Embryonic natural orif ce transluminal endoscopic surgery in the treatment of severe acute pancreatitis complicated by abdominal compartment syndrome
Department of Gastroenterology, Aff liated Donghua Hospital of Sun Yat-sen University, Dongguan 523110, Guangdong Province, China
Embryonic natural orif ce transluminal endoscopic surgery in the treatment of severe acute pancreatitis complicated by abdominal compartment syndrome
Hui-ming Zhu, Shao-qing Guo, Xiu-min Liao, Li Zhang, Li Cai
Department of Gastroenterology, Aff liated Donghua Hospital of Sun Yat-sen University, Dongguan 523110, Guangdong Province, China
BACKGROUND:The study aimed to estimate the value of embryonal natural orifice transluminal endoscopic surgery (ENOTES) in treating severe acute pancreatitis (SAP) complicated with abdominal compartment syndrome (ACS).
METHODS:The patients, who were randomized into an ENOTES group and an operative group, underwent ENOTES and laparotomy, respectively. The results and complications of the two groups were compared.
RESULTS:Enterocinesia was observed earlier in the ENOTES group than in the operative group. Acute Physiology and Chronic Health Evaluation II (APACHE II) score of patients in the ENOTES group was lower than that of the operative group on the 1st, 3rd and 5th post-operative day (P<0.05). The cure rate was 96.87% in the ENOTES group, which was statistically different from 78.12% in the operative group (P<0.05). There were significant differences in complications and mortality between the two groups (P<0.01).
CONCLUSION:Compared with surgical decompression, ENOTES associated with flexible endoscope therapy is an effective and minimal invasive procedure with less complications.
Embryonal natural orif ce transluminal endoscopic surgery; Flexible endoscope; Peritoneal lavage; Peritoneal dialysis; Severe acute pancreatitis; Abdominal compartment syndrome
INTRODUCTION
Abdominal compartment syndrome (ACS) is one of the complications of severe acute pancreatitis (SAP). Damaged pancreatic cells induce tissue necrosis and the release of multiple enzymes and various inflammatory mediators, which increase the permeability of blood capillaries, resulting in systemic capillary leak syndrome (SCLS), abdominal viscera edema, seroperitoneum, enteroplegia and eventually increased intra-abdominal pressure complicated with ACS. Multiple organ failure in patients with SAP is closely correlated with intraabdominal hypertension and ACS;[1–3]if abdominal decompression is not performed timely, it is easy to cause multiple organ failure with a mortality of over 42%.[4–6]Researches[7,8]have demonstrated that early interventions are necessary for abdominal decompression of SAP patients with ACS, and surgical decompression is commonly used. However, it does not reduce the mortality sometimes. Therefore, it is essential to explore effective measures to treat SAP complicated with ACS.
Embryonal natural orifice transluminal endoscopic surgery (ENOTES) has been an endoscopic procedure for the minimally invasive treatment of digestive diseases in recent years. ENOTES can be performed with a flexible endoscope through the stomach, large intestine, vagina and bladder into the abdominal cavityto diagnose and treat intra-abdominal diseases. At present, NOTES and flexible endoscope surgery have been successfully used in the diagnosis and treatment for a number of intra-abdominal diseases because of their high eff cacy and less invasiveness.[9–14]The umbilicus is an embryonic natural orif ce that closes after a delivery, thus the treatment using a flexible endoscope through the umbilicus into the abdominal cavity is defined as ENOTES. In this study, we aimed to compare the eff cacy of ENOTES and surgical abdominal decompression in the treatment of SAP complicated with ACS.
METHODS
Clinical data
A total of 64 patients meeting the inclusion criteria were randomized equally into two groups, ENOTES and operation. The clinical data, including age, gender, laboratory results and organ function of patients in the two groups are shown in Table 1. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Affiliated Donghua Hospital of Sun Yat-sen University. Written informed consent was obtained from all patients or relatives.
Inclusion criteria
Diagnostic criteria for ACS included persistent intra-abdominal pressure >20 mmHg, with or without abdominal perfusion pressure (APP) <60 mmHg, and organ dysfunction or failure.[15]
ENOTES and therapeutic peritoneoendoscopy
ENOTES was done within 4 hours after admission: 1) A 0.8 cm abdominal incision was made in patients with tracheal intubation under general anesthesia. The pneumoperitoneum was created by CO2insuff ation througha disposable needle. After puncture with a needle, a guide wire was inserted through the needle to the abdominal cavity. In the abdominal cavity a cannular sheath was placed. 2) An electronic endoscope was inserted into the abdominal cavity through the cannular sheath for examination. 3) Inf ammatory secretions were removed by the endoscope from the enterocoelia, which was washed with 4 000 mL normal saline. For patients with effusion of the lesser omentum sac, the gastrocolic ligament was cut off for drainage and the lesser omentum sac was cleansed with an endoscope. 4) An irrigatiion tube and a drain tube were respectively placed at the inferior margin of the liver and pelvic cavity under the endoscopic guidance. 5) Peritoneal lavage and dialysis were performed after administration of 2 000 mL of 1.5% glucose dialysate and retained for 1 hour in a 4-hour interval for 7 consecutive days.
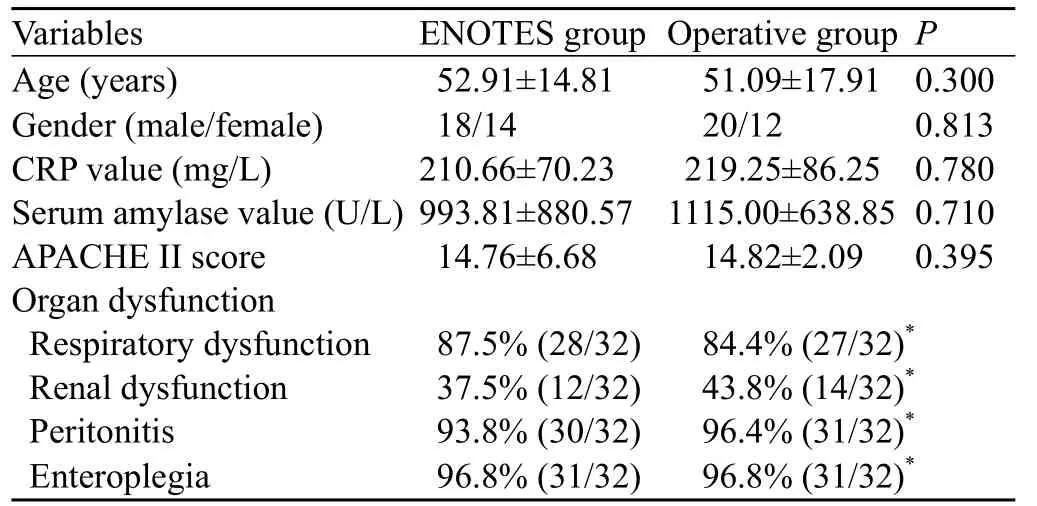
Table 1. Clinical data of patients in the two groups
Surgical decompression and drainage
Laparotomy was performed to remove necrotic tissue around the pancreas. Pancreatic capsular was cut to decompress and a drain tube was put into the intraabdominal cavity.
Routine treatment
Routine treatment included ambrosia, gastrointestinal decompression, oxygen therapy, inhibition of pancreatic enzyme secretion, fluid resuscitation, nutrition support and prophylactic anti-infection, etc.
Statistical analysis
Data analysis was made with SPSS17.0 software. The data were presented as mean±standard deviation and analyzed with Student's t test, and enumeration data were calculated using the Chi-square test. A P value less than 0.05 was considered statistically signif cant. The repeatedly measured data were analyzed using analysis of variance.
RESULTS
Treatment overview
There were differences in operative time and frequency, length of hospital stay and hospital expenses between the two groups (P<0.001, Table 2).
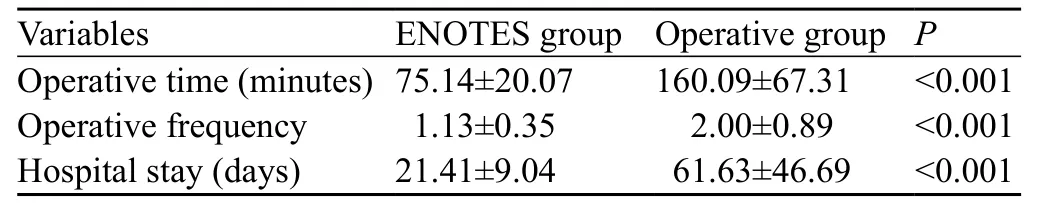
Table 2. Treatment overview in the ENOTES and operative groups

Table 3. Changes of bowl sounds (time/minute; mean±SD)
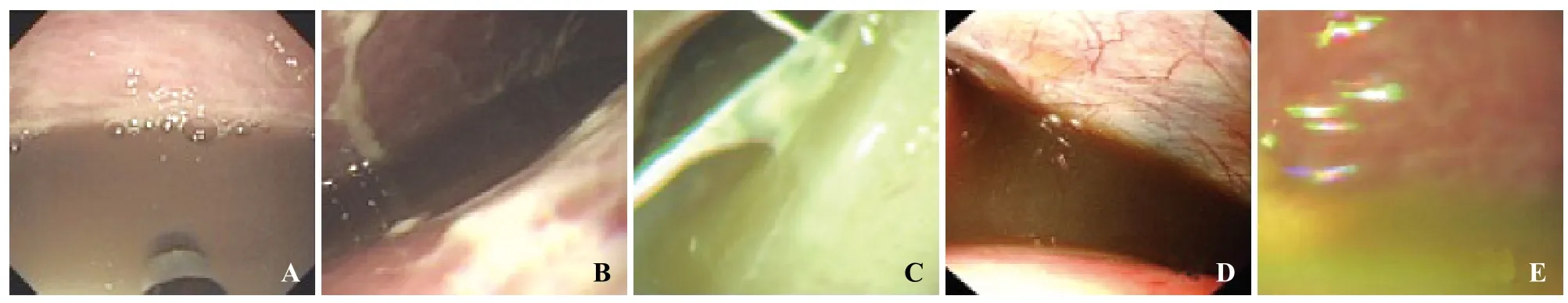
Figure 1. Abdominal cavity effusion. A: serous ascites; B: f brin ascites; C: suppurative ascites; D: bloody ascites; E: biliary ascites.
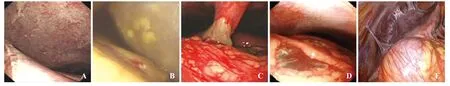
Figure 2. Peritoneal lesion. A: diffuse peritonitis; B: peritoneal fatty plaques; C: peritoneal ulcer; D: defect of the greater omentum; E: peritoneal adhesive band.
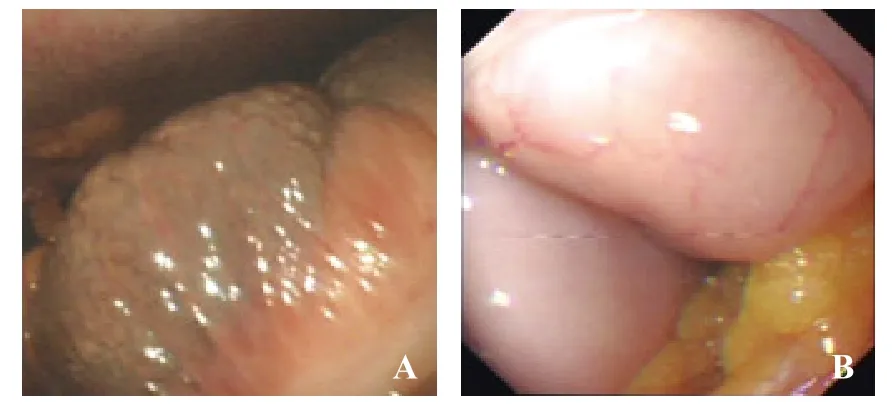
Figure 3. Lesion caused by the intestinal tube. A: intestinal congestion; B: intestinal f atulence.
Findings of abdominal exploration
In the ENOTES group (32 patients), peritoneoendoscopic findings included peritoneal congestion and edema in 46.9% (15) patients, peritoneal adhesive band in 18.7% (6), peritoneal ulcer in 15.5% (5), peritoneal fatty plaques in 12.5% (4), and defect of the greater omentum in 6.3% (2). The volume of abdominal cavity effusion in the 32 patients ranged from 300 to 2 100 mL, with a mean of 1 100 mL. In 32 patients with ascites, those with serous ascites accounted for 43.7% (14), fibrin ascites 21.8% (7), bloody ascites 18.7% (6), suppurative ascites 9.3% (3), and biliary ascites 6.2% (2). Twenty-nine (90.6%) patients had intestinal flatulence and 3 (9.4%) had intestinal congestion. Peritoneal lesions, abdominal cavity effusion, and lesion caused by the intestinal tube are shown in Figures 1–3.
Changes of bowel sounds and abdominal ressure
No significant difference in the number of bowel sounds was observed between the ENOTES and operative groups before the operation (P>0.05). Intestinal peristalsis was observed on the f rst day after operation in the patients who underwent endoscopic treatment, and peritoneal lavage or dialysis in the ENOTES group was done earlier than in the surgery group (the third day, Table 3). The bowel sounds in both groups were normal on the fifth day. Abdominal pressure in the ENOTES group was not markedly different from that of the operative group either before or after the operation (P> 0.05, Table 4).
Dynamic APACHE II score
APACHE II scores of patients in the ENOTES group were lower than those in the operative group on the f rst, third and fifth day after treatment (P<0.05), whereas there was no difference in APACHE II scores between the two groups before and the seventh day after the operation (P>0.05, Table 5).
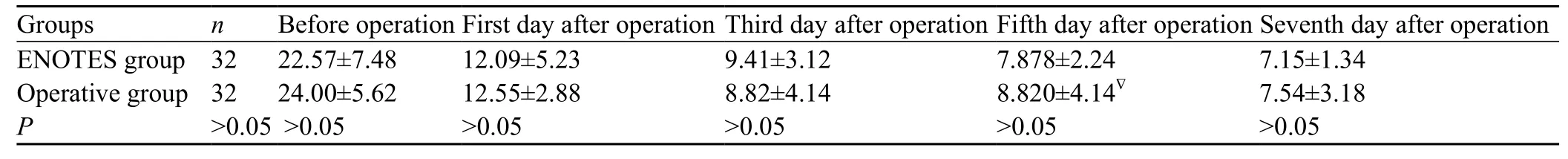
Table 4. Changes of abdominal pressure before and after the operation (mean±SD)

Table 5. Change of APACHE II scores in the two groups (mean±SD)
Therapeutic effects and complications
The cure rate was 96.87% in the ENOTES group, which was statistically different from 78.12% in the operative group (P<0.05). In the ENOTES group two patients had bleeding from abdominal incision and two had abdominal infection (4; 12.5%). But in the operative group, bleeding from abdominal incision was observed in 2 patients, abdominal infection in 7 patients, intraabdominal hemorrhage in two patients, intestinal f stula in 1 patient, biliary f stula in 1 patient and ventral hernia in 2 patients (15; 46.87%). There was a significant difference in complication rate between the two groups (P<0.01).
Therapeutic effects and complications
The cure rate in the ENOTES group was 96.87%, which was statistically different from 78.12% in the operative group (P<0.05). The complications in the ENOTES group included bleeding from abdominal incision (2 patients) and abdominal infection (2). The complications in the operative group included bleeding from abdominal incision (2 patients), abdominal infection (7), intra-abdominal hemorrhage (2), intestinal f stula (1), biliary f stula (1), and ventral hernia (2). The difference in complication rate was significant between the two groups (P<0.01).
DISCUSSION
In recent years, surgical abdominal decompression is commonly used in the treatment of SAP complicated with ACS. Although this method is effective, the mortality is still high. This study was to evaluate the effect of ENOTES in the treatment of SAP complicated by ACS. The cure rate was statistically higher in the ENOTES group than in the operative group, and there were a lower incidence of complications and an earlier recovery of intestinal peristalsis. APACHE II score in the ENOTES group was lower than that in the operative group on the 1st, 3rd and 5th day after treatment (P<0.05). This f nding indicated that in the treatment of SAP complicated with ACS, ENOTES is more eff cious than surgical decompression.
SAP initiates from the activation of mononuclear macrophages and neutrophils by pancreatic enzymes from abnormal pancreas and a release of a large number of cytokines and inf ammatory mediators. Thus inf ammatory effects are amplified to induce systemic inflammatory response syndrome (SIRS), and eventually lead to multiple organ dysfunction syndrome (MODS).[16–18]In SAP patients with SIRS, the release of abundant inf ammatory mediators causes capillary endothelial damage, leakage of plasma into the interstitial space and serous cavity, SCLS and positive fluid balance. However, fluid resuscitation offsets positive fluid balance, maintains effective blood volume, and finally induces peritoneal and progressive visceral edema. In addition, diffuse pancreatic fluid collection in the peritoneal cavity and paralytic ileus finally result in a rapid increase of intraabdominal pressure or ACS.[19–21]
ACS can be divided into effusion and flatulence types according to its clinical manifestations. Patients with abdominal effusion demonstrated abundant effusion in the abdominal cavity complicated by tissue or organ edema in the intra-abdominal omentum, mesangial, intestinal canal and posterior parietal peritoneum. Those with flatulence were characterized by intestinal paralysis and restricted intestinal movement, which lead to gastrointestinal pneumatosis and effusion as well as ileus. However, both types of ACS share the same pathophysiological effects on the body, i.e. a rapid increase of intra-abdominal pressure causes ACS, while inducing intra-abdominal and systemic organ dysfunction and organ failure.
The treatment for intra-abdominal hypertension and ACS has always been the academic focus. Different from ACS induced by abdominal trauma and hemorrhagic shock, SAP complicated by ACS has both primary and secondary factors at the same time. Severe SIRS complicated by ACS aggravates SIRS and MODS. Laparotomy not only has a high risk, but also increases the chance of abdominal infection. Some patients require multiple operations, which may result in high rates of complications and mortality. Although surgical abdominal decompression is considered as an effective procedure for ACS, trauma and intra-abdominal interference as well as anesthetic effects on the liver and other organs may destroy the defense function of the body and the stability of internal environment but increase the chance of tissue infection. In addition, laparotomy also produces diff culty in abdominal closure because of high intra-abdominal pressure. Therefore, surgical decompression for early SAP complicated by ACS should be carefully considered by weighing the pros and cons of the operation.[22]
ENOTES is valuable in the treatment of SAP complicated by ACS. First, it is important to remove various substances from the abdominal cavity such as pancreatic enzymes, inf ammatory mediators, vasoactive polypeptide, cellulose, necrotic tissue, blood and bile in the abdominal cavity and peripancreatic exudate. All of which may cause local or systemic damage in SAP patients with ACS. The local damage includes chemical peritonitis, enteroplegia, microcirculation disturbance and pancreatic necrosis induced by oppression of the pancreas, intra-abdominal hypertension, ACS and secondary infection at late course, in addition to adhesion, enveloping and separation formed in the abdominal cavity or around the pancreas. The systemic damage refers to the absorption of toxin in inf ammatory exudates, which aggravates inflammatory response, accelerates SAP process and induces MODS, especially acute respiratory distress syndrome (ARDS) and acute renal failure. Intra-abdominal harmful substances are effectively eliminated through drainage and flushing during ENOTES. Second, ENOTES could rapidly relieve intra-abdominal hypertension, restore peristalsis and normalize intra-abdominal pressure by removing peritoneal effusion and f ushing intra-abdominal harmful substances. Third, the operation could guide abdominal catheterization for continuous lavage and drainage, and timely remove postoperative inflammatory exudate in the abdominal cavity. Forth, peritoneal dialysis could be carried out to eliminate inflammatory mediators and alleviate SIRS induced by cascade amplification of inflammatory mediators. Fifth, hyperosmotic peritoneal dialysate can be used in ACS patients with f atulence to relieve edema of the bowel wall and restore peristalsis.
ENOTES with a flexible endoscope is minimally invasive in the treatment of SAP complicated by ACS. Damage control is emphasized in SAP patients with systemic inflammatory response, infection, stress and multi-organ functional disturbance. Therefore, complications such as blood loss and large surgical trauma are severe damages. Hence it is reasonable to determine minimal invasion during the treatment. ENOTES using a flexible endoscope has the following advantages: less pneumoperitoneum, single incision for endoscopy and small abdominal interference, etc which are extremely benef cial to SAP patients with ACS.
In conclusion, the present study demonstrated that ENOTES could rapidly remove intra-abdominal inflammatory exudates, relieve intra-abdominal hypertension, dialyze inf ammatory mediators and block the vicious circle in SAP complicated by ACS. Compared with surgical abdominal decompression, ENOTES using a flexible endoscope is a more effective and minimal invasive procedure, with less complications.
Funding:None.
Ethical approval:This study was conducted with approval from the Ethics Committee of the Affiliated Donghua Hospital of Sun Yat-sen University, Dongguan, China.
Conf icts of interest:No any benef ts have been received from a commercial party related directly or indirectly to the study.
Contributors:Zhu HM proposed and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts.
1 Al-Bahrani AZ, Abid GH, Holt A, McCloy RF, Benson J, Eddleston J, et al. Clinical relevance of intra-abdominal hypertension in patients with severe acute pancreatitis. Pancreas 2008; 36: 39–43.
2 De Waele JJ, Leppäniemi AK. Intra- abdominal hypertension in acute pancreatitis. World J Surg 2009; 33: 1128–1133.
3 Keskinen P, Leppaniemi A, Pettila V, Piilonen A, Kemppainen E, Hynninen M. Intra-abdominal pressure in severe acute pancreatitis. World J Emerg Surg 2007; 2: 2–6.
4 Chen H, Li F, Sun JB, Jia JG. Abdominal compartment syndrome in patients with severe acute pancreatitis in early stage. World J Gastroenterol 2008; 14: 3541–3548.
5 Dambrauskas Z, Parseliunas A, Gulbinas A, Pundzius J, Barauskas G. Early recognition of abdominal compartment syndrome in patients with acute pancreatitis. World JGastroenterol 2009; 15: 717–721.
6 Caronna R, Benedetti M, Morelli A, Rocco M, Diana L, Prezioso G, et al. Clinical effects of laparotomy with perioperative continuous peritoneal lavage and postoperative hemofiltration in patients with severe acute pancreatitis. World J Emerg Surg 2009; 4: 45–49.
7 Anand RJ, Ivatury RR. Surgical management of intra-abdominal hypertension and abdominal compartment syndrome. Am Surg 2011; 77: 42–45.
8 Mentula P, Hienonen P, Kemppainen E, Puolakkainen P, Leppäniemi A. Surgical decompression for abdominal compartment syndrome in severe acute pancreatitis. Arch Surg 2010; 145: 764–769.
9 Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, et al. Flexible transgastric peritoneoscopy: A novel approach to diagnostic andtherapeutic interventions in the peritoneal cavity. Gastrointest Endosc 2004; 60: 114–117.
10 Voermans RP, Faigel DO, van Berge Henegouwen MI, Sheppard B, Fockens P. Comparison of transcolonic NOTES and laparoscopic peritoneoscopy for the detection of peritoneal metastases. Endoscopy 2010; 42: 904–909.
11 Wu XL, Long D, Yu L, Yang JH, Zhang YC, Geng F. Urokinasetype plasminogen activator receptor as a predictor of poor outcome in patients with systemic inflammatory response syndrome. World J Emerg Med 2013; 4: 190–195.
12 Khashab MA, Kalloo AN. NOTES: current status and new horizons. Gastroenterology 2012; 142: 704–710.
13 Gumbs AA, Fowler D, Milone L, Evanko JC, Ude AO, Stevens P, et al. Transvaginal natural orifice translumenal endoscopic surgery cholecystectomy: early evolution of the technique. Ann Surg 2009; 249: 908–912.
14 Zorrón R, Soldan M, Filgueiras M, Maggioni LC, Pombo L, Oliveira AL. NOTES: transvaginal for cancer diagnostic staging: preliminary clinical application. Surg Innov 2008; 15: 161–165.
15 Terayama T, Hifumi T, Kiriu N, Kato H, Koido Y, Ichinose Y, et al. A minimally invasive multiple percutaneous drainage technique for acute necrotizing pancreatitis. World J Emerg Med 2014; 5: 310–312.
16 Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, et al. Pathophysiology of acute pancreatitis. Pancreatology 2005; 5: 132–144.
17 Menger MD, Plusczyk T, Vollmar B. Microcirculatory derangements in acute pancreatitis. J Hepatobiliary Pancreat Surg 2001; 83: 187–194.
18 Gunther A, Walmrath D, Grimminger F, Seeger W. Pathophysiology of acute lung injury. Semin Respir Crit Care Med 2001; 22: 247–258.
19 Dugernier T, Laterre PF, Reynaert MS. Ascites fluid in severe acute pancreatitis: from pathophysiology to therapy. Acta Gastroenterol Belg 2000; 63: 264–268.
20 An G, West MA. Abdominal compartment syndrome: a conciseclinical review. Crit Care Med 2008; 36: 1304–1310.
21 Leppaniemi A, Johansson K, De Waele JJ. Abdominal compartment syndrome:and acute pancreatitis. Acta Clin Belg Suppl 2007; 1: 131–135.
22 Diuzheva TG, Shefer AV. Intra-abdominal hypertension in patients with severe acute pancreatitis. Khirurgiia (Mosk) 2014; 1: 21–29.
Received August 10, 2014
Accepted after revision January 15, 2015
Hui-ming Zhu, Email: zhuhuiming212011@163.com
World J Emerg Med 2015;6(1):23–28
10.5847/wjem.j.1920–8642.2015.01.004
 World journal of emergency medicine2015年1期
World journal of emergency medicine2015年1期
- World journal of emergency medicine的其它文章
- Evaluation of a simulation-based workshop on clinical performance for emergency physicians and nurses
- Ultrasound diagnosis of malaria: examination of the spleen, liver, and optic nerve sheath diameter
- Effect of low-dose glucocorticoid on corticosteroid insuff cient patients with acute exacerbation of chronic obstructive pulmonary disease
- Prognostic value of CD4+CD25+Tregs as a valuable biomarker for patients with sepsis in ICU
- Effect of harmless acute pancreatitis score, red cell distribution width and neutrophil/lymphocyte ratio on the mortality of patients with nontraumatic acute pancreatitis at the emergency department
- The risk of wound infection after simple hand laceration
