NO对盐胁迫下苜蓿根系生长抑制及氧化损伤的缓解效应
周万海, 冯瑞章, 师尚礼, 寇江涛
1 宜宾学院生命科学与食品工程学院 西南特色经济植物实验室, 宜宾 644000 2 甘肃农业大学草业学院 草业生态系统教育部重点实验室, 兰州 730070
NO对盐胁迫下苜蓿根系生长抑制及氧化损伤的缓解效应
周万海1,2, 冯瑞章1, 师尚礼2,*, 寇江涛2
1 宜宾学院生命科学与食品工程学院 西南特色经济植物实验室, 宜宾 644000 2 甘肃农业大学草业学院 草业生态系统教育部重点实验室, 兰州 730070
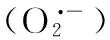
一氧化氮; 苜蓿; 盐胁迫; 抗氧化系统; 根系
根系是植物与土壤环境接触的主要界面,不仅在植物水分、养分吸收、土壤固着及内源激素合成中起关键作用,也是土壤盐渍危害中植物最直接的受害部位,因此改善和提高植物根系的生长对植物适应环境有重要意义[1- 2]。一氧化氮(NO)是一种重要的信号分子,在植物生长发育和抗逆反应中起关键作用[3],研究表明,一定浓度的NO对植物侧根、不定根和根毛的发育有促进作用, 但对主根的伸长具抑制效应[4- 5]。外源NO能提高盐胁迫下根组织的渗透调节能力、降低自由基积累,缓解根系氧化损伤,改善根系对离子的选择性吸收、促进根系生长,从而提高植株耐盐性[6- 8]。然而,植物能通过一氧化氮合酶(NOS)或硝酸还原酶(NR)催化的酶学途径或非酶学途径形成内源NO,且内源NO的产生受植物种、组织和细胞类型及环境条件的影响[9],使得NO在植物体中的来源及在抗逆中的作用有较大争议。因此,研究NO对根系耐盐性的调控效应及作用机制具有重要的理论意义和实践价值。
苜蓿(MedicagosativaL.)是我国西北地区广泛种植的一种优质豆科牧草,盐渍已成为限制苜蓿生产的主要土壤环境因子之一,虽然苜蓿耐盐性研究已有一些报道,但有关NO对苜蓿根系耐盐性的作用机制仍然模糊不清,且苜蓿根系中内源NO的来源和对根系抗逆性的影响亦无相关报道,为进一步阐明NO在这一过程中的作用,本试验以苜蓿为材料,研究外源NO对盐胁迫下苜蓿根系生长和氧化损伤的缓解效应;同时还研究了苜蓿根系中内源NO的来源及其在根系耐盐中的作用,为进一步理解盐胁迫对豆科双子叶植物根系生长抑制的作用机制及调控方法提供依据。
1 材料和方法
1.1 材料的培养和处理
供试苜蓿品种为甘农4号(MedicagosativaL. cv. Gannong No.4)。选取饱满均匀的苜蓿种子,用0.1% HgCl2溶液消毒5 min, 去离子水洗净,播种于灭菌的蛭石培养钵中,种子萌发后每盆定植10株,转移至光照培养室(相对湿度60%,光照时间为14 h, 光通量密度400 μmol m-2s-1,昼/夜温度为25 ℃/20 ℃),每3 d浇灌1次1/2 Hoagland营养液,生长35 d后,挑选整齐一致的幼苗洗净后移栽到盛有1/2 Hoagland营养液的塑料钵中预培养,预培养3 d后开始处理。试验中以亚硝基铁氰化钠(sodium nitroprusside,SNP)为NO供体,亚铁氰化钠(sodium ferrocyanide,SF)为SNP类似物(不产生NO),2- 4-羧苯基四甲基咪唑烷- 1-氧- 3-氧化物(2-(4-carboxyphenyl)- 4,4,5,5-tetramethylimidazoline- 1-oxyl- 3-oxide,c-PTIO)为NO特异清除剂,钨酸盐(tungstate,缩写为T)为硝酸还原酶活性抑制剂,N-硝基-L-精氨酸甲脂(NG-nitro-L-Arg-methyl ester,L-NAME)为一氧化氮合酶抑制剂,以上化学试剂均购于Sigma公司。试验设11种处理:1)CK,2)SNP(100 μmol/L SNP),3)NaCl(150 mmol/L NaCl),4)NaCl+SNP(150 mmol/L NaCl+100 μmol/L SNP),5)NaCl+SF(150 mmol/L NaCl+100 μmol/L 亚铁氰化钠),6)NaCl+c-PTIO(150 mmol/L NaCl+100 μmol/L c-PTIO),7)NaCl+c-PTIO+SNP(150 mmol/L NaCl+100 μmol/L c-PTIO+100 μmol/L SNP),8)NaCl+T(150 mmol/L NaCl+100 μmol/L Tungstate),9)NaCl+SNP+T(150 mmol/L NaCl+100 μmol/L Tungstate+100 μmol/L SNP),10)NaCl+L-NAME(150 mmol/L NaCl+100 μmol/L L-NAME),11)NaCl+SNP+L-NAME(150 mmol/L NaCl+100 μmol/L L-NAME+100 μmol/L SNP);所用溶液均用1/2 Hoagland营养液溶解配制,试验采用随机区组设计,每处理10盆,重复3次。为保证处理浓度稳定,每隔2 d更换1次处理液,用空气压缩泵给营养液间歇通入空气。处理第8天取根样测定各项指标,每处理取3个重复。
1.2 测定项目与方法
1.2.1 根系干重测定
处理结束后,将植株的地上部和地下部分开,取苜蓿幼苗根系,用去离子水冲洗干净,擦干水分,105 ℃杀青15 min,70 ℃烘干至恒重,称干质量。
1.2.2 生理生化指标及NO产生量测定
根系活力用氯化三苯基四氮唑(triphenyltetrazolium chloride,TTC)法测定[10],游离脯氨酸含量用水合茚三酮法测定[10],用蒽酮法测定可溶性糖含量[10],考马斯亮蓝G- 250法测定可溶性蛋白含量[10],硫代巴比妥酸法测定丙二醛(malondialdehyde,MDA)含量[10],羟自由基含量测定参考Halliwell的方法[11],过氧化氢(H2O2)含量测定采用Velikova的方法[12],超氧阴离子产生速率测定参考王爱国的方法[13];粗酶液的制备参考Azevedo-Neto的方法[14],超氧化物歧化酶(superoxide dismutase,SOD)活性测定参考Giannopolitis和Ries的方法[15],过氧化氢酶(catalase,CAT)活性测定参考Beers和Sizer的方法[16],抗坏血酸过氧化物酶(ascorbate peroxidase,APX)活性测定参考Nakano and Asada(1981)的方法[17],愈创木酚过氧化物酶(guaiacol peroxidase,GPX)活性测定参考Urbanek的方法[18],谷胱甘肽还原酶(glutathione reductase,GR)活性测定参考Foyer的方法[19];还原型抗坏血酸(reduced ascorbic acid,AsA)含量测定参考Law的方法[20],还原型谷胱甘肽(reduced glutathion,GSH)含量测定参考Griffith的方法[21];NO产生量测定参考Zhou的方法[22]。
1.3 数据处理
所有数据均为3个重复的平均值±标准误(±SE),SPSS(16.0)软件进行单因素方差(ANOVA)统计分析,Duncan 法多重比较,差异显著性定义为P<0.05,Excel 2003制作相应图表。
2 结果与分析
2.1 SNP对苜蓿根系生长和根系活力的影响
盐胁迫抑制了苜蓿幼苗根系生长和根系活力(图1),与CK相比,根系干重和活力分别降低15.9%和58.7%(P<0.05);SNP缓解了盐胁迫对根系生长和活力的抑制,干重和根系活力分别比CK降低8.1%和16.7%(P<0.05)。NO的特异清除剂c-PTIO、硝酸还原酶活性抑制剂钨酸盐和一氧化氮合酶活性抑制剂L-NAME分别与NaCl共处理进一步抑制了苜蓿根系干重和根系活力(P<0.05),与CK相比根系干重分别降低26.7%、24.9%和32.7%,根系活力降低63.3%、62.5%和68.9%;c-PTIO、钨酸盐和L-NAME对根系干重和活力的抑制效应能被SNP缓解,各处理根系干重和活力与NaCl处理无差异;盐胁迫对苜蓿幼苗根系生长和根系活力的抑制不能被SNP类似物亚铁氰化钠缓解(P>0.05)。
二是要有闲。时间是保证自驾游的前提。这次去西北,我们两口子是退休,小弟是休年假,还带了正放暑假的侄女。时间不受限制。自驾游的特点是宽松自由,一个好的景点甚至一个好的画面,想看了,可以随时停车欣赏,时间长一点短一点,自己掌握,无关紧要。
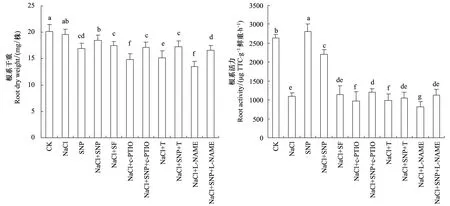
图1 SNP对盐胁迫下苜蓿根系干重和根系活力的影响
2.2 SNP对盐胁迫下苜蓿根系渗透调节物质含量的影响
盐胁迫下苜蓿根系中游离脯氨酸和可溶性糖含量分别比CK增加3.4倍和2.1倍,可溶性蛋白含量降低43.7%,表明盐胁迫引起苜蓿根系的渗透胁迫。添加SNP使可溶性蛋白含量升高,游离脯氨酸和可溶性糖含量降低。c-PTIO、钨酸盐和L-NAME与NaCl共处理进一步提高了游离脯氨酸和可溶性糖含量,降低了可溶性蛋白含量(P<0.05),与CK相比,游离脯氨酸增加3.8—4.1倍,可溶性糖含量提高2.3—2.6倍,可溶性蛋白分别降低56.1%、52.3%和58.7%,添加SNP能逆转c-PTIO、钨酸盐和L-NAME处理对苜蓿根系游离脯氨酸、可溶性糖、可溶性蛋白的影响。亚铁氰化钠处理使苜蓿根系中游离脯氨酸、可溶性糖和可溶性蛋白含量与NaCl处理无差异(P>0.05)(表1)。
2.3 SNP对盐胁迫下苜蓿根系自由基和MDA含量的影响


表1 SNP对盐胁迫下苜蓿根系可溶性蛋白、游离脯氨酸和可溶性糖含量的影响

图2 SNP对盐胁迫下苜蓿根系MDA、H2O2、OH·含量和产生速率的影响
2.4 SNP对盐胁迫下苜蓿根系抗氧化系统的影响
与CK相比,正常条件下添加SNP能在一定程度上提高苜蓿根系抗氧化酶活性和抗氧化物含量(表2);盐胁迫使苜蓿根系SOD、CAT、GPX和APX活性比CK降低48.5%、50.2%、23.9%和57.4%, GR活性升高34.4%(P<0.05),AsA和GSH含量分别提高1.17和1.28倍。SNP提高根系抗氧化系统活性,SOD、CAT、GPX、APX和GR活性分别比NaCl处理提高54.2%、83.3%、125.9%、78.3%和25.6%,AsA和GSH含量也进一步提高(P<0.05)(表2)。c-PTIO、钨酸盐和L-NAME处理能抑制SOD、GPX、CAT、APX和GR活性,降低AsA和GSH含量(P<0.05)。添加SNP能缓解c-PTIO、钨酸盐和L-NAME处理对抗氧化系统活性的抑制;亚铁氰化钠处理对苜蓿幼苗根系抗氧化系统的影响与NaCl处理类似(P>0.05)(表2)。

表2 SNP对盐胁迫下苜蓿根系抗氧化系统活性和NO含量的影响
2.5 SNP处理对盐胁迫下苜蓿根系NO含量的影响
3 结论与讨论
根系生长受抑制是盐胁迫下植物最早和最明显的症状[23],本试验中NaCl胁迫明显抑制苜蓿幼苗根系生长,用100 μmol/L SNP处理苜蓿幼苗,根系干重显著增加,NO清除剂c-PTIO处理则进一步降低了苜蓿幼苗根系干重,添加SNP能逆转c-PTIO对根生长的抑制,由于SNP被广泛用做NO供体,且SNP类似物亚铁氰化钠处理对苜蓿根的生长无促进效应,表明NO能够缓解盐胁迫对苜蓿幼苗根生长的抑制效应。
根系活力是判断植物对逆境适应能力的优良指标。本研究中,盐胁迫导致苜蓿幼苗根系活力显著降低,说明盐胁迫破坏了苜蓿根系的功能。NO作为生长素下游的一个信号分子,能替代IAA诱导植物侧根发育并促进根系对水分和养分的吸收[4,24];此外,NO还能调控生长素在根器官中的转运和平衡[25],抑制IAA氧化酶的活性,提高膜的转运活性和根系吸收功能[26],从而促进细胞的分裂和生长[27],增强根系活力和植株对逆境的适应能力,本研究也得到类似结果。
植物能通过积累可溶性蛋白、氨基酸和可溶性糖等一些小分子有机物缓解盐胁迫的不利影响[28]。其中可溶性蛋白除参与渗透调节外[29],还在一定程度上代表植物器官功能的变化[30];脯氨酸含量的增加也能调控植物渗透势, 提高植株耐性[31]。本试验中盐胁迫显著降低苜蓿幼苗根系中可溶性蛋白含量,提高了游离脯氨酸含量,说明盐胁迫破坏了根系功能,但可溶性蛋白的分解会产生大量的游离氨基酸,这有利于根系进行渗透调节。因此,盐胁迫下根系中可溶性蛋白的分解可能是游离脯氨酸含量升高的原因之一。SNP处理显著降低了根系中游离脯氨酸含量,但提高了可溶性蛋白含量,表明NO促进了植株蛋白的合成,这有助于维持根系渗透压和恢复根系功能。盐胁迫也提高了苜蓿根系中可溶性糖含量,但SNP处理使其含量显著降低,由于可溶性糖在植物体内具有渗透保护、碳储存和清除自由基的作用[32],这种变化可能与NO对碳水化合物的代谢调控有关,需做进一步研究。因此,NO通过渗透调节来维持根系渗透压和功能是促进盐胁迫下苜蓿根系生长的原因之一。

NOS和NR是植物体内源NO的最主要来源,但仍存在争议[9]。为明确苜蓿根系内源NO的来源和其对苜蓿根系耐盐性的影响,本试验用NO特异清除剂c-PTIO、NOS活性抑制剂和NR活性抑制剂处理苜蓿幼苗,结果表明,c-PTIO处理进一步提高根系中自由基含量,加剧膜脂过氧化作用,添加SNP能逆转c-PTIO的效应,SNP类似物处理对苜蓿根系的膜脂过氧化和ROS代谢无缓解效应,表明NO能缓解盐胁迫对苜蓿幼苗的氧化胁迫,且内源NO对根系耐盐也有一定作用。与c-PTIO处理类似,NOS抑制剂 L-NAME降低了根组织中NO的产生量和根系活力,加剧根系中ROS的积累和膜脂过氧化作用,抑制了根系的抗氧化系统活性和根系生长。但添加SNP则能提高根系中NO含量,缓解L-NAME的处理效应,表明根系中内源NO的合成可能依赖于NOS途径;用NR活性抑制剂钨酸盐处理,也得到与L-NAME类似的结果,然而,需要注意的是,钨酸盐不仅抑制NR活性,还能抑制植物根系生长、影响皮层微管的形成和诱导细胞的程序性死亡[41],且通过NR途径来源的NO也受培养液中氮源的影响,因此,本研究中,钨酸盐处理下根系伤害的加剧和内源NO来源及代谢变化的原因还需被进一步研究和阐明。
逆境胁迫可以改变内源NO的代谢[42],本试验中盐胁迫降低了苜蓿根系中NO的积累,这与一年生蒺藜苜蓿[26]上的研究结果一致,但与拟南芥[43]上的研究结果相反,这可能与研究中使用的材料类型,胁迫因子及处理时间有关。然而,内源NO必须积累达到一定浓度才能启动根的生长[44]。因此,内源NO浓度降低也可能是盐胁迫下苜蓿根系生长降低的原因,而添加外源SNP后,促进了内源NO的积累,达到启动根系生长所需的浓度阈值,从而促进苜蓿植株根系的生长,但相关机制还应做进一步研究。
综上所述,SNP处理能明显促进根系中NO的积累,提高苜蓿幼苗根系可溶性蛋白含量、根系活力和根系中抗氧化系统活性,降低自由基含量和膜脂过氧化作用,缓解盐胁迫对苜蓿幼苗根系的氧化伤害和生长抑制。NO特异清除剂c-PTIO、NOS活性抑制剂和NR活性抑制剂处理盐胁迫下苜蓿幼苗,进一步降低了根系活力和根系中NO产量,抑制了根系抗氧化系统活性,提高了根系中自由基含量和膜脂过氧化作用,加剧了盐渍对苜蓿根系生长的抑制,表明依赖于NOS活性和/或NR活性产生的内源NO也参与盐胁迫下苜蓿幼苗根系耐盐性的调节。
[1] 闻玉, 赵翔, 张骁. 水分胁迫下一氧化氮对小麦幼苗根系生长和吸收的影响. 作物学报, 2008, 34(2): 344- 348.
[2] Chen C W, Yang Y W, Lur H S, Tsai Y G, Chang M C. A novel function of abscisic acid in the regulation of rice (OryzasativaL.) root growth and development. Plant and Cell Physiology, 2006, 47(1): 1- 13.
[3] Qiao W H, Fan L M. Nitric oxide signaling in plant responses to abiotic stresses. Journal of Integrative Plant Biology, 2008, 50(10): 1238- 1246.
[4] Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta, 2004, 218(6): 900- 905.
[5] 张美玲, 廖伟彪, 肖洪浪. 一氧化氮和过氧化氢对万寿菊不定根形成的影响. 中国沙漠, 2012, 32(1): 105- 111.
[6] 陈明, 沈文飚, 阮海华, 徐朗莱. 一氧化氮对盐胁迫下小麦幼苗根生长和氧化损伤的影响. 植物生理与分子生物学学报, 2004, 30(5): 569- 576.
[7] 刘建新, 胡浩斌, 王鑫. 外源NO对盐胁迫下黑麦草幼苗根生长抑制和氧化损伤的缓解效应. 植物研究, 2008, 28(1): 7- 13.
[8] Shi Q H, Ding F, Wang X F, Wei M. Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiology and Biochemistry, 2007, 45(8): 542- 550.
[9] Moreau M, Lindermayr C, Durner J, Klessig D F. NO synthesis and signaling in plants-where do we stand. Physiologia Plantarum, 2010, 138(4): 372- 383.
[10] 邹琦. 植物生理学实验指导. 北京: 中国农业出版社, 2006.
[11] Halliwell B, Grootveld M, Gutteridge J M C. Methods for the measurement of hydroxyl radicals in biochemical systems: deoxyribose degradation and aromatic hydroxylation. Methods of Biochemical Analysis, 2006, 33: 59- 90.
[12] Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Science, 2000, 151(1): 59- 66.
[13] 王爱国, 罗广华. 植物的超氧物自由基与羟胺反应的定量关系. 植物生理学通讯, 1990, (6): 55- 57.
[14] de Azevedo N A, Priscob J T, Enéas-Filho J, Rolim Medeiros J V, Gomes-Fiho E. Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants. Journal of Plant Physiology, 2005, 162(10): 1114- 1122.
[15] Giannopolitis C N, Ries S K. Superoxide dismutases. I. Occurrence in higher plants. Plant Physiology, 1977, 59(2): 309- 314.
[16] Beers R F, Sizer I W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. Journal of Biological Chemistry, 1952, 195(1): 133- 140.
[17] Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidases in spinach chloroplasts. Plant and Cell Physiology, 1981, 22(5): 867- 880.
[18] Urbanek H, Kuzniak-Gebarowska E, Herka K. Elicitation of defense responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiologiae Plantarum, 1991, 13(1): 43- 50.
[19] Foyer C H, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta, 1976, 133(1): 21- 25.
[20] Law M Y, Charles S A, Halliwell B. Glutathione and ascorbic acid in spinach (Spinaciaoleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochemical Journal, 1983, 210(3): 899- 903.
[21] Griffith O W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Analytical Biochemistry, 1980, 106(1): 207- 212.
[22] Zhou B Y, Guo Z F, Xing J P, Huang B R. Nitric oxide is involved in abscisic acid-induced antioxidant activities inStylosanthesguianensis. Journal of Experimental Botany, 2005, 56(422): 3223- 3228.
[23] 弋良朋, 王祖伟. 盐胁迫下3种滨海盐生植物的根系生长和分布. 生态学报, 2011, 31(5): 1195- 1202.
[24] Chen W W, Yang J L, Qin C, Jin C W, Mo J H, Ye T, Zheng S J. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiology, 2010, 154(2): 810-819.
[25] Fernández-Marcos M, Sanz L, Lewis D R, Muday G K, Lorenzo O. Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proceeding of the National Academy of Science of the United States of America, 2011, 108(45): 18506- 18511.
[26] Xu J, Wang W Y, Yin H X, Liu X J, Sun H, Mi Q. Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots ofMedicagotruncatulaseedlings under cadmium stress. Plant Soil, 2010, 326(1/2): 321- 330.
[27] Zhang Y H, Liu H, Yin H, Wang W X, Zhao X M, Du Y G. Nitric oxide mediates alginate oligosaccharides-induced root development in wheat (TriticumaestivumL.). Plant Physiology and Biochemistry, 2013, 71: 49- 56.
[28] 周万海, 师尚礼, 寇江涛. 盐胁迫下外源NO对苜蓿幼苗生长及氮代谢的影响. 应用生态学报, 2012, 23(11): 3003- 3008.
[29] Sadeghipour O, Monem R, Tajal A A. Production of mungbean (VignaradiataL.) as affected by nitrogen and phosphorus fertilizer application. Journal of Applied Sciences, 2010, 10(10): 843- 847.
[30] Cernusak L A, Aranda J, Marshall J D, Winter K. Large variation in whole-plant water-use efficiency among tropical tree species. New Phytologist, 2007, 173(2): 294- 305.
[31] Wang X Z, Wang H, Wu F Z, Liu B. Effects of cinnamic acid on the physiological characteristics of cucumber seedlings under salt stress. Frontiers of Agriculture China, 2007, 1(1): 58- 61.
[32] 廖岩, 彭友贵, 陈桂珠. 植物耐盐性机制研究进展. 生态学报, 2007, 27(5): 2077- 2085.
[33] Lu S Y, Zhuo C L, Wang X H, Guo Z F. Nitrate reductase (NR)-dependent NO production mediates ABA- and H2O2-induced antioxidant enzymes. Plant Physiology and Biochemistry, 2014, 74: 9- 15.
[34] 刘伟成, 郑春芳, 陈琛, 彭益全, 曾国权, 冀德伟, 陈少波, 谢起浪, 於俊琦. 花期海蓬子对盐胁迫的生理响应. 生态学报, 2013, 33(17): 5184- 5193.
[35] Noctor G, Foyer C H. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology, 1998, 49(1): 249- 279.
[36] Tanou G, Molassiotis A, Diamantidis G. Induction of reactive oxygen species and necrotic death-like destruction in strawberry leaves by salinity. Environmental and Experimental Botany, 2009, 65(2/3): 270- 281.
[37] Horváth E, Szalai G, Janda T. Induction of abiotic stress tolerance by salicylic acid signaling. Journal of Plant Growth Regulation, 2007, 26(3): 290- 300.
[38] Yamasaki H, Sakihama Y, Takahashi S. An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends in Plant Science, 1999, 4(4): 128- 129.
[39] Ungar I A. Ecophysiology of Vascular Halophytes. Boca Raton: CRC Press, 1991.
[40] Innocenti G, Pucciariello C, Gleuher M L, HopkiNs J, Stefano M D, Delledonne M, Puppo A, Baudouin E, Frendo P. Glutathione synthesis is regulated by nitric oxide inMedicagotruncatularoots. Planta, 2007, 225(6): 1597- 1602.
[41] Xiong J, Fu G F, Yang Y J, Zhu C, Tao L X. Tungstate: is it really a specific nitrate reductase inhibitor in plant nitric oxide research. Journal of Experimental Botany, 2012, 63(1): 33- 41.
[42] Méndez-Bravo A, Raya-González J, Herrera-Estrella L, López-Bucio J. Nitric oxide is involved in alkamide-induced lateral root development in Arabidopsis. Plant and Cell Physiology, 2010, 51(10): 1612- 1626.
[43] Besson-Bard A, Gravot A, Richaud P, Auroy P, Duc C, Gaymard F, Taconnat L, Renou J P, Pugin A, Wendehenne D. Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiology, 2009, 149(3): 1302- 1315.
[44] Wang P, Du Y, Li Y, Ren D, Song C P. Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. The Plant Cell, 2010, 22(9): 2981- 2998.
Nitric oxide protection of alfalfa seedling roots against salt-induced inhibition of growth and oxidative damage
ZHOU Wanhai1,2, FENG Ruizhang1, SHI Shangli2,*, KOU Jiangtao2
1LaboratoryofSouthwesternEconomicPlants,CollegeofLifeScience&FoodEngineering,YibinUniversity,Yibin644000,China2KeyEcosystemLaboratoryoftheMinistryofEducation,CollegeofGrasslandScience,GansuAgriculturalUniversity,Lanzhou730070,China
Roots play important roles in anchoring plants in the soil and absorbing water and nutrients from the soil. Soil salinity is one of the major abiotic stresses that adversely affect root growth and development. The exogenous application of some bioactive substances is a simple and effective approach to improve plant stress tolerance in agricultural production. Nitric oxide (NO) is a bioactive, gaseous, multifunctional molecule which plays a central role and mediates a variety of physiological processes and responses to biotic and abiotic stresses, including salt. However, information on the effects of exogenous SNP on alfalfa root systems under salt stress conditions and its physiological mechanisms is lacking. In this study,MedicagosativaL. cv. Gannong No.4 was used as the experimental material and NO-donor sodium nitroprusside (SNP), NO-scavenger 2-(4-carboxyphenyl)- 4,4,5,5-tetramethylimidazoline- 1-oxyl- 3-oxide (c-PTIO), tungstate, the nitrate reductase (NR) inhibitor, nitric oxide synthase (NOS) inhibitor NG-nitro-L-Arg-methyl ester (L-NAME) and sodium ferrocyanide (SNP analogue, does not release NO) were used to investigate the effects of NO on growth, root vigor, osmoregulatory molecules, membrane lipid peroxidation, reactive oxygen species (ROS) accumulation and the activity of antioxidant enzyme in alfalfa roots under NaCl stress. Results showed that when 100 μmol/L SNP was applied in the nutrient solution under NaCl stress, the growth of alfalfa roots was significantly improved for eight days, the root vigor value and the content of free proline were increased and the content of soluble protein was decreased throughout the treatment period. SNP also caused an increase in the activities of ascorbate peroxidase (APX), catalase (CAT), glutathione reductase (GR), guaiacol peroxidase (GPX), superoxide dismutase (SOD), and the contents of reduced ascorbic acid (AsA) and reduced glutathion (GSH) under salt stress. Whereas, the content of H2O2, OH·, production rate of O·-2and MDA level were decreased in alfalfa roots. Meanwhile, SNP pretreatment improved the endogenous NO accumulation. Sodium ferrocyanide, the NO-donor SNP analogue, has no noticeable effect on the damage caused by NaCl stress for alfalfa roots. When applied with the NO-scavenger c-PTIO, under NaCl stress, the NR inhibitor tungstate and NOS inhibitor L-NAME both reduced the accumulation of endogenous NO in roots, further inhibited the activity of the antioxidant system, aggravated membrane lipid peroxidation and root growth, however, their harmful effects on growth, antioxidant system and membrane lipid peroxidation could be reversed by the addition of SNP. Thus, the results showed that application of SNP significantly increased root vigor, activated the antioxidant system, alleviated the oxidative root damage induced by salt stress and promoted root growth under NaCl stress. Endogenous NO may also take part in the regulation of salt-tolerance of alfalfa roots under NaCl stress, and the release of NO according to the NOS and NR pathways may play an important role in alleviating the inhibitive effect in alfalfa root growth. These results may provide a theoretical basis for the mechanisms of salt resistance, help research into NO in the chemical regulation of alfalfa salt tolerance, the breeding of salt tolerant alfalfa cultivars, and the utilization of saline land in the future.
Nitric oxide; alfalfa; salt stress; antioxidant systems; roots
牧草种质资源保护利用(NB2130135); 国家牧草产业技术体系专项(CARS- 35); 宜宾学院重点科研项目(2013QD06)
2013- 10- 14;
2014- 08- 22
10.5846/stxb201310142472
*通讯作者Corresponding author.E-mail: shishl@gsau.edu.cn
周万海, 冯瑞章, 师尚礼, 寇江涛.NO对盐胁迫下苜蓿根系生长抑制及氧化损伤的缓解效应.生态学报,2015,35(11):3606- 3614.
Zhou W H, Feng R Z, Shi S L, Kou J T.Nitric oxide protection of alfalfa seedling roots against salt-induced inhibition of growth and oxidative damage.Acta Ecologica Sinica,2015,35(11):3606- 3614.

