Analysis of Genetic Differences between Luffa acutangula(L.)Roxb.and Luffa cylindrica(L.)Roem.by ISSR Markers
Bo SONG,Longzheng CHEN,Hai XU,Tao ZHENG,Yueyue FEI
1.Vegetable Research Institute,Jiangsu Academy of Agricultural Sciences/Jiangsu Key Laboratory for Horticultural Crop Genetic Improvement,Nanjing 210014,China;2.Bureau of Rural Development in Xinjin County,Chengdu City,Chengdu 611400,China;3.Xinyang Agricultural Experiment Station in Yancheng,Yancheng 224049,China
Luffa acutangula(L.)Roxb.andLuffa cylindrical(L.)Roem.are two loofah cultivars widely planted in China.There are great differences in flower color,flowering period,fruit traits and other agronomic traits between them.At the same time,there are also many good complementary traits in the two loofah cultivars.For example,L.cylindricalturns brown easily after its peel is grazed or its flesh is cooked,butL.acutanguladoes not change its color after cooking.L.acutangulahas strict requirements for length of sunshine,and it has poor early ripeness and low yield.However,L.cylindricalhas good early ripeness,and its yield is high.Both the two loofah cultivars can be improved by utilizing each other’s good traits through interspecific hybridization[1-2].At present,the domestic and foreign researches aboutinterspecific hybridization of loofah cultivars mainly focus on hybrid technology,agronomic traits,etc[1-4].There has been no report on interspecific hybridization of loofah cultivars at the molecular level.In this study,the genetic differences and similaritiesingenomicDNAbetweenL.acutangulaandL.cylindricaland among the F1s and F2s of their hybrid were analyzed by ISSR technology,thereby providing certain reference for revealing the genetic characteristics of loofah interspecific hybrids.
Materials and Methods
Materials
The test materials includedL.acutangula(P1),L.cylindrical(P2),L.acutangula×L.cylindricalF1 hybrid of and 20L.acutangula×L.cylindricalF2hybrids.The genomic DNA of the test materials was extracted from their leaves collected at the three-leaf stage by CTAB method.
ISSR analysis
The ISST reaction system (20 μl)was as follows:double distilled water 11.8 μl,10 × Buffer 2 μl,25 mmol/L MgCl22 μl,2 mmol/L dNTPs 2.5 μl,10 mmol/L 20-bp random primer 0.5 μl,TaqDNA polymerase 1 U,20-40 ng template DNA 1 μl.The reactive components above were purchased from ShanghaiSangonBiologicalEngineering Technology&Services Co.,Ltd.The reaction program was as follows:denaturation at 94℃for 5 min;94℃for 30 s,55.8℃for 45 s,72℃for 2 min,35 cycles;extension at 72℃for 10 min.The amplified products were separated by 1.2% agarose gel electrophoresis at voltage of 5 V/cm for 1.5 h.The agar gel was stained by 0.5 μg/L ethidium bromide and analyzed by gel imaging analysis system(Peiqing,Shanghai).The used DNA marker was GeneRulerTM100 bp DNA Ladder Plus.From the 70 primers,total 6 primers with good repeatability and wide adaptability were screened for ISSR analysis,including ISSR17,ISSR22,ISSR23,ISSR26,ISSR33 and ISSR59.
Data processing and statistics
If there was one band at certain molecular weight,the value was assigned as 1;but if there was no band,the value was assigned as 0.The genetic similarity coefficient between materials was calculated by Nei's method[5].
In the formula,GS(i,j)represents the genetic similarity coefficient;N(i,j)represents the number of bands shared byiandjmaterials;N(i)represents the number of bands of materiali;N(j)represents the number of bands of materialj.
Results and Analysis
ISSR analysis of test materials
Total6 ISSR primers were screened due to their stable reactions.A total of 652 bands were amplified from the 23 test materials.The lengths of ISSR fragments ranged from 500 to 2 900 bp,and total 38 amplified bands were obtained among the 23 test materials.Among the 38 amplified bands,34 bands were polymorphic with polymorphic rate of 89.5%.The total bands amplified from each testmaterial ranged from 22 to 35,and the amplified total fragments differed(1-8)among different primers(Table 1).
ISSR analysis of parents and F1hybridsAs shown in Table 1,total 30 and 23 bands were amplified fromL.acutangulaandL.cylindricalby the 6 primers.There were 15 and 8 bands were specific toL.acutangulaandL.cylindrical,respectively,accounting for 50.0% and 34.8% of the total bands.Thus theL.acutangulaandL.cylindricalcould be distinguished easily.Total 35 bands were amplified fromL.acutangula×L.cylindricalF1hybrid by the 6 primers.Among them,12 bandswere specifictoL.acutangula,accounting for 34.3% of the total bands,while 8 bands were specific toL.cylindrical,accounting for 22.9%.The F1hybrid lost 3L.acutangula-specific bands,but lost none ofL.cylindrical-specific bands.Fig.1 showed that the F1hybrid had the bands specific to eitherL.acutangulaorL.cylindrical,such as ISSR33-1500 and ISSR33-1800,indicating that the band pattern ofL.acutangula×L.cylindricalF1hybrid is characterized by complementation between those of both the parents.
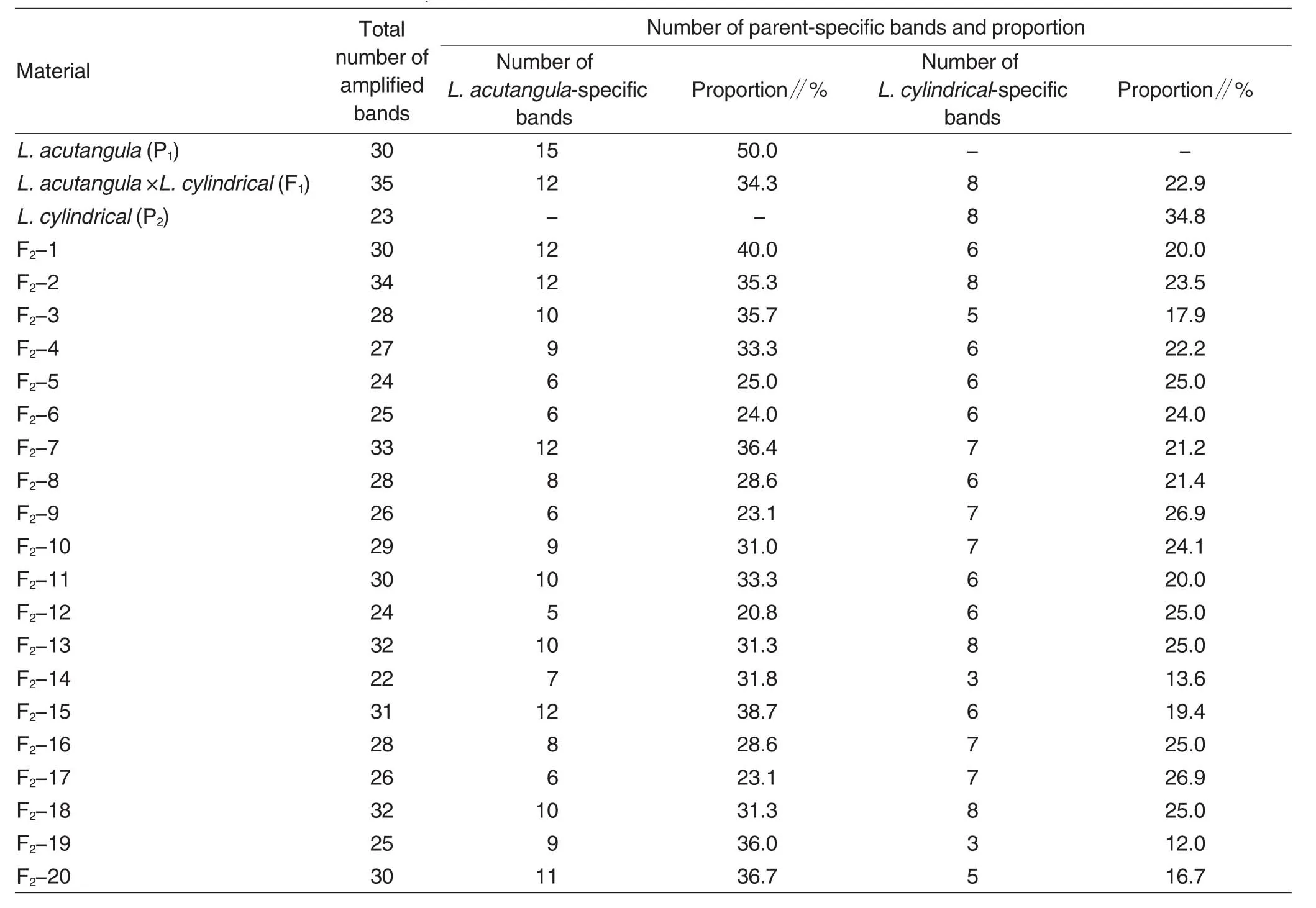
Table 1 Statistical results of ISSR-based amplification bands of test materials

ls ria te a m h fa o lo st te g n o m a ts n fficie e co rity ila sim tic e n e G 2 le b a T 0 2-F2 9 1-F2 8 1-F2 7 1-F2 6 1-F2 5 1-F2 4 1-F2 3 1-F2 2 1-F2 1 1-F2 0 1-F2 9-F2 8-F2 7-F2 6-F2 5-F2 4-F2 3-F2 2-F2 1-F2 P2 F1 P1 l st ria e te T a m 0 0.0 1 P1 0 0.0 1 1 1.7 0 F1 0 0.0 1 4 8.6 0 5 9.3 0 P2 0 0.0 1 3 5.5 0 3 6.7 0 4 8.6 0 1-F2 0 0.0 1 0 9.7 0 8 5.6 0 8 6.8 0 4 8.6 0 2-F2 0 0.0 1 4 8.6 0 9 7.5 0 5 0.6 0 1 1.7 0 4 8.6 0 3-F2 0 0.0 1 1 1.7 0 6 1.8 0 8 5.6 0 2 3.6 0 7 3.7 0 5 0.6 0 4-F2 0 0.0 1 3 5.5 0 9 7.5 0 2 3.6 0 9 7.5 0 1 1.7 0 1 1.7 0 6 2.5 0 5-F2 0 0.0 1 1 1.7 0 7 3.7 0 8 5.6 0 3 6.7 0 5 0.6 0 7 3.7 0 7 3.7 0 3 5.5 0 6-F2 0 0.0 1 4 8.6 0 5 0.6 0 4 8.6 0 1 1.7 0 3 6.7 0 8 5.6 0 2 3.6 0 5 9.8 0 1 1.7 0 7-F2 0 0.0 1 3 6.7 0 3 6.7 0 0 9.7 0 0 0.5 0 4 8.6 0 2 3.6 0 9 7.5 0 1 1.7 0 3 6.7 0 2 3.6 0 8-F2 0 0.0 1 2 4.8 0 1 1.7 0 3 6.7 0 7 3.7 0 3 5.5 0 9 7.5 0 4 8.6 0 2 3.6 0 3 6.7 0 3 6.7 0 4 2.5 0 9-F2 0 0.0 1 5 0.6 0 5 0.6 0 4 8.6 0 4 8.6 0 5 0.6 0 2 3.6 0 5 0.6 0 3 6.7 0 1 1.7 0 4 8.6 0 7 3.7 0 5 0.6 0 0 1-F2 0 0.0 1 8 5.6 0 0 9.7 0 7 3.7 0 6 1.8 0 1 1.7 0 4 8.6 0 1 1.7 0 4 8.6 0 0 9.7 0 2 3.6 0 8 5.6 0 8 6.8 0 4 8.6 0 1 1-F2 0 0.0 1 7 3.7 0 5 0.6 0 5 9.8 0 0 9.7 0 1 1.7 0 3 6.7 0 4 8.6 0 5 0.6 0 4 8.6 0 2 3.6 0 9 7.5 0 3 6.7 0 1 1.7 0 6 2.5 0 2 1-F2 0 0.0 1 7 3.7 0 0 9.7 0 1 1.7 0 7 3.7 0 7 3.7 0 6 1.8 0 1 1.7 0 7 3.7 0 1 1.7 0 4 8.6 0 2 4.8 0 7 3.7 0 1 1.7 0 8 6.8 0 2 3.6 0 3 1-F2 0 0.0 1 2 3.6 0 4 8.6 0 2 3.6 0 5 0.6 0 2 3.6 0 4 8.6 0 8 5.6 0 3 6.7 0 4 8.6 0 8 5.6 0 2 3.6 0 6 2.5 0 9 7.5 0 5 0.6 0 8 5.6 0 2 3.6 0 4 1-F2 0 0.0 1 5 0.6 0 3 6.7 0 1 1.7 0 6 1.8 0 2 3.6 0 3 6.7 0 1 1.7 0 2 4.8 0 4 8.6 0 5 0.6 0 2 3.6 0 5 0.6 0 3 6.7 0 6 1.8 0 9 7.5 0 5 9.8 0 1 1.7 0 5 1-F2 0 0.0 1 1 1.7 0 0 9.7 0 2 4.8 0 0 9.7 0 7 3.7 0 1 1.7 0 4 8.6 0 7 3.7 0 3 6.7 0 1 1.7 0 0 9.7 0 8 5.6 0 7 3.7 0 4 8.6 0 4 8.6 0 1 1.7 0 6 1.8 0 9 7.5 0 6 1-F2 0 0.0 1 0 9.7 0 3 5.5 0 4 8.6 0 4 8.6 0 0 9.7 0 2 3.6 0 1 1.7 0 0 9.7 0 7 3.7 0 3 5.5 0 1 1.7 0 0 9.7 0 3 5.5 0 2 3.6 0 4 8.6 0 2 3.6 0 3 6.7 0 8 5.6 0 6 2.5 0 7 1-F2 0 0.0 1 4 8.6 0 4 8.6 0 3 6.7 0 9 7.5 0 0 9.7 0 2 3.6 0 0 9.7 0 1 1.7 0 7 3.7 0 7 3.7 0 3 6.7 0 3 6.7 0 4 8.6 0 3 6.7 0 2 3.6 0 5 9.8 0 4 8.6 0 1 1.7 0 8 6.8 0 2 3.6 0 8 1-F2 0 0.0 1 3 6.7 0 5 0.6 0 5 0.6 0 9 7.5 0 5 0.6 0 5 0.6 0 5 0.6 0 1 1.7 0 6 2.5 0 5 0.6 0 1 1.7 0 2 3.6 0 7 3.7 0 3 5.5 0 4 8.6 0 3 6.7 0 1 1.7 0 0 0.5 0 9 7.5 0 2 3.6 0 1 1.7 0 9 1-F2 0 0.0 1 0 1.7 0 2 4.8 0 4 8.6 0 4 8.6 0 6 1.8 0 4 8.6 0 7 3.7 0 4 8.6 0 7 3.7 0 8 5.6 0 4 8.6 0 7 3.7 0 3 6.7 0 6 1.8 0 4 8.6 0 1 1.7 0 4 8.6 0 0 9.7 0 4 8.6 0 5 0.6 0 6 1.8 0 7 3.7 0 0 2-F2
ISSR analysis of F2populationAs shown in Table 1,the total bands amplified by the 6 primers ranged from 22 to 34 among the F2population.F2-14 showed 22 bands,and it lost 8L.acutangula-specific bands,5L.cylindricalspecific bands and 3 bands shared by bothL.acutangulaandL.cylindrical.F2-2 showed total 34 bands.It lost only 3L.acutangula-specific bands and one band shared by both the parents,but inherited all the bands specific toL.cylindrical.The F2population showed 5-12 bands shared byL.acutangula,accounting for 20.8% -40.0% ofthe totalamplified bands;they showed 3-8 bands shared byL.cylindrical,accounting for 12% -26.9% of the total amplified bands.The F2population inherited 12-14 bands shared by both the parents,indicating certain stability in inheriting common bands betweenL.acutangulaandL.cylindrical.However,no new bands were amplified from the F2population.
Comparison of genetic similarity coefficient among parents and interspecific hybrids
As shown in Table 2,the genetic similarity coefficient betweenL.acutangulaandL.cylindricalwas 0.395,indicating greater genetic differences between both the parents.The genetic similarity coefficient betweenL.acutangulaand F1hybrid was 0.711,while betweenL.cylindricaland F1hybrid was 0.684(P>0.05).The genetic similarity coefficients betweenL.acutangulaand F2population ranged from 0.524 to 0.737,while betweenL.cylindricaland F2population ranged from 0.553 to 0.763.It indicated that the F1hybrid and F2population all showed no significant tendency to inherit from either male or female parent.The genetic similarity coefficients among the F2population ranged from 0.500 to 0.895,indicating greater genetic variations among the F2population.
Conclusions and Discussion
This study shows that the genetic similarity coefficient betweenL.acutangulaandL.cylindricalis relatively low,which explains why there are great differences in agronomic traits between the two loofah cultivars.This finding is consistent with the study result of Xiaet al.[6]aboutL.acutangulaandL.cylindricalby RAPD analysis.
In this study,the genetic characteristics of interspecific progenies of loofah were studied by ISSR markers.The results showed that the bands of F1hybrid mainly consisted of both parents’.However,the bands of F2population were all inherited from their parents,and no new bands were amplified.It suggested that F2population had a high stability in inheriting common bands of both parents.In theory,new bands should be generated in F2population after meiosis,but the truth was opposite,which might be caused by fewer primers in this study.ISSR molecular marker technology has a high polymorphism in loofah,so it can be better used in molecular marker studies on loofah.This study will lay a foundation forthe development of molecular markers for studies on flesh browning.
[1]SONG B(宋波),SU XJ(苏小俊),CHEN J (陈洁),et al.Effect analysis of reciprocal crosses betweenLuffa cylindricalandLuffa acutangula(普通丝瓜与有棱丝瓜亚种间杂交的正反交效应分析)[J].Jiangsu Agricultural Sciences (江苏农业科学),2008,5:139-142.
[2]SONG B(宋波).Study on genetic characterization of the interspecific hybrid progenies(有棱丝瓜与普通丝瓜种间杂种后代的遗传分析)[D].Nanjing:Nanjing Agricultural University(南京:南京农业大学),2008.
[3]YUAN XH(袁希汉),SU XJ(苏小俊),GAO J(高军),et al.Effects of low-temperature storage on pollen viability and fruiting of loofah(低温贮藏对丝瓜花粉活力和结实的影响)[J].Jiangsu Agricultural Sciences(江苏农业科学),2008,2:122-124.
[4]XIE WJ(谢文军).Studies on heterosis and characteris heredity in different types of luffa(不同类型丝瓜杂种优势及性状遗传特性的研究)[D].Tyan:Shandong Agricultural University(泰安:山东农业大学),2003.
[5]NEI M,LI WH.Mathematical model for studying genetic variation in terms of restriction endonucleases[J].Proceedings of the National Academy of Sciences of the United States of America,1979,76(10):5269-5273.
[6]XIA JH(夏军辉).Studies on genetic diversity ofLuffaspp.germplasm resources(丝瓜种质资源遗传多样性研究)[D].Wuhan:Huazhong Agricultural University(武汉:华中农业大学),2007.
 Agricultural Science & Technology2015年11期
Agricultural Science & Technology2015年11期
- Agricultural Science & Technology的其它文章
- Chemical Property Variation Trend Analysis and Quality Evaluation of Water in Wei River
- Effects of Plastic Film Mulching on Physical Characters of Soil and Yield and Yield Components of Sweet Potato
- Effect of Soil Organic Matter Content on Soil Physical and Chemical Indexes and Plant Diversity Indexes of Natural Secondary Karst Forest in Southern Guizhou Province,China
- Effects of Different Harvesting Dates on Yield and Quality of Silage Maize
- The Dynamic Changes in Cold Tolerance of Ground-cover Chrysanthemum Growing in the Open Field during the Overwintering
- Effects of Ppc Gene Construction of Monocotyledon on Seedling Growth of Transgenic Nicotiana tabacum
