油菜素内酯对盐渍下油菜幼苗生长的调控效应及其生理机制
马 梅,刘 冉,郑春芳,刘伟成,尹晓明,刘金隆,王长海,郑青松,*
1 南京农业大学资源与环境科学学院 江苏省海洋生物学重点实验室, 南京 210095 2 浙江省海洋水产养殖研究所 浙江省永兴水产种业有限公司, 温州 325005
油菜素内酯对盐渍下油菜幼苗生长的调控效应及其生理机制
马 梅1,刘 冉1,郑春芳2,刘伟成2,尹晓明1,刘金隆1,王长海1,郑青松1,*
1 南京农业大学资源与环境科学学院 江苏省海洋生物学重点实验室, 南京 210095 2 浙江省海洋水产养殖研究所 浙江省永兴水产种业有限公司, 温州 325005
为了探讨油菜素内酯对植物耐盐性的调控,以甘蓝型油菜“南盐油1号”为试验材料,研究了外源24-表油菜素内酯(24-EBL)对100、200 mmol/L NaCl胁迫下油菜幼苗干重(DW)、相对含水量(RWC)、渗透调节能力(OAA)、叶片气体交换参数、气孔限制值(Ls)等的调节效应,还测定了不同器官的Na+、K+、Cl-含量,并计算各器官的K+/Na+和SK,Na。结果表明: (1)在不同浓度的盐胁迫下,油菜幼苗DW显著下降,胁迫下外源喷施10-12、10-10、10-8、10-6mol/L 24-EBL作用下,油菜植株干重均不同程度的上升,且植株干重都在10-10mol/L 24-EBL(EBL2)处理下达到最大值,分别比100、200 mmol/L NaCl胁迫下增加29%和20%。与对照相比,非盐胁迫下外源喷施10-12、10-10、10-8、10-6mol/L 24-EBL,油菜幼苗植株干重与对照相比均无显著变化。(2)不同NaCl浓度胁迫下,油菜叶片的RWC显著下降,外施EBL2可显著提高油菜叶片的RWC和OAA。(3)不同浓度NaCl胁迫下,油菜幼苗叶片净光合速率(Pn)、气孔导度(Gs)、胞间CO2浓度 (Ci)和蒸腾速率(Tr)均不同程度下降,而Ls显著上升,而外喷EBL2可不同程度的提高Pn、Gs、Ci、Tr,降低Ls。(4)与对照相比,NaCl胁迫下油菜幼苗叶片、叶柄和根的Na+和Cl-含量均显著上升,NaCl浓度愈高,Na+和Cl-含量上升愈显著。 而K+含量均下降,外源EBL2可显著降低幼苗各器官的Na+和Cl-含量,对幼苗叶片K+含量没有影响,但提高了叶柄和根中的K+含量。上述表明,合适浓度的24-EBL外喷可明显提高油菜的耐盐水平,且不同浓度NaCl胁迫下,最适24-EBL浓度均为10-10mol/L。主要是因为外源喷施24-EBL能显著改善离子稳态和渗透调节能力,从而改善盐胁迫下油菜幼苗的光合作用、水分状况,提高其耐盐性。而24-EBL对盐处理下油菜植株气孔限制的显著改善是其促进其光合、水分利用的重要原因,也是其对100 mmol/L NaCl处理的油菜生长调控效果优于200 mmol/L NaCl处理的重要原因之一。结果还显示,在叶片中,24-EBL外施可通过排Na+和Cl-来维持植株离子稳态,而对K+影响不大;在根、茎中可通过排Na+、排Cl-、吸K+维持稳态。
油菜; 幼苗; 24-表油菜素内酯; 盐胁迫; 光合作用; 渗透调节; 离子吸收
土壤盐渍化是降低作物产量的主要原因之一,世界上20%的灌溉用地都面临土壤盐渍化的影响,由于高盐分危害作物生长,进而影响了世界陆地的可种植面积[1]。造成土壤盐渍化的原因有自然因素,但更主要因素是垦荒和不合理灌溉引起的[2]。滴灌和轮作等措施可以适当缓解土壤盐渍化,但都有赖于植物耐盐性的提高,因为土壤中总有一定程度的盐存在[3-4]。油菜作为世界第三大油料作物和我国第一大食用植物油料作物种植范围非常广泛[5-6]。其中,甘蓝型油菜(BrassicanapusL.)比较耐盐,近年来,油菜在半干旱地区的种植面积不断增加,因此研究油菜耐盐性十分重要[6]。
油菜素甾醇类化合物(brassinosteroids, BRs)是迄今为止国际上公认的活性最高、最广谱的一类植物生长激素,植物经极低浓度处理便能表现出显著的生理效应[7]。自1979年首次从油菜花粉中分离油菜素内酯(brassinolide, BL)以来,已先后从植物中分离了约70种BRs[8]。它能调节植物的生长发育,如茎的伸长、花粉管生长、果实成熟、乙烯的合成、木质部的分化、植物光合作用等[9-11]。对BRs信号传导途径的研究一直都是热点,除了对其生长调节的研究外,BRs也参与一系列非生物胁迫,如重金属胁迫、高温胁迫、干旱胁迫、氧化胁迫、盐胁迫等,但其抗性机制尚不清楚[12-14]。BRs介导植物耐盐性调节的研究主要集中在抗氧化能力、渗透保护物质、光合作用等方面,且集中在水稻[15]、菜豆[16]、大麦[16]、绿豆[17]、胡椒[18]等材料上,在其它方面的研究相对还比较薄弱,特别是在离子稳态方面,其机理仍然很不清晰。而在植物的盐适应机制上,离子稳态与渗透调节、光合作用等代谢过程相互交织[2-3,19]。24-表油菜素内酯(24-Epibrassinolide, 24-EBL)对150 mmol/L NaCl胁迫下油菜生长的调节效应和植物色素在油菜耐盐性中的作用,发现外源喷施适宜浓度24-EBL 能够显著促进盐渍条件下油菜幼苗光合能力,并提高其抗氧化能力,从而增强其对盐渍的适应性,而光合色素和花青素水平被24-EBL诱导上升在油菜幼苗抗氧化过程中起着重要的作用[14]。在本研究中,设置了多个水平(0、100、200 mmol/L NaCl)盐处理,并喷施多个24-EBL浓度(0、10-6、10-8、10-10、10-12mol/L),探讨外源24-EBL调节不同水平盐胁迫下油菜幼苗生长的浓度效应,并探讨了24-EBL对油菜幼苗光合作用、渗透调节和离子稳态等调控效应,以明确24-EBL诱导油菜幼苗抗盐性的效果及可能的生理机制,为作物的抗盐栽培以及应用BRs缓解作物盐害提供更多的理论和实践依据。
1 材料与方法
1.1 试验材料
甘蓝型油菜(BrassicanapusL.)品种:南盐油1号。
1.2 试验设计和处理
挑选大小一致、饱满的种子经0.1% HgCl2消毒3 min,清水冲洗干净后清水浸种12 h,放入垫有吸水纸的瓷盘内催芽。挑选露白一致的种子播种于装有20—40目石英砂的塑料盆(直径16 cm,高24 cm)中,每盆6粒种子。用1/4 Hoagland溶液培养,砂培至3叶期,间苗以确保齐苗,每盆2株。转入含100和200 mmol/L NaCl的Hoagland溶液进行处理,编号为S100和S200,同时外喷10-12、10-10、10-8、10-6mol/L 24-EBL,依次编号为EBL1、EBL2、EBL3、EBL4。不含NaCl的Hoagland培养液的、喷施去离子水的幼苗为对照(Control)。 每个处理4盆,处理期间每隔1 d更换处理液,于每天早上和傍晚分别叶面喷施去离子水或不同浓度的24-EBL,处理15 d后采样进行指标测定。整个培养期间温度为(25.0±4.0) ℃(白天),(22.0±2.0) ℃(夜间),相对湿度(55.2±10.3)%(白天)及(58.1±6.2)%(夜间)。
1.3 测定指标和方法
1.3.1 植株生物量测定
将油菜幼苗从砂中取出,先用自来水缓慢冲洗根部的砂粒,再用蒸馏水将鲜样洗净,用吸水纸吸干,子叶节处分开地上部和根部,在105 ℃杀青15 min后于75 ℃烘干至恒重,称得干重。
1.3.2 叶片相对含水量和渗透调节能力的测定
将新完全展开叶(即倒3叶)从植株分离后立即称其重量得叶片鲜重(FW),再将其浸入蒸馏水中, 4 ℃,过夜,使叶片吸水成饱和状态,称重,得饱和时的重量(TW),然后将样品75 ℃烘干,称重,得干重(DW),根据公式求得RWC:
RWC (%) = [(FW-DW)/(TW-DW)]×100。
采用 Zheng等[20]方法测定饱和渗透势(π100)和渗透调节能力(OAA),根据公式计算OAA:

1.3.3 气体交换参数的测定
采用美国LI-COR公司的LI-6400 XT便携式光合仪测定系统,在处理的第15 天(晴天)9:00—11:00测定其新完全展开叶的净光合速率(Pn)、气孔导度(Gs)、胞间CO2浓度(Ci)、蒸腾速率(Tr)。气孔限制值(Ls)的测定按照Yang等[21]文献。
1.3.4 植株Na+和K+浓度、K+/Na+和SK,Na的测定
将烘干的油菜叶片(倒3叶)及其叶柄和植株根系磨碎后过40目不锈钢筛,采用HNO3∶HClO4(3∶1)消化,利用电感耦合等离子体广谱发生仪(ICP)测定并计算样品中的Na+和K+含量 (mmol/g干重),每个样品重复3次。然后计算K+/Na+比值,按下列公式计算离子吸收和运输的K+、Na+的选择性比率[22],即SK,Na:
根SK,Na= (根K+/Na+)/(介质K+/Na+)
叶柄SK,Na= (叶柄K+/Na+)/(根K+/Na+)
叶SK,Na= (叶K+/Na+)/(根K+/N a+)
1.4 数据处理与统计分析
利用Microsoft Excel、SPSS17.0软件进行数据的处理、统计分析,数据均为“平均数±标准差”格式,采用Duncan新复极差测验法(P<0.05)进行显著性分析。
2 结果与分析
2.1 24-EBL对盐胁迫下油菜幼苗生长的效应
表1所示,与对照相比,10-12mol/L 24-EBL处理下,油菜幼苗植株干重增加,但未达显著水平,随着24-EBL浓度的增加,油菜幼苗植株干重逐渐下降,但均未达显著水平;而100 mmol/L NaCl胁迫(S100)下,10-6—10-12mol/L 24-EBL处理均提高植株干重,其中10-10mol/L 24-EBL处理作用最为明显,干重增加29%;而200 mmol/L NaCl胁迫(S200)下,EBL2处理作用最为明显,干重增加20%,而其它浓度24-EBL处理对干重的提高不显著。

表1 不同水平的24-表油菜素内酯(24-EBL)和NaCl交互作用对油菜幼苗植株干重的影响Table 1 Interaction effects of different concentrations of 24-epibrassinolide (24-EBL) and NaCl on plant dry weight of canola seedlings
同列不同的小写字母表示差异显著(P<0.05)
2.2 24-EBL对盐胁迫下油菜幼苗叶片水分含量和渗透调节能力的效应
与对照相比,NaCl胁迫下油菜叶片相对含水量(RWC)显著下降;而不同盐度胁迫下,外加EBL2处理均明显提高叶片RWC(图1);100、200 mmol/L NaCl胁迫下油菜叶片均显示出一定的渗透调节能力(OAA),而EBL2处理均极显著提高其叶片的OAA(图1)。
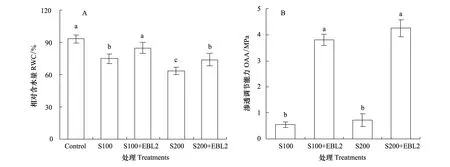
图1 100、200mmol/L NaCl胁迫下不同浓度24-EBL对油菜叶片相对含水量和渗透调节能力(OAA)的影响 Fig.1 Effects of different concentrations of 24-EBL on relative water content (RWC) and osmotic adjustment ability (OAA) the dry weight of canola seedlings under 100 and 200 mmol L-1 NaCl stress conditions
2.3 24-EBL对盐胁迫下油菜幼苗叶片气体交换参数和气孔限制值(Ls)的效应
NaCl胁迫均降低油菜幼苗叶片的Pn、Gs、Ci和Tr,其中Gs和Tr的降幅要大于Pn和Ci,外源EBL2则可不同程度的部分逆转这一效应(图2)。而图3显示,NaCl胁迫均明显提高叶片的Ls,盐度愈高,Ls愈大;外源EBL2可降低盐胁迫植株叶片的Ls,其中在100 mmol/L NaCl胁迫下,EBL2降低Ls的效应达到显著水平(图3)。
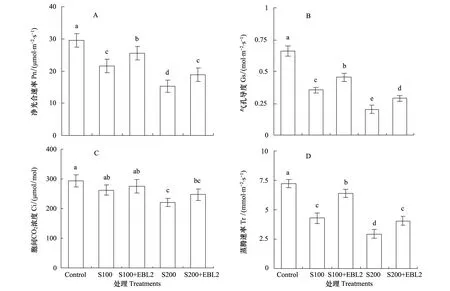
图2 100、200 mmol/L NaCl胁迫下10-10 mol/L 24-EBL (EBL2)对油菜Pn、Gs、Ci和Tr的影响Fig.2 Effects of 10-10 mol/L 24-EBL (EBL2) on the net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci) and transpiration rate (Tr) of canola seedlings under 100 and 200 mmol/L NaCl stress conditions

图3 100、200 mmol/L NaCl胁迫下10-10 mol/L 24-EBL (EBL2)对油菜Ls的影响Fig.3 Effects of 10-10 mol/L 24-EBL (EBL2) on the stomata limitation values (Ls) of canola seedlings under 100 and 200 mmol/L NaCl stress conditions
2.4 24-EBL对盐胁迫下油菜幼苗离子含量和选择性吸收或运输的效应
与对照相比,NaCl胁迫下油菜幼苗叶片、叶柄和根的Na+和Cl-含量均显著上升,NaCl浓度愈高,Na+和Cl-含量上升愈显著。 而K+含量均下降,但是叶片中K+含量下降的幅度要显著低于叶柄和根。因此,在NaCl胁迫下油菜幼苗每个器官K+/Na+均显著下降(表2)。盐胁迫下,外源EBL2可显著降低幼苗各器官的Na+和Cl-含量,在100、200 mmol/L NaCl胁迫下,外加EBL2,分别降低叶片中Na+含量49%和18%,及Cl-含量43%和15%;分别降低叶柄中Na+含量28%和16%,及Cl-含量13%和和25%;分别降低根中Na+含量23%和17%,Cl-含量21%和和18%;盐胁迫下,外源EBL2对幼苗叶片K+含量没有影响,在100、200 mmol/L NaCl胁迫下,外加EBL2,分别提高叶柄中K+含量17%和13%,分别提高根中K+含量34%和31%(表2)。因此,盐胁迫下,外源EBL2不同程度地提高油菜不同器官的K+/Na+。SK,Na反映植物对不同离子吸收或运输的选择性程度。盐胁迫下,外源EBL2明显提高叶片和根的SK,Na,而显著降低叶柄的SK,Na。
表2 100、200 mmol/L NaCl胁迫下10-10mol/L 24-EBL (EBL2)对油菜幼苗不同器官Na+、Cl-、K+含量,以及K+/Na+和SK,Na的影响
Table 2 Effects of 10-10mol/L 24-EBL (EBL2) on contents of Na+, Cl-and K+, K+/Na+andSK,Naof canola seedlings under 100 and 200 mmol/L NaCl stress conditions
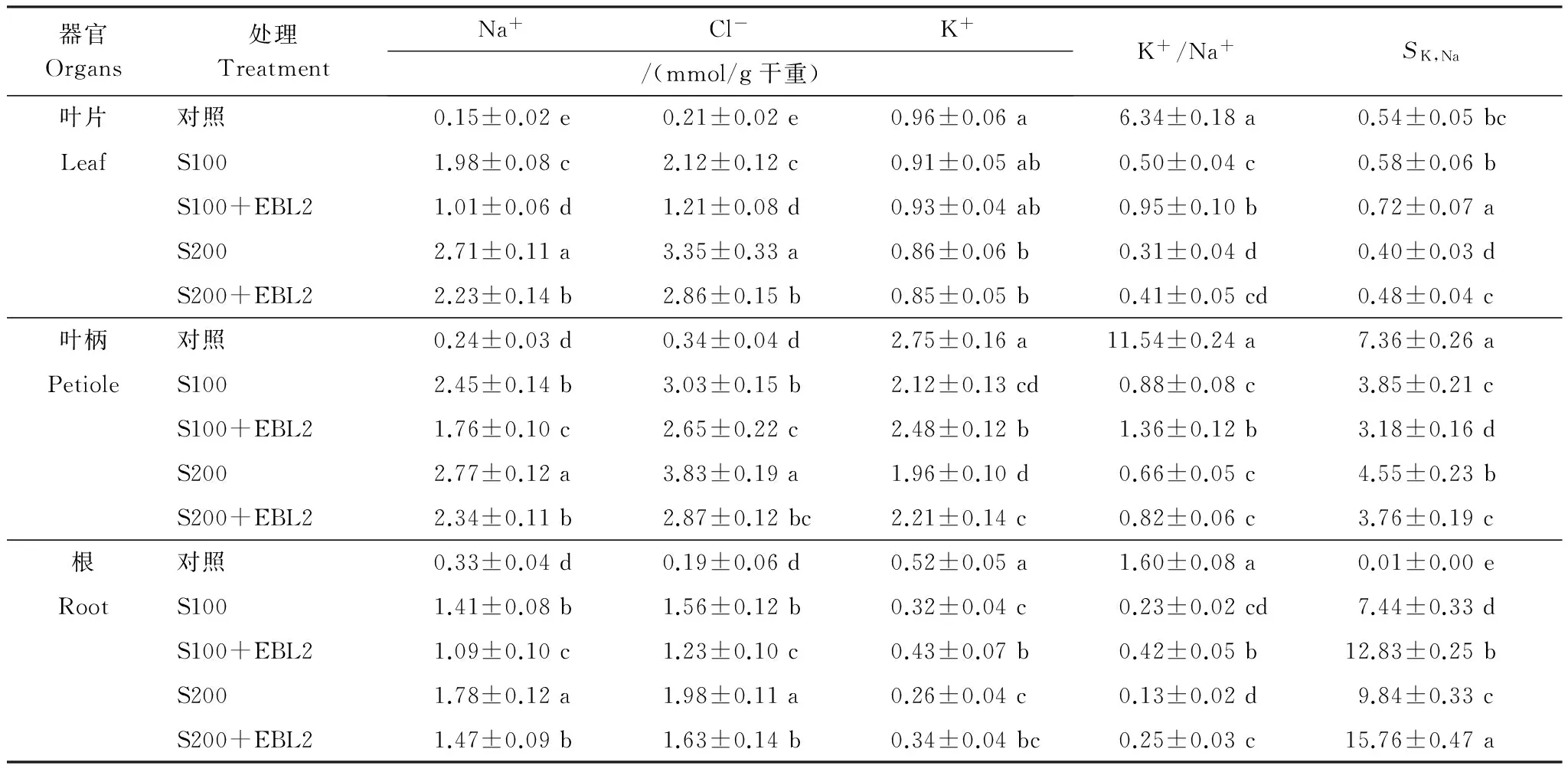
器官Organs处理TreatmentNa+Cl-K+/(mmol/g干重)K+/Na+SK,Na叶片对照0.15±0.02e0.21±0.02e0.96±0.06a6.34±0.18a0.54±0.05bcLeafS1001.98±0.08c2.12±0.12c0.91±0.05ab0.50±0.04c0.58±0.06bS100+EBL21.01±0.06d1.21±0.08d0.93±0.04ab0.95±0.10b0.72±0.07aS2002.71±0.11a3.35±0.33a0.86±0.06b0.31±0.04d0.40±0.03dS200+EBL22.23±0.14b2.86±0.15b0.85±0.05b0.41±0.05cd0.48±0.04c叶柄对照0.24±0.03d0.34±0.04d2.75±0.16a11.54±0.24a7.36±0.26aPetioleS1002.45±0.14b3.03±0.15b2.12±0.13cd0.88±0.08c3.85±0.21cS100+EBL21.76±0.10c2.65±0.22c2.48±0.12b1.36±0.12b3.18±0.16dS2002.77±0.12a3.83±0.19a1.96±0.10d0.66±0.05c4.55±0.23bS200+EBL22.34±0.11b2.87±0.12bc2.21±0.14c0.82±0.06c3.76±0.19c根对照0.33±0.04d0.19±0.06d0.52±0.05a1.60±0.08a0.01±0.00eRootS1001.41±0.08b1.56±0.12b0.32±0.04c0.23±0.02cd7.44±0.33dS100+EBL21.09±0.10c1.23±0.10c0.43±0.07b0.42±0.05b12.83±0.25bS2001.78±0.12a1.98±0.11a0.26±0.04c0.13±0.02d9.84±0.33cS200+EBL21.47±0.09b1.63±0.14b0.34±0.04bc0.25±0.03c15.76±0.47a
同一器官不同小写字母表示差异显著(P<0.05)
3 讨论
24-EBL对植物耐盐性的调节的浓度效应报道不一。Zeng等[23]研究发现,在100 mmol/L NaCl胁迫下,10-9和10-8mol/L 24-EBL能明显促进拟南芥(Arabidopsisthaliana)根的伸长,缓解其盐害。Divi等[24]在150 mmol/L NaCl处理下,也发现10-9mol/L 24-EBL能明显促进拟南芥(A.thaliana)种子萌发。Anurdha和Rao[25]研究表明在150 mmol/L NaCl胁迫下,3×10-6mol/L 24-EBL浸种能明显促进水稻(Oryzasativa)种子萌发及其幼苗生长;Özdemir等[15]在120 mmol/L NaCl处理水稻(O.sativa),也采用同样的浓度浸种处理,得到同样的效应,但非盐胁迫下,浸种处理反而起抑制作用。Houimli等[18]在0.4 g/L NaCl处理下外喷0.01、0.05、0.1、0.5 mg/L 24-EBL(24-EBL:0.5 mg/L相当于1.04×10-6mol/L),可不同程度的增加胡椒(Pipernigrum)的耐盐性,且随着24-EBL浓度上升,耐盐性的增强愈明显。非盐胁迫或者盐胁迫(150 mmol/L NaCl)下,外喷0.001、0.025、0.050、0.075、0.10、0.125、0.150、0.175、0.20 mg/L 24-EBL均能不同程度促进小麦(Triticumaestivum)幼苗生长,其中0.125 mg/L(2.6×10-7mol/L)24-EBL处理的效果最为显著[26]。Kagale等[27]研究表明,盐胁迫下1×10-6和2×10-6mol/L 24-EBL促进油菜(Brassicanapus)种子萌发。研究[14]表明,外喷10-6、10-8、10-10、10-12mol/L 24-EBL均能不同程度促进150 mmol/L NaCl处理下的甘蓝型油菜(B.napus)幼苗的生长,其中以10-10mol/L 24-EBL浓度效果最好,然而在非盐胁迫下外喷10-10mol/L 24-EBL,对其根系的生长还有抑制作用,对其地上部无明显影响。在100 mmol/L NaCl处理下,10-6—10-12mol/L 24-EBL处理均可提高植株干重,其中10-10mol/L 24-EBL(EBL2)处理作用最为明显,而胁迫浓度增加到200 mmol/L NaCl,24-EBL处理效应的趋势还是一样,但是除了EBL2处理对其生物量的提升达到显著水平外,其它浓度的24-EBL的促进效应均不显著(表1)。同时,非盐胁迫下,除了外喷10-12mol/L 24-EBL促进油菜幼苗生长外,外喷10-6—10-10mol/L 24-EBL对植株生长均没有显著作用(表1)。上述结果与以往研究[14]类似。因此,24-EBL在不同植物上、不同盐胁迫处理下得到的结果有所差异。
盐胁迫下的许多植物具有一定的渗透调节作用,从而维持叶片的含水量,但是尽管这样,植株在盐胁迫下仍然会表现出失水状况[3,28]。研究表明,100和200 mmol/L NaCl胁迫下,EBL2处理均明显增加油菜叶片RWC(图1),这显示外喷24-EBL可明显改善油菜植株的水分状况。在江苏菜豆(Phaseolusvulgaris)[26]、大麦(Hordeumvulgare)[26]和胡椒(Capsicumannuum)[29]上也有类似的研究报告。然而逆境下有关BRs对植物渗透调节效应的文献鲜有触及。盐胁迫下,EBL2处理明显增加油菜叶片的OAA(图1),从而改善植株的水分状况。而OAA的改善也势必促进植物的光合作用[3,30],在外喷24-EBL条件下,油菜幼苗光合作用的气孔限制得到缓解(图3),从而盐渍下被抑制的光合作用得到明显改善(图2)。
重建体内离子平衡来抵御盐渍伤害是植物耐盐的一大特征,在盐渍环境,植物对K+的维持对其生存至关重要[30]。不同器官的K+/Na+在盐胁迫下显著下降,盐处理强度越大,降幅越大。然而,外施24-EBL可不同程度的提高油菜不同器官的K+/Na+。盐胁迫下24-EBL对植物K+/Na+、Ca2+/Na+的这种提高效应在大麦、玉米和草莓上也有文献报告[26,31-32]。盐渍下,外施24-EBL可明显提高油菜叶柄和根中的K+含量,而对叶片中的K+影响不大。这一结果和在芥菜(Brassicajuncea)[33]和小麦(Triticumaestivum)[34]上的结果相似。盐渍下24-EBL对植物叶片K+积累的这一特征很可能正如Haubrick等[35]所推断,即BRs可抑制保卫细胞的内整流K+通道。因此,证明了24-EBL对油菜叶片K+/Na+的提高主要是其对植株Na+外排的结果。就SK,Na来看,盐胁迫下,外源EBL明显提高油菜叶片和根的SK,Na,而显著降低叶柄的SK,Na,说明EBL可以调节油菜叶片和根对K+、Na+的选择性吸收或运输来更好的维持植株的离子稳态,增强植株耐盐性。从Na+和Cl-主要分布上看,盐渍下油菜的生存策略正如Majeed等[36]所描述,即Na+和Cl-主要积累在叶柄(或茎),而根和叶片中较少(尤其是本研究中油菜的根)。外源24-EBL处理进一步增强了这一效应,而且24-EBL对不同器官矿质营养离子的促进和盐离子的外排,改善了根、叶片等器官的离子稳态,对植株器官的OAA的改善也起着很好的作用。已有研究[14]表明,外源喷施10-10mol/L 24-EBL 能够显著促进盐渍条件下油菜幼苗抗氧化能力,其实这一促进与植株离子稳态和渗透调节的改善是分不开的[30](表2)。
本研究探讨了外源24-EBL对盐胁迫下油菜幼苗生长、光合作用、渗透调节和离子稳态等调控效应,发现24-EBL外施同样存在着明显的浓度效应,且可以通过改善渗透调节和离子稳态,促进其在盐胁迫下的光合作用和生物量积累。在叶片中,24-EBL外施可通过排Na+和Cl-来维持离子稳态,在根、茎中可通过排Na+和Cl-、吸K+来维持离子稳态。从整体看,根或叶片可维持较高的对K+的选择性吸收或运输,维持离子稳态和渗透调节。对Na+、Cl-、K+的吸收、运输和分配机制,以及其在渗透调节中的角色或作用,高亲和性钾离子转运蛋白(HKT)、质膜Na+/H+逆向运输蛋白(SOS1)、液泡膜Na+/H+逆向运输蛋白(NHX)、H+-ATPase等膜蛋白的作用和功能有待深入研究。
[1] Yamaguchi T, Blumwald E. Developing salt-tolerant crop plants: challenges and opportunities. Trends in Plant Science, 2005, 10(12): 615-620.
[2] Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology, 2008, 59: 651-681.
[3] Munns R. Comparative physiology of salt and water stress. Plant, Cell and Environment, 2002, 25(2): 239-250.
[4] Zhao Y Y, Hu X H, Zou Z R, Yan F. Effects of seed soaking with different concentrations of 5-aminolevulinic acid on the germination of tomato (Solanumlycopersicum) seeds under NaCl stress. Acta Ecologica Sinica, 2013, 33(1): 62-70.
[5] Zheng Q S, Liu H Y, Long X H, Liu Z P, Niu D D, Gao Y Y. Effects of salt stress on ionic absorption and distribution of rapeseed seedlings. Chinese Journal of Oil Crop Sciences, 2010, 32(1): 65-70.
[6] Akbari G A, Hojati M, Modarres-Sanavy S A M, Mussig F G C, Altmann T. Exogenously applied hexaconazole ameliorates salinity stress by inducing an antioxidant defense system inBrassicanapusL. plants. Pesticide Biochemistry and Physiology, 2011, 100: 244-250.
[7] Choudhary S P, Oral H V, Bhardwaj R, Yu J Q, Tran L S P. Interaction of brassinosteroids and polyamines enhances copper stress tolerance inRaphanussativus. Journal of Experimental Botany, 2012, 63(15): 5659-5675.
[8] Bajguz A, Hayat S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiology and Biochemistry, 2009, 47 (1): 1-8.
[9] Arteca J M, Arteca R N. Brassinosteroid-induced exaggerated growth in hydroponically grownArabidopsisplants. Physiologia Plantarum, 2001, 112(1): 104-112.
[10] Yu J Q, Hu W H, Zhou Y H, Mao W H, Ye S F, Nogues S. A role for brassinosteroids in the regulation of photosynthesis inCucumissativus. Journal of Experimental Botany, 2004, 55(399): 1135-1143.
[11] Fu F Q, Mao W H, Shi K, Zhou Y H, Asami T, Yu J Q. A role of brassinosteroids in early fruit development in cucumber. Journal of Experimental Botany, 2008, 59(9): 2299-2308.
[12] Xia X J, Wang Y J, Zhou Y H, Tao Y, Mao W H, Shi K, Asami T, Chen Z, Yu JQ. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiology, 2009, 150(2): 801-814.
[13] Gao H L, Liu J L, Zheng Q S, Hong L Z, Wang C H, Ma M, Zhao S X, Zheng C F. Regulation of exogenous brassinosteroid on growth and photosynthesis ofHelianthustuberosusseedlings and cadmium biological enrichment under cadmium stress. Acta Ecologica Sinica, 2012, 33(6): 1935-1943.
[14] Liu J L, Gao H L, Hong L Z, Wang C H, Zhao G M, Wang X Y, Zheng Q S. Role of plant pigments in the 24-epibrassinolide ameliorating salt stress in canola. Acta Botanica Boreali-Occidentalia Sinica, 2013, 33(1): 90-100.
[15] Özdemir F, Bor M, Demiral T, Turkan I. Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (OryzasativaL.) under salinity stress. Plant Growth Regulation, 2004, 42 (3): 203-211.
[16] Abdel-Fattah RI. Osmolytes-antioxidant behaviour inPhaseolusvulgarisandHordeumvulgarewith brassinosteroid under salt stress. American-Eurasian Journal of Agricultural and Environmental Science, 2007, 2(6): 639-647.
[17] Hayat S, Hasan SA, Yusuf M, Hayat Q, Ahmad A. (2010) Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature inVignaradiata. Environmental and Experimental Botany, 2010, 69(2): 105-112.
[18] Houimli S I M, Denden M, Mouhandes BD. Effects of 24-epibrassinolide on growth, chlorophyll, electrolyte leakage and proline by pepper plants under NaCl-stress. EurAsian Journal of BioSciences, 2010, 4: 96-104.
[19] He X L, Chen S F, Wang Z H, Jia W J, He J B, Wang H Y, Yang G. Effect of saline water irrigation on sand soil salt and the physiology and growth ofPopuluseuphraticaOliv. Acta Ecologica Sinica, 2012, 32(11): 3449-3459.
[20] Zheng Q S, Liu Z P, Chen G, Gao Y Z, Li Q, Wang J Y. Comparison of osmotic regulation in dehydration and salinity stressed sunflower seedlings. Journal of Plant Nutrition, 2010, 33 (7): 966-981.
[21] Yang Y, Zheng Q S, Liu M, Long X H, Liu Z P, Shen Q R, Guo S W. Difference in sodium spatial distribution in the shoot of two canola cultivars under saline stress. Plant and Cell Physiology, 2012, 53(6): 1083-1092.
[22] Shao J, Zheng Q S, Liu Z P, Ning J F. Effects of phosphorus application on ion distribution in aloe seedlings under seawater stress. Acta Ecologica Sinica, 2005, 25(12): 3167-3171.
[23] Zeng H T, Tang Q, Hua XJ.Arabidopsisbrassinosteroid mutantsdet2-1 andbin2-1 display altered salt tolerance. Journal of Plant Growth Regulation, 2010, 29(1): 44-52.
[24] Divi UK, Rahman T, Krishna P. Brassinosteroid-mediated stress tolerance inArabidopsisshows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biology, 2010, 10: 151-164.
[25] Anuradha S, Rao S S R. Effect of brassinosteroids on salinity stress induced inhibition of seed germination and seedling growth of rice (OryzasativaL.). Plant Growth Regulation, 2001, 33 (2): 151-153.
[26] Ali A A, Abdel-Fattah R I. Osmolytes-antioxidant behaviour inPhaseolusvulgarisandHordeumvulgarewith brassinosteroid under salt stress. Journal of Agronomy, 2006, 5(1): 167-174.
[27] Kagale S, Divi U K, Krochko J E, Keller W A, Krishna P. Brassinosteroid confers tolerance inArabidopsisthalianaandBrassicanapusto a range of abiotic stresses. Planta, 2007, 225(2): 353-364.
[28] You J, Wang W R, Lu J, Jia P X, Miao J S, Yang Y L. Effects of salinity on seed germination and seedling growth in halophyteLimoniumaureum(L.) Hill. Acta Ecologica Sinica, 2012, 32(12): 3825-3833.
[29] Houimli S I M, Denden M, El Hadj S B. Induction of salt tolerance in pepper (Capsicumannuum) by 24-epibrassinolide. EurAsian Journal of BioSciences, 2008, 2: 83-90.
[30] Parida A K, Das A B. Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety, 2005, 60(3): 324-349.
[31] El-Khallal S M, Hathout T A, Ashour A A, Kerrit A A. Brassinolide and salicylic acid induced growth, biochemical activities and productivity of maize plants grown under salt stress. Research Journal of Agriculture and Biological Sciences, 2009, 5 (4): 380-390.
[32] Karlidag H, Yildirim E, Turan M. Role of 24-epibrassinolide in mitigating the adverse effects of salt stress on stomatal conductance, membrane permeability, and leaf water content, ionic composition in salt stressed strawberry (Fragria×ananassa). Scientia Horticulturae, 2011, 130(1): 133-140.
[33] Hayat S, Ahmad A, Mobin M, Hussain A, Fariduddin Q. Photosynthetic rate, growth, and yield of mustard plants sprayed with 28-homobrassinolide. Photosynthetica, 2001, 38 (3): 469-471.
[34] Shahbaz M, Ashraf M. Influence of exogenous application of brassinosteroid on growth and mineral nutrients of wheat (TriticumaestivumL.) under saline conditions. Pakistan Journal of Botany, 2007, 39 (2): 513-522.
[35] Haubrick L L, Torsethaugen G, Assmann S M. Effect of brassinolide, alone and in concert with abscisic acid on control of stomatal aperture and potassium currents ofViciafabaguard cell protoplasts. Physiologia Plantarum, 2006, 128(1): 134-143.
[36] Majeed A, Nisar M F, Hussain K. Effect of saline culture on the concentration of Na+, K+and Cl-inAgrostistolonifera. Current Research Journal of Biological Sciences, 2009, 2(1): 76-82.
Regulation of exogenous brassinosteroid on growth of salt-stressed canola seedlings and its physiological mechanism
MA Mei1, LIU Ran1, ZHENG Chunfang2, LIU Weicheng2, YIN Xiaoming1, LIU Jinlong1, WANG Changhai1, ZHENG Qingsong1,*
1CollegeofResourcesandEnvironmentalScience,KeyLaboratoryofMarineBiology,NanjingAgriculturalUniversity,Nanjing210095,China2ZhejiangMaricultureResearchInstitute,ZhejiangYongxingAquaticProductsIndustryCo.,Ltd.,Wenzhou325005,China
Brassinosteroids are a new group of steroid phytohormones that have high bioactivity and are widely spread in the plant kingdom. They are not only essential regulators of plant normal growth and development, but also can alleviate various abiotic stresses at very low concentration. This study evaluated effects of foliar spraying 24-epibrassinoide (24-EBL) on dry weight (DW), relative water content (RWC), osmotic adjustment ability (OAA), leaf gas exchange parameters, stomatal limitation values (Ls), the contents of Na+, K+, Cl-, K+/Na+ratio andSK,Nain different organs of canola seedlings under 100 and 200 mmol/L NaCl. The results showed that: (1) DW of canola seedlings was decreased significantly under 100 and 200 mmol/L NaCl, however, 10-12,10-10, 10-8, 10-6mol/L 24-EBL foliar spraying application all increased the DW of stressed plant. Also, 10-10mol/L 24-EBL (EBL2) treatments made the seedling DW peaked in both salt-stressed canola plants, and 29% and 20% higher than the values of only 100 or 200 mmol/L NaCl stress treatment, respectively. It was suggested that 10-12, 10-10, 10-8, 10-6mol/L 24-EBL foliar spraying application alone made no significant difference to biomass accumulation of canola under non-salt treatment. (2) RWC of canola leaves was decreased under NaCl treatments, and EBL2 application promoted RWC and OAA of salt-stressed canola. (3) Under NaCl treatments, net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2concentration (Ci) and transpiration rate (Tr) of canola leaves were declined at varying degree and limitation values (Ls) was increased markedly. However, EBL2 application partly reversed all the parameters at varying degree. (4) Compared with control, the contents of Na+and Cl-in leaves, petioles and roots of canola were increased significantly, while its K+was decreased significantly. EBL2 application reduced the contents of Na+and Cl-in all canola organs, did not affected K+content in the leaves significantly, and increased K+content in petioles and roots. In general, the appropriate range concentration of foliar spraying 24-EBL obviously promoted salt resistance of canola seedlings. however, the optimal 24-EBL concentration of canola growth promotion was 10-10mol/L at each NaCl treatment level. The promotion of canola salt resistance was due to 24-EBL promoting the plant ion homeostasis and osmotic adjustment, then, improved plant water status and photosynthesis. Promotions of photosynthesis and water use efficiency in salt-stressed canola were owing to significant alleviation on stomatal limitation of stressed plant leaves caused by 24-EBL. The results also suggested that improvement of ion homeostasis in leaves caused by 24-EBL was as the result of exclusion of Na+and Cl-. However, not only exclusion of Na+and Cl-, but also K+uptake increase was as the result of 24-EBL application contributing to ion homeostasis improvement in roots and stems.
canola; seedlings; 24-epibrassinoide; salt stress; photosynthesis; osmotic adjustment; ion homeostasis
浙江省重大科研专项(2012C12017-3);浙江省科技厅创新团队建设与人才培养项目(2011F20032);浙海渔计(2013)108; 浙海渔计(2012)146; 温州市科技计划项目(S20110010)
2013-05-23;
日期:2014-04-25
10.5846/stxb201305231157
*通讯作者Corresponding author.E-mail: qszheng@njau.edu.cn
马梅,刘冉,郑春芳,刘伟成,尹晓明,刘金隆,王长海,郑青松.油菜素内酯对盐渍下油菜幼苗生长的调控效应及其生理机制.生态学报,2015,35(6):1837-1844.
Ma M, Liu R, Zheng C F, Liu W C, Yin X M, Liu J L, Wang C H, Zheng Q S.Regulation of exogenous brassinosteroid on growth of salt-stressed canola seedlings and its physiological mechanism.Acta Ecologica Sinica,2015,35(6):1837-1844.

