co., Ltd., China) to the general seawater. During the experiment, water temperature ranged from 19.5℃ to 21.9℃, and the pH was approximately 8.0. The amount of ammonia nitrogen in water was less than 0.18 mg L-1. Photoperiod was 12 h light:12 h dark provided by artificial lighting with a light intensity at 100–120 lx. Light aeration was continuously provided to maintain dissolved oxygen levels at above 6 mg L-1. The salinity (measured by SevenGoTMconductivity SG3-ELK, Mettler Toledo Pte Ltd, Singapore), pH (measured by pH meter FE20, Mettler Toledo Pte Ltd, Singapore), and ammonia (measured by ML820 Ammonia portable photometer, Shanghai Milian Electronic Technology CO., LTD, China) levels were monitored daily.
2.3 Determination of Parameters
Plasma osmolality was measured using a Micro-Osmometer (Model 210; Fiske Associates). Its value was expressed in mOsm kg-1H2O. Plasma cortisol was determined by radioimmunoassay (RIA), as described by Wen et al. (2006), using commercial radioimmunoassay kits ([125I] cortisol; Tianjin Nine Tripods Medical & Bioengineering Co., Ltd.). Pellets were quantified using a gamma counter (SN-695B; Shanghai Hesuo Rihuan Photo electricity Instrument Co., Ltd., China), and their concentrations were expressed in µg dL-1. Plasma samples were measured using an L-8800 amino acid analyzer (Hitatchi, Tokyo, Japan). To determine the amount of free amino acids, peptidesand proteins in plasma were removed through the precipitation with sulfosalicylic acid in case they can block the column. Quantification was performed by cation exchange chromatography with lithium citrate buffer, the subsequent post-column reaction with ninhydrin, and photometric detection using the Hitachi amino acid analyzer. Plasma protein was measured using the Coomassie blue method (Bradford, 1976), with bovine plasma albumin as a standard.
2.4 Data Analysis
The data were analyzed with the SPSS for Windows (version 13.0) statistical package. The different parameters among treatments were compared using one-way ANOVA, which was followed by Duncan’s multiple range tests for post-hoc pairwise comparisons. Differences were considered statistically significant when P <0.05. All results were expressed as the mean ± SE (n = 5).
3 Results
No fish died during the experiment. Variation of the plasma osmolality levels was positively correlated with the environmental salinity. S0 and S10 decreased and S40 and S50 increased as compared with the pre-transfer control group (Fig.1). The peak values of plasma osmolality in S0, S10, S40 and S50 appeared at 2 d post-transfer, recovered by 8 d post-transfer, and remained constant thereafter. At the termination of the experiment (32 d post-transfer), plasma osmolality was observed in three significantly different levels (F = 6.95, P = 0.017). One group included fish at S40 and S50. The second group was composed of fish at S10, S20, and S30, whereas S0 comprised the last group.
The plasma cortisol concentrations of S0, S40, and S50 were significantly elevated (F = 9.67, P = 0.003) relative to S10, S20, and S30, and peaked at 6 h, 6 h, and 1 h posttransfer, respectively (Fig.2). Then the plasma cortisol levels gradually decreased until 8 d post-transfer. Significant differences were observed throughout the remainder of the experiment for salinity levels of 0, 40, and 50, as compared with S10, S20, and S30 (F = 6.28, P = 0.005). The plasma cortisol levels were not significantly changedin fish transferred from a salinity of 30 to 10 or 20 during the experiment.

Fig.1 Time course of changes in the plasma osmolality of C. semilaevis after transfer from salinity of 30 to 0, 10, 20, 30, 40, or 50. Each point represents the mean ± SE (n = 5). Different letters (a, b, c, or d) indicate significant differences (P <0.05) between groups at the same sampling time.
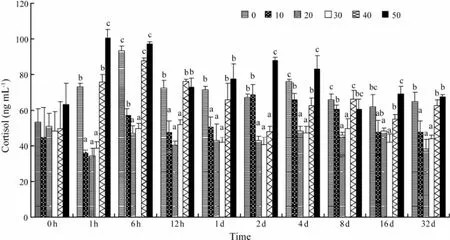
Fig.2 Time course of changes in the plasma cortisol of C. semilaevis after transfer from a salinity of 30 to 0, 10, 20, 30, 40, or 50. Each point represents the mean ± SE of n = 5 fish per group at each sampling. Different letters (a, b, or c) indicate significant differences (P < 0.05) between groups at the same sampling time.
Changes of plasma free amino acids, indispensable amino acids and dispensable amino acids were shown in Fig.3. The concentrations of plasma free amino acid were affected by the salinity levels, which showed a ‘U-shaped’ relationship from approximately 4 d post-transfer and onward (Fig.3A). The levels of plasma free amino acid in S50 at 4 d, 8 d, 16 d and 32 d were significantly higher than those in S0, S10, S20 and S30 (F = 6.15, P = 0.013). While the concentration of plasma amino acid in S0 was significantly higher than in S30 at 16 d and 32 d (F = 3.92, P = 0.037). At 32 d post-transfer, plasma amino acid levels were observed in three significantly different levels (F = 8.75, P = 0.003). One group included fish at S40 and S50. The second group was composed of fish at S0 and S10, while S20 and S30 comprised the last group. The plasma indispensable amino acid concentrations showed a similar pattern as free amino acid concentrations (Fig.3B). The ‘U-shaped’ relationship was also detected in the concentrations of plasma indispensable amino acid from approximately 4 d post-transfer to the end of the trial, and the plasma indispensible amino acid levels of S50 were significantly higher than those of S0 and S10 at 1 d post-transfer and thereafter (Fig.3B). The concentrations of plasma indispensable amino acid of S0, S10, S40 and S50 peaked at 16 d, 8 d, 16 d and 8 d, respectively. No clear trend of the plasma dispensable amino acid concentration was observed during the acclimation to hyperosmotic or hypo-osmotic conditions (Fig.3C).
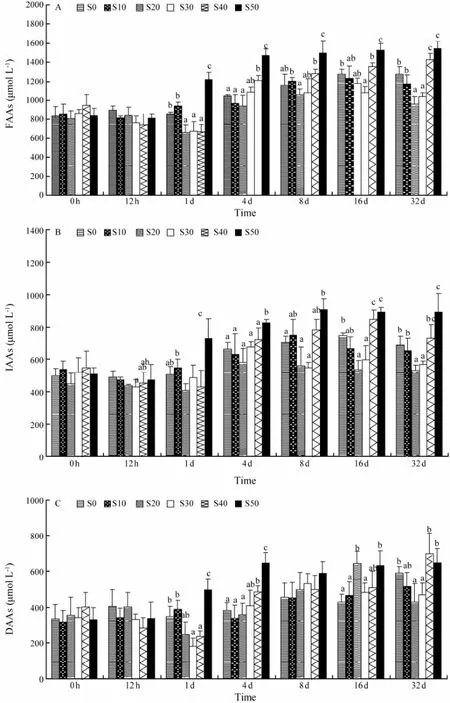
Fig.3 Time course of changes in the plasma free amino acids (A), indispensable amino acids (B), and dispensable amino acids (C) of C. semilaevis after transfer from a salinity of 30 to 0, 10, 20, 30, 40, or 50. Each point represents the mean ± SE (n = 5). Different letters (a, b, and c) indicate significant differences (P < 0.05) between groups with the same sampling time.
The effects of salinity on the concentrations of individual amino acids are shown in Fig.4. The concentrations of valine, isoleucine, lysine, glutamic acid, glycine, proline and taurine were increased with rising salinity. The plasma levels of threonine, leucine, arginine, serine, and alanine demonstrated a ‘U-shaped’ relationship with salinity. Significant changes were observed in the plasma levels of threonine, valine, isoleucine, leucine, lysine, arginine, serine, glutamic acid, glycine, alanine, proline and taurine, with respect to the environmental salinities.

Fig.4 Plasma concentrations for the different indispensable (A) and dispensable (B) amino acids of C. semilaevis at 32 d after transfer from salinity 30 to 0, 10, 20, 30, 40, and 50. Each point represents the mean ± SE (n = 5). Different letters (a, b, or c) indicate significant differences (P < 0.05) among different salinity levels for each amino acid.
4 Discussion
We can distinguish between fully euryhaline teleosts that are capable of surviving salinities ranging from freshwater to high salinity water, and partially euryhaline teleosts that survive in a more limited range of salinities ranging from low to high salinity water (Arjona et al., 2007). The results of this study demonstrated that juvenile tongue soles were able to survive a wide range of environmental salinities from 0 to 50 in a short time (32 d). During this process, tongue sole was able to adjust to the different salinity levels by the changes of plasma osmolality, cortisol and amino acid. After 8 d post-transfer to a different level of salinity, the plasma concentrations of osmolality, cortisol and free amino acids were stable. However, as significantly higher cortisol levels were still maintained in extreme salinities and plasma osmolality was in three significantly different levels, tongue sole can be defined as partially euryhaline teleost.
Cortisol has an important role in the acclimation to hyper- and hypo-osmotic environments (McCormick, 2001). The changes in cortisol concentration were previously associated with the mobilization of energy substrates in fish (Mommsen et al., 1999). Cortisol may regulate energy metabolism by affecting important metabolic pathways, such as protein synthesis or amino acid catabolism (Soengas and Aldegunde, 2002). Moreover, cortisol affects the plasma concentration of certain indispensable amino acids in fish (Vijayan et al., 1997). In the present study, the increase in plasma osmolality at 6 h–2 d following the initial rise in cortisol levels at 1–6 h posttransfer indicated a pattern of osmoregulatory for the fish. This suggested that salinity stimulated the cortisol response, and then plasma solute concentrations peaked later.
A comparison was conducted between cortisol and amino acids on their ‘time courses’ in the present study. Tongue sole transferred abruptly to extremely high or low salinity showed immediate increase in plasma cortisol, followed by increased levels of plasma free amino acids and indispensable amino acides (from day 4 d to 32 d). This suggested that salinity stimulated the response of cortisol, which might boost metabolism in teleost (Soengas and Aldegunde, 2002). Accordingly, plasma solutes such as free amino acids rose to its peak subsequently. Increased cortisol and plasma solutes might act in concert to promote salinity acclimation of tongue sole. After 8 d, cortisol concentrations decreased to a stable level very close to its original state, which may ascribe to the regulation response afterwards, such as increased level of free amino acid.
Plasma amino acid patterns reflect the net results of digestion, absorption, and subsequent utilization (Jürss et al., 1983). A decline in the plasma amino acid concentrations may be caused by increased catabolism or protein synthesis. Changes in specific amino acids may provide clues to the dominant processes (Jarvis and Ballantyne,2003). Increased plasma amino acid concentrations may arise from a number of conditions, such as decreased amino acid degradation, reduced amino acid incorporation into new proteins, or increased catabolism of skeletal muscle proteins (Sadok et al., 2004). In the current study, tongue sole exposed to different salinities (0 to 50) demonstrated a ‘U-shaped’ relationship of the free amino acids and the indispensable amino acids in the plasma. More pronounced changes occurred when tongue sole was exposed to hyper-osmotic conditions compared to hypo-osmotic conditions, because the acclimation of euryhaline marine fish species to low-salinity water requires less energy than their acclimation to hyper-osmotic conditions (Sangiao-Alvarellos et al., 2005).
There are two major functions of free amino acids during the salinity acclimation of teleost. Firstly, amino acids play a central role on energy metabolism and their oxidization would promote production of ATP during osmoregulation (Tseng and Hwang, 2008). Secondly, free amino acids are used to synthesize macromolecules in osmoregulatory organs during seawater acclimation (Auerswald et al., 1997). In the current study, significant changes were observed on concentration of threonine, valine, isoleucine, leucine, lysine, arginine, serine, glutamic acid, glycine, alainine, proline and taurine. The concentrations of threonine, leucine, arginine, serine and alainine in tongue sole cultured in extreme salinities (S0, S40 and S50) were significantly higher than those in S10, S20 and S30. This indicated that these amino acids might be related to the increased demand for oxidizable substrates of fishes during salinity acclimation (Jarvis and Ballantyne, 2003). Mommsen et al. (1999) proposed that alanine may be the preferred carrier of amino acid nitrogen for inter-tissue transport, because several amino acids can be converted to alanine, which is then released into the blood stream to work as fuel sources by other tissues (Ballantyne, 2001). In present study, alanine level was higher in extreme salinity treatments than others, which might indicate the high demand of tongue sole for energy in hyper- and hypo-osmotic environments. Moreover, leucine has also been proved to be a paramount energy source. In rainbow trout, 35%–40% of leucine can be oxidized while the rest were converted to protein (Fauconneau and Arnal, 1985). The same tendency appeared in present study. The concentrations of valine, isoleucine, lysine, glutamic acid, glycine, proline and taurine were rising with increasing salinity, showing more mobilization of these amino acids under hyper-osmotic environments in tongue sole. It seemed that those amino acids might participate in osmotic regulation. It has been confirmed that taurine is an important osmolyte in euryhaline fish species (Huxtable, 1992; Schaarschmidt et al., 1999). An increase in the concentration of taurine in the plasma of tongue sole was concomitant with an increase in salinity in the current study. This might ultimately contribute to a higher plasma osmolality in fish maintained at 40 and 50 than at the other environmental salinities. Glycine also had a critical role in the osmoregulatory response to environmental stress for fish and shellfish. For example, oysters immediately absorbed free glycine from the surrounding water in response to sudden changes in salinity (Powell et al., 1982). As regard to other plasma amino acids, methionine, a compound with a critical role in cellular peroxidative protection (Obled et al., 2002), is the precursor of cysteine and glutathiomine. Histidine might be an important energy source in Arctic char gill as it can be converted to glutamate (Bystriansky et al., 2007). However, no significant changes of methionine and histidine were detected in the present study, which might owe to the species-specific differences of osmotic acclimation pattern in flatfish.
In present study, tongue sole demonstrated the capacity of salinity acclimation. No fish died after acute salinity transfer. The plasma cortisol levels were increased immediately after the abrupt salinity transfer, followed by plasma osmolality and free amino acids. All three plasma parameters settled out after 8 d post-transfer. Free amino acids demonstrated to be important effectors on osmotic acclimation of tongue sole, which might relate to energy metabolism and their oxidization during salinity acclimation.
Acknowledgements
This work was supported by the National Great Project of Scientific and Technical Supporting Programs (Grant No. 2011BAD13B03), the program for Excellent Youth Foundation of Shandong province (Grant No. JQ201009), and the Major Project for Agricultural Application Technology Innovation of Shandong Province (Grant No. 2013-136).
Arjona, F. J., Vargas-Chacoff, L., Ruiz-Jarabo, I., Martín del Río, M. P., and Mancera, J. M., 2007. Osmoregulatory response of Senegalese sole (Solea senegalensis) to changes in environmental salinity. Comparative Biochemistry and Physiology-Part A, 148 (2): 413-421.
Auerswald, L., Jürss, K., Schiedek, D., and Bastrop, R., 1997. The influence of salinity acclimation on free amino acids and enzyme activities in the intestinal mucosa of rainbow trout, Oncorhynchus mykiss (Walbaum). Comparative Biochemistry and Physiology–Part A, 116 (2): 149-155.
Ballantyne, J. S., 2001. Amino acid metabolism. In: Fish Physiology, Vol. 20 Nitrogen Excretion. Wright, P. A., and Anderson, A. J., eds., Academic Press, New York, 77-107.
Boeuf, G., and Payan, P., 2001. How should salinity influence fish growth? Comparative Biochemistry and Physiology–Part C, 130 (4): 411-423.
Bradford, M. M., 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72 (1-2): 218-254.
Bystriansky, J. S., Frick, N. T., and Ballantyne, J. S., 2007. Intermediary metabolism of Arctic char Salvelinus alpinus during short-term salinity exposure. The Journal of Experimental Biology, 210 (Pt 11): 1971-1985.
Fauconneau, B., and Arnal, M., 1985. Leucine metabolism in trout (Salmo gairdneri Richardson.). Influence of temperature.Comparative Biochemistry and Physiology–Part A, 82 (2): 435-445.
Gilles, R., 1997. ‘Compensatory’ organic osmolytes in high osmolarity and dehydration stresses: History and perspectives. Comparative Biochemistry and Physiology–Part A, 117 (3): 279-290.
Gonzalez, R. J., 2012. The physiology of hyper-salinity tolerance in teleost fish: A review. Journal of Comparative Physiology B, 182 (3): 321-329.
Huxtable, R. J., 1992. Physiological actions of taurine. Physiological Reviews, 72 (1): 101-144.
Jamil, K., Shoaib, M., Ameer, F., and Lin, H., 2004. Salinity tolerance and growth response of juvenile Oreochaomis mossambicus at different salinity levels. Journal of Ocean Univerity of China, 3 (1): 53-55.
Jarvis, P. L., and Ballantyne, J. S., 2003. Metabolic responses to salinity acclimation in juvenile shortnose sturgeon Acipenser brevirostrum. Aquaculture, 219 (1-4): 891-909.
Jürss, K., Bittorf, T., Vökler, T., and Wacke, R., 1983. Influence of nutrition on biochemical sea water adaptation of the rainbow trout (Salmo gairdneri Richardson). Comparative Biochemistry and Physiology–Part B, 75 (4): 713-717.
Ma, A. J., Liu, X. Z., Xu, Y. J., Liang, Y., Zhuang, Z. M., Zhai, J. M., and Li, B., 2005. Study on feeding behavior and growth of tongue sole Cynoglossus semilaevis in early development stage. Oceanologia et Limnologia Sinica, 36 (2): 130-138.
Ma, A. J., Wang, X. A., Zhuang, Z. M., and Liu, X. Z., 2007. Study on relationship of the special sense organ and feeding behaviour of Cynoglossus semilaevis Güther. Oceanologia et Limnologia Sinica, 38 (3): 240-245.
McCormick, S. D., 2001. Endocrine control of osmoregulation in teleost fish. American Zoologist, 41: 781-794.
Mommsen, T. P., Vijayan, M., and Moon, T., 1999. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Reviews in Fish Biology and Fisheries, 9 (3): 211-268.
Obled, C., Papet, I., and Breuillee, D., 2002. Metabolic bases of amino acid requirements in acute diseases. Current Opinion in Clinical Nutrition & Metabolic Care, 5: 189-197.
O’Connor, E. A., Pottinger, T. G., and Sneddon, L. U., 2011. The effects of acute and chronic hypoxia on cortisol, glucose and lactate concentrations in different populations of three-spined stickleback. Fish Physiology and Biochemistry, 37 (3): 461-469.
Pankhurst, N. W., 2011. The endocrinology of stress in fish: An environmental perspective. General and Comparative Endocrinology, 170 (2): 265-275.
Powell, E. N., Kasschau, M., Chen, E., Koenig, M., and Pecon, J., 1982. Changes in the free amino acid pool during environmental stress in the gill tissue of the oyster, Crassostrea virginica. Comparative Biochemistry and Physiology–Part A, 71 (4): 591-598.
Robinson, J. W., Yanke, D., Mirza, J., and Ballantyne, J. S., 2011. Plasma free amino acid kinetics in tainbow trout (Oncorhynchus mykiss) using a bolus injection of 15N-labeled amino acids. Amino Acids, 40 (2): 689-696.
Sadok, S., M’Hetli, M., Abed, A., and Uglow, R. F., 2004. Changes in some nitrogenous compounds in the blood and tissues of freshwater pikeperch (Sander lucioperca) during salinity acclimation. Comparative Biochemistry and Physiology-part A, 138 (1): 9-15.
Sangiao-Alcarellos, S., Arjona, F. J., MartínRío, M. P., Míguez, J. M., Mancera, J. M., and Soengas, J. L., 2005. Time course of osmoregulatory and metabolic changes during osmotic acclimation in Sparus auratus. The Journal of Experimental Biology, 208 (Pt 22): 4291-4304.
Schaarschmidt, T., Meyer, E., and Jürss, K., 1999. A comparison of transport related gill enzyme activities and tissue-specific free amino acid concentrations of Baltic Sea (brackish water) and freshwater three spine sticklebacks, Gasterosteus aculeatus, after salinity and temperature acclimation. Marine Biology, 135 (4): 689-697.
Soengas, J. L., and Aldegunde, M., 2002. Energy metabolism of fish brain. Comparative Biochemistry and Physiology–Part B, 131 (3): 271-296.
Tseng, Y. C., and Hwang, P. P., 2008. Some insights into energy metabolism for osmoregulation in fish. Comparative Biochemistry and Physiology–Part C, 148 (4): 419-429.
Tian, X. L., Wang, G. D., and Dong, S. L., 2011. Effect of salinity on plasma osmolality and gill Na+/K+-ATPase activity of the tongue sole (Cynoglossus semilaevis). Marine Science, 35 (2): 27-31.
Vijayan, M. M., Pereira, C., Gordon, G. E., and Iwama, G. K., 1997. Metabolic response associated with confinement stress in tilapia: The role of cortisol. Comparative Biochemistry and Physiology–Part C, 116 (1): 89-95.
Webb, S. D., Woodcock, S. H., and Gillanders, B. M., 2012. Sources of otolith barium and strontium in estuarine fish and the influence of salinity and temperature. Marine Ecology, 453: 189-199.
Wen, H. S., Song, H. X., Yang, L. T., Mao, X. K., and Gao, L., 2006. A study on the effects of exogenous hormone on the plasma testosterone and estradiol levels in cultured Japanese flounder. Acta Oceanologica Sinica, 28 (4): 115-120.
Wilson, R. P., 2002. Amino acids and proteins. In: Fish Nutrition. Halver, J. E., and Hardy, R.W., eds., Elsevier Science, San Diego, 144-179.
Yamashita, Y., Tominaga, O., Takami, H., and Yamada, H., 2003. Comparison of growth, feeding and cortisol level in Platichthys bicoloratus juveniles between estuarine and nearshore nursery grounds. Journal of Fish Biology, 63 (3): 617-630.
(Edited by Qiu Yantao)
(Received February 13, 2014; revised April 30, 2014; accepted June 5, 2015)
J. Ocean Univ. China (Oceanic and Coastal Sea Research)
DOI 10.1007/s11802-015-2598-9
ISSN 1672-5182, 2015 14 (5): 881-887
http://www.ouc.edu.cn/xbywb/
E-mail:xbywb@ouc.edu.cn
* Corresponding author. Tel: 0086-532-82032117
E-mail: xianglitian@ouc.edu.cn
 Journal of Ocean University of China2015年5期
Journal of Ocean University of China2015年5期
- Journal of Ocean University of China的其它文章
- Seasonal Dynamics of Turbidity Maximum in the Muthupet Estuary, India
- Decadal Variability of Global Ocean Significant Wave Height
- An Observational and Modeling Study of Extratropical Transition of Hurricane Sandy in 2012
- The Nonlinear Bifurcation and Chaos of Coupled Heave and Pitch Motions of a Truss Spar Platform
- Beach Morphology and Coastline Evolution in the Southern Bohai Strait
- Comparison of Meiofaunal Abundance in Two Mangrove Wetlands in Tong’an Bay, Xiamen, China
